Abstract
BACKGROUND
We sought to define the impact of cortisol-secreting status on outcomes after surgical resection of adrenocortical carcinoma (ACC).
METHODS
The U.S ACC group database was queried to identify patients who underwent ACC resection between 1993 and 2014. The short-term and long-term outcomes were assessed.
RESULTS
The incidence of all functional and cortisol-secreting tumors was 40.6% and 22.6%, respectively. On multivariable analysis, cortisol secretion remained associated with an increased risk of postoperative complications (odds ratio = 2.25, 95 % confidence interval = 1.04 to 4.88; P = .04). At a median follow-up of 17.6 months, 118 patients (50.4%) had developed a recurrence. On multivariable analysis, after adjusting for patient and disease-related factors cortisol secretion independently predicted shorter recurrence-free survival (Hazard ratio = 2.05, 95% confidence interval = 1.16 to 3.60; P = .01).
CONCLUSIONS
Cortisol secretion was associated with an increased risk of postoperative morbidity. Recurrence remains high among patients with ACC after surgery; cortisol secretion was independently associated with a shorter recurrence-free survival. Tailoring postoperative surveillance of ACC patients based on their cortisol secreting status may be important.
Keywords: Adrenocortical, Carcinoma, Cortisol, Outcomes
Although adrenocortical carcinoma (ACC) is often diagnosed as an incidental finding on cross-sectional imaging, up to 40% to 60% of patients present with symptoms.1 Clinical syndromes are typically secondary to the autonomous production of hormones that include androgens, estrogens, mineralocorticoids, and corticosteroids.2,3 Unlike patients with nonfunctional tumors who may present with nonspecific symptoms related to local mass effects of the tumor such as abdominal pain,4,5 patients with functional tumors often present with very hormone-specific symptoms. For example, androgen-secreting tumors can lead to gynecomastia and testicular atrophy,6 whereas excessive mineralocorticoid secretion commonly induces hypertension.7 In addition, cortisol-secreting tumors commonly present with rapidly progressing Cushing’s syndrome.8,9
Cortisol-secreting tumors are the most common hormonal-functional ACCs.3 However, the impact of cortisol-secreting tumors and their distinct biologic behavior remain poorly defined. Cortisol has been demonstrated in general to suppress immune surveillance by blunting the cellular immune response possibly leading to tumor growth and recurrence.10 Of note, recurrence after ACC surgery is particularly common, occurring in 50% to 90% of patients.6,11–13 To date, only a handful of single-institution studies have explicitly examined the effect of cortisol-secretion on the long-term outcomes of patients with ACC.14–17 More importantly, these studies have suffered from small sample size, conflicting results, and variable inclusion criteria.14–17 As such, the objective of the present study was to examine the outcomes of patients with cortisol-secreting ACC tumors using a large, multicenter collaborative database. In particular, given that cortisol-secreting ACC tumors are the most common hormonal tumor, and the importance of determining prognosis after surgical management of ACC, we sought to define the short-term outcomes and the long-term prognostic impact of cortisol secretion among patients who underwent resection of ACC.
Methods
Study design
Patients were identified from a retrospective, multi-institutional database of 234 patients who underwent surgery for ACC between 1993 and 2014 at 13 major cancer centers in the United States. The 13 institutions participating in the study included Johns Hopkins Hospital, Baltimore, MD; Emory University, Atlanta GA; Stanford University, Palo Alto, CA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; The Ohio State University, Columbus, OH; Medical College of Wisconsin, Milwaukee, WI; New York University, New York, NY; University of California at San Diego, San Diego, CA; University of California at San Francisco, San Francisco, CA; University of Texas Southwestern Medical Center, Dallas, TX; and Vanderbilt University Medical Center, Nashville, TN. Only patients with histologically confirmed ACC were included in the study group. Overall, patients less than 18 years old were excluded in the present study. The Institutional Review Board of each institution approved the study.
Data on patient demographic and clinicopathologic features including age, sex, race, and tumor-specific characteristics were collected. In particular, data were obtained on ACC tumor size, weight, laterality, lymph node status, and presence of capsular invasion. In addition, we recorded the presence of clinical signs and symptoms. Data on T stage were also collected according to 7th edition American Joint Committee on Cancer staging system.18 Resection margin status was recorded and classified as microscopically negative (R0), microscopically positive (R1), or macroscopically positive (R2). Mitotic rate was defined as number of mitoses/50 high-powered field (HPF). Definition of the functional status of ACC was based on the standard biochemical evaluation of hormone excess. Patients with elevated hormone levels were considered to be hyper secreting irrespective of the presence of clinical symptoms. Furthermore, data regarding treatment details of ACC were collected, including surgical approach and receipt of adjuvant chemotherapy and mitotane. Perioperative complications and mortality were considered within 30 days from the operation. Complications were categorized based on the Clavien-Dindo classification system, with minor complications defined as grade I or II and major complications as grade III or IV.19 Additional data on the occurrence of postoperative adrenal insufficiency were collected. In addition, the length of hospital stay, date of the last follow-up visit, as well as recurrence and survival information was collected. Recurrence was defined as unequivocal radiologic evidence of a new tumor during follow-up or as a biopsy-proven ACC.
Statistical analysis
Discrete variables were described as medians with interquartile range (IQR) and categorical variables were described as totals and frequencies. Univariate comparisons for discrete variables were assessed using the Chi-square test and Fisher’s exact test, as appropriate. The Kruskal-Wallis test was used to compare continuous variables. Univariable and multivariable logistic regression models were assessed to determine specific prognostic factors for perioperative outcomes. Variables significant on univariable analysis (P < .05) were entered into the multivariable regression models. Overall survival (OS) and recurrence-free survival (RFS) were estimated using the Kaplan-Meier methods. Univariable and multivariable Cox proportional hazards models were built to determine factors predictive of risk of recurrence or death. For multivariable Cox proportional hazards models, variables with missing data were subjected to multiple imputation and all variables of clinical importance were included. Comparisons of survival between groups were made using the log-rank test. All analyses were performed with STATA, version 12.0 (StataCorp, College Station, TX), and P < .05 (2 tailed) was considered statistically significant.
Results
Demographic and clinicopathologic features
A total of 234 patients were identified. Table 1 shows the baseline characteristics of the entire cohort stratified by functional status and cortisol-secreting status. The median patient age was 52 years (IQR 44 to 63); most of the patients were female (n = 144, 61.5%) and Caucasian (n = 185, 81.1%). The median tumor size was 11.5 cm (IQR 8.0 to 15.0 cm). At the time of surgery, most of the patients underwent an open abdominal adrenalectomy (n = 152, 67%). The remaining patients underwent either an open thoraco-abdominal (n = 34, 15%) or a minimally invasive surgery (n = 41, 18.1%). On final histopathology, an R0 resection was achieved in most patients (n = 143, 68.4%). Most of the patients had T3/4 stage disease (n = 113, 52.8%). Most of the tumors (20.1%) had a mitotic rate of greater than 10 mitoses/50 HPF, whereas 14.5% had a mitotic rate of 6 to 10 mitoses/50 HPF and 10.7% had a mitotic rate of less than 5 mitoses/50 HPF. Overall, 36 patients (16.7%) received postoperative systemic chemotherapy, whereas 78 patients received adjuvant mitotane (42.2%). Preoperative chemotherapy was administered only to 4 patients (1.8%). Regarding the secretory status, 53 patients had cortisol- , 29 had estrogen/androgen- , and 13 patients had mineralocorticoid-secreting tumors.
Table 1.
Baseline characteristics of patients undergoing surgery for adrenocortical carcinoma
| Characteristic | Total (n = 234) |
Cortisol secreting (n = 53) |
Other functional (n = 42) |
Nonfunctional (n = 139) |
|---|---|---|---|---|
| Age, y, median (IQR) | 52 (44–63) | 53 (46–59) | 50.5 (36.0–60.0) | 52.0 (45.0–64.0) |
| Sex | ||||
| Female | 144 (61.5) | 34 (64.2) | 32 (76.2) | 78 (56.1) |
| Male | 90 (38.5) | 19 (35.8) | 10 (23.8) | 61 (43.9) |
| Race (n = 228) | ||||
| White | 185 (81.1) | 39 (78.0) | 35 (85.4) | 111 (81.0) |
| Black | 12 (5.3) | 2 (4.0) | 1 (2.4) | 9 (6.6) |
| Others | 31 (13.6) | 9 (18.0) | 5 (12.2) | 17 (12.4) |
| Symptomatic (n = 228) | 143 (62.7) | 34 (65.4) | 22 (53.7) | 87 (64.4) |
| Weight loss (n = 224) | 30 (13.4) | 4 (7.7) | 5 (12.2) | 21 (16.0) |
| Abdominal pain (n = 222) | 100 (45.0) | 18 (35.3) | 12 (30.0) | 70 (53.4)* |
| Palpable mass (n = 220) | 24 (10.9) | 4 (7.8) | 6 (15.4) | 14 (10.8) |
| Leg edema (n = 216) | 42 (19.4) | 21 (41.2) | 9 (22.5) | 12 (9.6)* |
| Tumor size, cm, median (IQR) | 11.5 (8.0–15.0) | 11.2 (9.0–14.0) | 13.2 (9.8–18.0)* | 11.1 (8.0–14.5) |
| Tumor weight, mg, median (IQR) | 337 (165–1,000) | 310 (174–620) | 330 (180.6–1,225) | 382.5 (150–1,020) |
| Laterality (n = 230) | ||||
| Left | 127 (55.2) | 30 (57.7) | 26 (63.4) | 71 (51.8) |
| Right | 103 (44.8) | 22 (42.3) | 15 (36.6) | 66 (48.2) |
| Capsular invasion (n = 161) | ||||
| No | 64 (39.8) | 14 (35.0) | 13 (52.0) | 37 (38.5) |
| Yes | 97 (60.2) | 26 (65.0) | 12 (48.0) | 59 (61.5) |
| Nodal status | ||||
| Negative | 52 (22.2) | 8 (15.1) | 12 (28.6) | 32 (23.0) |
| Positive | 25 (10.7) | 7 (13.2) | 6 (14.3) | 12 (8.6) |
| Not harvested | 157 (67.1) | 38 (71.7) | 24 (57.1) | 95 (68.3) |
| Margin (n = 209) | ||||
| R0 | 143 (68.4) | 26 (54.2) | 27 (77.1) | 90 (71.4)* |
| R1 | 51 (24.4) | 20 (41.7) | 6 (17.1) | 25 (19.8) |
| R2 | 15 (7.2) | 2 (4.2) | 2 (5.7) | 11 (8.7) |
| Metastatic disease | 43 (18.4) | 18 (34.0) | 10 (23.8) | 15 (10.8)* |
| American Joint Committee on Cancer T stage (n = 214) | ||||
| T1 | 11 (5.1) | 3 (5.8) | 0 (.0) | 8 (6.4) |
| T2 | 90 (42.1) | 17 (32.7) | 18 (48.6) | 55 (44.0) |
| T3 | 82 (38.3) | 24 (46.2) | 12 (32.4) | 46 (36.8) |
| T4 | 31 (14.5) | 8 (15.4) | 7 (18.9) | 16 (12.8) |
| Mitosis in 50 HPF, n (%) | ||||
| <5 | 25 (10.7) | 6 (11.3) | 6 (14.3) | 13 (9.4) |
| <10 | 34 (14.5) | 6 (11.3) | 12 (28.6) | 16 (11.5) |
| >10 | 47 (20.1) | 15 (28.3) | 4 (9.5) | 28 (20.1) |
| Missing | 128 (54.7) | 26 (49.1) | 20 (47.6) | 82 (59.0) |
| Operation approach (n = 227) | ||||
| Open abdominal or posterior | 152 (67.0) | 34 (64.2) | 29 (69.0) | 89 (67.4) |
| Minimally invasive surgery | 41 (18.1) | 11 (20.8) | 4 (9.5) | 26 (19.7) |
| Thoraco-abdominal surgery | 34 (15.0) | 8 (15.1) | 9 (21.4) | 17 (12.9) |
| Complication (n = 176) | 66 (37.5) | 21 (51.2) | 12 (37.5) | 33 (32.0)* |
| Grade of complications | ||||
| Grade I and II | 45 (68.2) | 12 (57.1) | 11 (91.7) | 22 (66.7) |
| Grade III and IV | 21 (31.8) | 9 (42.9) | 1 (8.3) | 11 (33.3) |
| Postoperative adrenal insufficiency (n = 200) |
47 (23.5) | 24 (50.0) | 8 (21.1)* | 15 (13.2)* |
| Length of stay, d, median (IQR) | 6.0 (4.0–8.0) | 7.0 (5.0–12.0) | 6.0 (5.0–8.0) | 5.5 (4.0–8.0)* |
| Neoadjuvant chemotherapy (n = 223) | 4 (1.8) | 1 (1.9) | 1 (2.4) | 2 (1.5) |
| Adjuvant chemotherapy (n = 215) | 36 (16.7) | 13 (26.0) | 11 (27.5) | 12 (9.6)* |
| Postoperative Mitotane (n = 185) | 78 (42.2) | 28 (62.2) | 17 (50.0) | 33 (31.1)* |
The percentages of patients who received adjuvant chemotherapy and mitotane as described previously cannot be added to calculate the percentage of patients who received adjuvant therapy because some patients received both treatments and overlap.
IQR = interquartile range.
P < .05 compared to cortisol-secreting group.
The distribution of cortisol excess according to clinical symptoms demonstrated that patients with cortisol-secreting tumors were more likely to present with leg edema vs patients with nonfunctional tumors (cortisol-secreting, 41.2% vs nonfunctional, 9.6%; P < .001). Conversely, patients with cortisol-secreting tumors were less likely to present with abdominal pain vs patients with nonfunctional tumors (cortisol secreting, 35.3% vs nonfunctional 53.4%; P < .05). The size of ACCs in patients with cortisol-secreting tumors was smaller compared with patients who had other functional tumors (cortisol secreting, 11.2 cm vs other functional, 13.2 cm; P < .05). On histopathology, patients with cortisol-secreting tumors were more likely to have metastatic disease (cortisol secreting, 34.0% vs nonfunctional, 10.8%; P < .001) and to undergo an R1 resection compared with patients who had nonfunctional tumors (cortisol secreting, 41.7% vs nonfunctional, 19.8%; P < .05). Patients with cortisol-secreting tumors were also more likely to receive postoperative mitotane vs patients who had nonfunctional tumors (cortisol secreting, 62.2% vs nonfunctional, 31.1%; P < .001).
Short-term clinical outcomes
A total of 66 patients (37.5%) experienced a postoperative complication (Table 1). In examining the entire cohort, 45 patients (68.2%) had a minor complication and 21 (31.8%) had a major complication. Patients with cortisol-secreting tumors had a more pronounced risk of postoperative complications vs patients with nonfunctional tumors (cortisol secreting, 51.2% vs nonfunctional, 32.0%; P < .05). Postoperative adrenal insufficiency was more common in patients with cortisol-secreting tumors compared with patients who had either other functional or nonfunctional tumors (cortisol secreting, 50.0% vs nonfunctional, 13.2 %; P < .001). Similarly, patients with cortisol-secreting tumors had a prolonged length of hospital stay vs patients with nonfunctional tumors (cortisol secreting, 7 days vs nonfunctional, 5.5 days; P < .05). In a multivariable logistic regression model that accounted for competing risk factors, cortisol secretion was independently associated with a higher risk of overall complications (odds ratio = 2.25, 95%, confidence interval (CI) = 1.04 to 4.88; P = .04) (Table 2). Moreover, on multivariable analysis cortisol secretion was associated with the severity of postoperative complications (odds ratio = 3.09, 95%, CI = 1.10 to 8.71; P = .03).
Table 2.
Univariate and multivariate analyses of factors associated with the incidence and severity of postoperative complications
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Overall complication (vs no complication) | ||||||
| Age | 1.01 | .98–1.03 | .62 | - | - | - |
| Surgical margin status | ||||||
| R0 | Ref | |||||
| R1/R2 | 1.66 | .83–3.32 | .15 | - | - | - |
| Operation approach | ||||||
| Open abdominal or posterior | Ref | Ref | ||||
| Minimally invasive surgery | .37 | .14–.97 | .04 | .35 | .13–.92 | .03 |
| Thoraco-abdominal surgery | 2.18 | .92–5.18 | .08 | 2.23 | .92–5.40 | .08 |
| Other organ resected | ||||||
| No | Ref | |||||
| Yes | 1.34 | .72–2.51 | .36 | - | - | - |
| Hormone secreting tumor | ||||||
| Nonfunctional | Ref | Ref | ||||
| Other functional | 1.27 | .56–2.92 | .57 | 1.06 | .45–2.50 | .89 |
| Cortisol secreting | 2.23 | 1.06–4.67 | .03 | 2.25 | 1.04–4.88 | .04 |
| Major complication (vs no complication) | ||||||
| Age | .99 | .96–1.01 | .33 | - | - | - |
| Surgical margin status | ||||||
| R0 | Ref | Ref | ||||
| R1/R2 | 2.4 | .85–6.75 | 0.1 | 1.40 | .49–3.98 | .52 |
| Operation approach | ||||||
| Open abdominal or posterior | Ref | |||||
| Minimally invasive surgery | .18 | .02–1.48 | .11 | - | - | - |
| Thoraco-abdominal surgery | 2.18 | .66–7.24 | 0.2 | - | - | - |
| Other organ resected | ||||||
| No | Ref | Ref | ||||
| Yes | 2.68 | .91–7.94 | .07 | 4.83 | 1.22–19.2 | .03 |
| Hormone secreting tumor | ||||||
| Nonfunctional | Ref | Ref | ||||
| Other functional | .32 | .04–2.64 | .29 | NA | NA | NA |
| Cortisol secreting | 2.86 | 1.04–7.90 | .04 | 3.09 | 1.10–8.71 | .03 |
CI = confidence interval; OR = odds ratio.
Long-term clinical outcomes
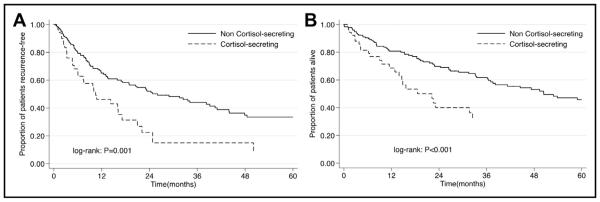
At the last follow-up, 118 patients recurred and 101 patients died. The median follow-up time for the cortisol-secreting group was 9.6 months, whereas the median follow-up for the noncortisol-secreting group was 21.7 months (P = .01). Median RFS was 20.9 months. The 1-, 3-, and 5-year RFS was 61.0%, 38.5%, and 28.8%, respectively. On univariable survival analysis, patients with cortisol-secreting tumors had a worse RFS compared with other patients (median survival: cortisol-secreting tumors, 10.5 months vs noncortisol secreting, 26 months; P = .001) (Fig. 1A). On multivariable analysis, cortisol secretion remained associated with an increased risk of recurrence (Hazard ratio [HR] 2.05, 95% CI 1.16-3.60; P = .01) after controlling for patient- and disease-specific factors (Table 3).
Figure 1.
(A) Recurrence-free survival stratified by cortisol-secreting status (B) OS stratified by cortisol-secreting status.
Table 3.
RFS (without metastasis group)
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | .99 | .98–1.01 | .31 | .99 | .98–1.01 | .77 |
| Surgical margin status | ||||||
| R0 | Ref | Ref | ||||
| R1/R2 | 1.61 | .99–2.60 | .05 | 1.01 | .59–1.71 | .98 |
| T stage | ||||||
| I–II | Ref | Ref | ||||
| III–IV | 2.07 | 1.35–3.17 | .001 | 2.15 | 1.34–3.46 | .002 |
| Hormone-secreting tumor | ||||||
| Nonfunctional | Ref | Ref | ||||
| Other functional | 1.08 | .63–1.83 | .78 | .76 | .41–1.41 | .39 |
| Cortisol secreting | 2.59 | 1.57–4.27 | ,.001 | 2.05 | 1.16–3.60 | .01 |
Follow-up median (IQR): overall 17.6 months (6.2 to 42.7).
Follow-up median (IQR): cortisol-secreting 9.6 months (3.9 to 22.5).
Follow-up median (IQR): noncortisol-secreting 21.7 months (7.0 to 47.3).
CI = confidence interval; RFS = recurrence-free survival; IQR = interquartile range; HR = hazard ratio.
Median overall survival was 44.5 months. The 1-, 3-, and 5-year OS was 78.1%, 55.8%, and 43.2%, respectively. On univariate survival analysis, patients with cortisol-secreting tumors had a worse OS compared with other patients (median survival: cortisol-secreting tumors, 18.6 months vs noncortisol secreting, 50.4 months; P < .001; Fig. 1B). Of note, on multivariable analysis, cortisol secretion was not associated with an increased risk of death (HR = 1.2, 95% CI = .74 to 1.93; P = .46) after controlling for patient- and disease-specific factors. In contrast, advanced T stage (HR = 1.62, 95% CI = 1.05 to 2.50; P = .03), R1/2 surgical margin status (HR = 2.53, 95% CI = 1.66 to 3.88; P < .001), and metastatic disease (HR = 3.01, 95% CI = 1.90 to 4.78; P < .001) remained independent predictors of worse OS.
Comments
Complete tumor extirpation of localized ACC offers the best hope at potential cure, with reported 5-year survival of up to 50% in most contemporary series.20,21 Almost 50% to 90% of patients with ACC will; however, develop recurrence and may not derive long-term benefit from surgical resection.12,13 As such, identifying patients at high risk for recurrence after surgical resection, and patients who might benefit from adjuvant treatment represents, is important for clinicians. Most studies that have reported on curative intent surgery for ACC have focused largely on overall survival. Data on recurrence have been scarce, and these studies have typically examined relatively small, single institution cohorts.14–17 In addition, many previous studies have not specifically focused on the outcome of patients with cortisolsecreting tumors.22–24 The present study is important as it defined the risk of recurrence associated with cortisol secretion among patients with ACC. Furthermore, we characterized the association of cortisol secretion with the risk of postoperative complications. Specifically, data from the present study demonstrated that cortisol-secreting ACC tumors were associated with both an increased risk of postoperative complications, and worse long-term outcomes.
The incidence of all functional and cortisol-secreting tumors in the present study was 40.6% and 22.6%, respectively. These data are comparable to the incidence reported in previous studies that investigated functional ACC tumors.3,25–28 Of interest, we noted that patients with cortisol-secreting tumors presented with somewhat different clinical symptoms and signs compared with patients who had nonfunctional tumors (Table 1). In addition, patients with cortisol-secreting tumors were more likely to experience a postoperative complication vs patients who had nonfunctional tumors (P < .05). Cortisol secretion remained associated with overall complications as well severity of complications even after adjusting for other patient and treatment-related factors (Table 2). In particular, cortisol-secreting tumors were associated with an increased risk of postoperative adrenal insufficiency compared with patients who had either another type of functional tumor or patients who had nonfunctional tumors (Table 1). This finding may have been partially attributed to the adrenolytic activity of mitotane treatment, which was administered more often to patients with cortisol-secreting tumors vs those patients with nonfunctional tumors. Moreover, mitotane is a well-known inducer of Cytochrome P450 3A4 leading to rapid inactivation of more than 50% of postoperatively administered hydrocortisone that, in turn, increases the need for hydrocortisone replacement in mitotane-treated patients.29
Of note, the subset of patients with cortisol-secreting tumors also had a worse OS when compared with patients who had other types of ACC tumors (Fig. 1B). However, after adjusting for patient and disease-related factors the difference did not remain significant. We did note that the overall incidence of recurrence after curative intent surgery was high, as 118 of 234 (50.43%) patients developed a recurrence at the time of last follow-up. In analyzing the entire cohort, RFS was 61% at 1 year and only 28.8% at 5 years. The overall incidence of recurrence reported in the present study was similar to the 50% to 90% overall recurrence rate reported previously.12,13 Factors associated with an overall shorter any-site RFS included advanced tumor stage and cortisol secretion (Table 3). Interestingly, Abiven et al15 1st reported the negative prognostic role of cortisol secretion on OS; however, these investigators did not evaluate the impact of cortisol secretion on RFS. In contrast, Berruti et al16 did report on RFS after ACC resection and noted that disease stage and cortisol secretion was associated with RFS. Of note, Berruti et al excluded patients with an R1 resection, and patients who received adjuvant systemic therapies other than mitotane. In addition, only clinically symptomatic ACC patients who were evaluated for hormone hyper-secretion with a complete hormone workup were included. Given this, results from the Berruti et al may not be generalized to all patients undergoing surgical resection for ACC. Rather findings from the present study corroborate those from Else et al17 who identified cortisol secretion status, disease staging, and grade as significant factors influencing RFS. In a separate study, Grubbs et al13 identified the receipt of adjuvant mitotane as the sole significant factor influencing RFS. Whether cortisol excess exerts a direct tumorigenic effect or acts as a surrogate for unfavorable biological behavior is difficult to determine. In other solid malignancies cortisol secretion itself has been shown to exert stimulatory effects on vascular endothelial growth factor that can in turn mediate tumor growth.30 Such an effect has been reported in patients with prostate cancer in which cortisol was demonstrated to promote growth of prostate cancer cells.31 In addition to a tumor promoting action, cortisol has also been shown to suppress immune surveillance by blunting the cellular immune response in breast cancer that, in turn, could lead to tumor recurrence.10 Collectively, these data suggest that cortisol secretion may similarly confer a worse prognosis among patients with ACC tumors. Given that only about 60% of patients with cortisol-secreting tumors received any kind of adjuvant therapy, increasing this relatively low percentage of patients who received adjuvant therapy may be warranted given the worse outcomes for patients with cortisol-secreting tumors.
The present study had several limitations. As with all retrospective studies, there was undoubtedly some selection bias. Although the multi-institutional nature of the present study was strength, it is conceivable that the incidence of recurrences was under-represented because of the surgical nature of the databases used in the present study. Another strength of the present study was that it represented a collaboration of 13 major academic centers in the United States and therefore reflected a more general experience with cortisol-secreting tumors. All participating centers were tertiary, academic centers in which a complete hormone workup was routinely performed, irrespective of the clinical symptoms. As such, the data may not be completely generalizable (eg, incidence of functional tumors) to the community hospital setting. In addition, the data were derived from a surgical database so the overall resectability rate of cortisol secreting tumors could not be defined. Finally, the relatively small sample size of the study precluded further subanalyses.
Conclusions
In conclusion, although 5-year survival after curative intent surgery for ACC now approaches 50%, the problem of recurrence remains a serious clinical challenge. In fact, 1 of nearly 2 patients experienced a recurrence. Patients with cortisol-secreting tumors had a higher risk of perioperative complications, and a 67% higher risk of recurrence on long term follow-up. Collectively, the data suggest a need for increased awareness of clinicians for detecting postoperative complications and tailoring postoperative surveillance of ACC patients based on their cortisol secreting status.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Fassnacht M, Libe R, Kroiss M, et al. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7:323–35. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 2.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–7. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez Ranvier GG, Inabnet WB., 3rd Surgical management of adrenocortical carcinoma. Endocrinol Metab Clin North Am. 2015;44:435–52. doi: 10.1016/j.ecl.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Veytsman I, Nieman L, Fojo T. Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma. J Clin Oncol. 2009;27:4619–29. doi: 10.1200/JCO.2008.17.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zini L, Porpiglia F, Fassnacht M. Contemporary management of adrenocortical carcinoma. Eur Urol. 2011;60:1055–65. doi: 10.1016/j.eururo.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–89. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Seccia TM, Fassina A, Nussdorfer GG, et al. Aldosterone-producing adrenocortical carcinoma: an unusual cause of Conn’s syndrome with an ominous clinical course. Endocr Relat Cancer. 2005;12:149–59. doi: 10.1677/erc.1.00867. [DOI] [PubMed] [Google Scholar]
- 8.Koschker AC, Fassnacht M, Hahner S, et al. Adrenocortical carcinoma—dimproving patient care by establishing new structures. Exp Clin Endocrinol Diabetes. 2006;114:45–51. doi: 10.1055/s-2006-923808. [DOI] [PubMed] [Google Scholar]
- 9.Libe R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 10.Elenkov IJ. Systemic stress-induced Th2 shift and its clinical implications. Int Rev Neurobiol. 2002;52:163–86. doi: 10.1016/s0074-7742(02)52009-2. [DOI] [PubMed] [Google Scholar]
- 11.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–80. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 12.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–80. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 13.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–70. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 14.Favia G, Lumachi F, D’Amico DF. Adrenocortical carcinoma: is prognosis different in nonfunctioning tumors? Results of surgical treatment in 31 patients. World J Surg. 2001;25:735–8. doi: 10.1007/s00268-001-0024-5. [DOI] [PubMed] [Google Scholar]
- 15.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–5. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 16.Berruti A, Fassnacht M, Haak H, et al. Prognostic role of overt hyper-cortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65:832–8. doi: 10.1016/j.eururo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99:455–61. doi: 10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. John Wiley & Sons; Chichester: 2011. [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169:891–9. doi: 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip JC, Pang TC, Glover AR, et al. Improving outcomes in adrenocortical cancer: an Australian perspective. Ann Surg Oncol. 2015;22:2309–16. doi: 10.1245/s10434-014-4133-4. [DOI] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–6. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 23.Beuschlein F, Weigel J, Saeger W, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100:841–9. doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]
- 24.Loncar Z, Djukic V, Zivaljevic V, et al. Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol. 2015;15:43. doi: 10.1186/s12894-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crucitti F, Bellantone R, Ferrante A, et al. The Italian Registry for adrenal cortical carcinoma: analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry study group. Surgery. 1996;119:161–70. doi: 10.1016/s0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 26.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92:1113–21. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol. 2003;169:5–11. doi: 10.1016/S0022-5347(05)64023-2. [DOI] [PubMed] [Google Scholar]
- 28.Lafemina J, Brennan MF. Adrenocortical carcinoma: past, present, and future. J Surg Oncol. 2012;106:586–94. doi: 10.1002/jso.23112. [DOI] [PubMed] [Google Scholar]
- 29.Chortis V, Taylor AE, Schneider P, et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5alpha-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98:161–71. doi: 10.1210/jc.2012-2851. [DOI] [PubMed] [Google Scholar]
- 30.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed] [Google Scholar]
- 31.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]