Abstract
CLN2 disease is one of a group of lysosomal storage disorders called the neuronal ceroid lipofuscinoses (NCLs). The disease results from mutations in the TPP1 gene that cause an insufficiency or complete lack of the soluble lysosomal enzyme tripeptidyl peptidase-1 (TPP1). TPP1 is involved in lysosomal protein degradation, and lack of this enzyme results in the accumulation of protein-rich autofluorescent lysosomal storage bodies in numerous cell types including neurons throughout the central nervous system and the retina. CLN2 disease is characterized primarily by progressive loss of neurological functions and vision as well as generalized neurodegeneration and retinal degeneration. In children the progressive loss of neurological functions typically results in death by the early teenage years. A Dachshund model of CLN2 disease with a null mutation in TPP1 closely recapitulates the human disorder with a progression from disease onset at approximately 4 months of age to end-stage at 10–11 months. Delivery of functional TPP1 to the cerebrospinal fluid (CSF), either by periodic infusion of the recombinant protein or by a single administration of a TPP1 gene therapy vector to the CSF, significantly delays the onset and progression of neurological signs and prolongs life span but does not prevent the loss of vision or modest retinal degeneration that occurs by 11 months of age. In this study we found that in dogs that received the CSF gene therapy treatment, the degeneration of the retina and loss of retinal function continued to progress during the prolonged life spans of the treated dogs. Eventually the normal cell layers of the retina almost completely disappeared. An exception was the ganglion cell layer. In affected dogs that received TPP1 gene therapy to the CSF and survived an average of 80 weeks, ganglion cell axons were present in numbers comparable to those of normal Dachshunds of similar age. The selective preservation of the retinal ganglion cells suggests that while TPP1 protein delivered via the CSF may protect these cells, preservation of the remainder of the retina will require delivery of normal TPP1 more directly to the retina, probably via the vitreous body.
Keywords: Neuronal ceroid lipofuscinosis, Gene therapy, Retinal degeneration, Optic nerve
1. Introduction
The CLN2 form of neuronal ceroid lipofuscinosis (NCL) is an autosomal recessively inherited, lysosomal storage disorder, usually with an early childhood onset of symptoms (Mole et al., 2011). The disease is characterized by vision loss culminating in blindness, seizures, progressive loss of motor and cognitive function, and premature death. Neurological and visual symptoms are associated with widespread, progressive degeneration of the brain and retina. CLN2 disease results from mutations in the TPP1 gene that cause deficiencies of functional tripeptidyl-peptidase 1 (TPP1), a lysosomal enzyme involved in protein degradation. Without functional TPP1, neurons develop inclusions of abnormal storage material and the retina and central nervous system (CNS) undergo progressive degeneration (Mole et al., 2011).
Multiple forms of NCL occur in people as a result of mutations in at least 13 different genes (Cotman et al., 2013; Mole and Cotman, 2015; Warrier et al., 2013). NCLs have also been reported in a number of dog breeds, with causative mutations identified in the canine orthologs of genes that contain mutations causing corresponding diseases in people (Gilliam et al., 2015). A disorder analogous to CLN2 disease was discovered in a miniature long-haired Dachshund. Genomic DNA sequencing revealed a null mutation in the canine ortholog to the TPP1 gene (Awano et al., 2006). Dogs with this mutation produce no functional TPP1 enzyme and develop symptoms similar to those found in children with this disease (Awano et al., 2006). Affected dogs also develop multifocal retinal detachments that increase in size and number as the disease progresses (Whiting et al., 2015).
Employing the Dachshund model, periodic infusion of recombinant TPP1 enzyme into the cerebrospinal fluid (CSF) proved effective in delaying neurologic symptoms of disease and extending lifespan (Katz et al., 2014; Vuillemenot et al., 2015; Whiting et al., 2014). Indeed, this treatment recently completed combined phase 1/2 clinical trials with promising preliminary results (https://clinicaltrials.gov/ct2/results?term=CLN2&Search=Search). However, this approach to treatment did not delay the progressive loss of retinal function as measured by electroretinography (Whiting et al., 2014). More recently, similar success in delaying neurological disease progression has been achieved in the dog model using AAV2-mediated TPP1 gene therapy targeted to cells lining the ventricles of the brain, again relying on the circulating CSF for widespread delivery of the enzyme (Katz et al., 2015). Because TPP1 gene therapy was effective in substantially delaying the onset and progression of neurological signs in TPP1-null Dachshunds, experiments were conducted to determine whether administration of the AAV2-TPP1 vector to the CSF was also effective in inhibiting retinal degeneration that has been documented in the TPP1-null dogs (Katz et al., 2008; Whiting et al., 2013a).
2. Materials and methods
2.1. Animals
Purpose-bred miniature longhaired Dachshunds housed in a research facility at the University of Missouri were used in this study. CLN2-affected dogs are homozygous for a one nucleotide deletion in TPP1 (c.325delC) that causes a shift in the reading frame and a premature stop codon (Awano et al., 2006). This study includes additional data obtained from CLN2-affected dogs treated with CNS gene therapy (n = 3; 1 male, 2 females) (Katz et al., 2015). Data reported by Whiting et al., 2013a are utilized as control data from normal dogs and untreated, CLN2-affected dogs (Whiting et al., 2013a). All studies were performed in compliance with the EU Directive 2010/63/EU for animal experiments and were approved by the University of Missouri Animal Care and Use Committee.
2.2. CNS gene therapy treatment
As previously described (Katz et al., 2015), we tested whether infusion into the CSF of a rAAV2 vector directing expression of canine TPP1 (AAV2.caTPP1) can provide enzyme replacement broadly through the brain for clinical benefit. At 11–14 weeks of age TPP1-null Dachshunds (n = 3) received unilateral, intraventricular injection of rAAV2.caTPP1. This resulted in nearly complete transduction of ependyma lining the lateral ventricles, and additional transduction of ependyma in the 3rd and 4th ventricles (Katz et al., 2015). Cyclosporine was administered continuously to dogs in this study starting one to two weeks prior to the gene therapy vector administration. Dogs were also treated with mycophenolate starting 5 days prior to rAAV.caTPP1 delivery. Mycophenolate administration was eventually discontinued after 2–4 months in all of the dogs.
2.3. Clinical examinations and in vivo retinal imaging
All dogs received a complete ophthalmic examination prior to inclusion in the study. Animals were excluded from the study if they exhibited any retinal abnormalities that would compromise vision prior to the onset of retinal or neurological disease due to CLN2 disease. Ophthalmic examination was performed monthly on TPP1 −/− dogs. Examination consisted of vision-mediated behavior assessment, slit lamp biomicroscopy and indirect ophthalmoscopy (Whiting et al., 2015).
In vivo imaging was performed approximately every 2 months in the left eye of each dog using a combined confocal scanning laser ophthalmoscope (SLO) and spectral-domain optical coherence tomography (OCT) instrument (Spectralis HRA/OCT, Heidelberg, Germany) (Whiting et al., 2015). SLO fundus images were analyzed quantitatively with Adobe Photoshop to determine the percentage of retinal area covered by detachment lesions and were scored as follows: Grade 1 indicating that less than 15% of the retina was affected by lesions; Grade 2 indicating that greater than 15% but less than 30% of the retina was affected by lesions; and Grade 3 indicating that 30% or greater of the retina was affected by lesions (Whiting et al., 2015).
OCT cross-sectional images were analyzed for total retinal thickness using the Heidelberg Eye Explorer software. Retinal thickness measurements were taken at 1 optic disc diameter (OD) distance superior to the optic nerve head (ONH) and 2OD distance superior to the ONH for each timepoint. Measurements were also taken 1OD and 2OD inferior to the ONH. Thickness measurements were only taken from retina closely apposed to the RPE and not from areas of the retina affected by localized detachment. Total retinal thickness values in the treated, CLN2-affected dogs were compared to those from normal, age-matched Dachshunds.
2.4. Electroretinography and pupillary light response recordings
For two dogs treated with CNS gene therapy, ERG recordings and pupillary light reflex (PLR) recordings were performed as previously described (Whiting et al., 2013a), approximately every two months between 6 and 14 months of age. PLR recordings were also performed in one of these dogs at 18 months of age.
ERGs were bilaterally elicited and simultaneously recorded with a portable unit (HMsERG model 2000; RetVet Corp., Columbia, MO). Each ERG session consisted of scotopic and photopic recordings in accordance with the Dog Diagnostic Protocol, recommended by the European College of Veterinary Ophthalmology, primarily for evaluation of rod and cone function (Ekesten et al., 2013). For each stimulus condition in a given recording session, the amplitudes from the right and left eye were averaged to give a single value per dog for each flash intensity.
All PLR recordings were done during the light period of the daily 12:12 light-dark cycle. The detailed methods for obtaining the PLRs have been described previously (Whiting et al., 2013b). Recordings were performed in dark-adapted, anesthetized dogs with a custom apparatus (Whiting et al., 2013b) which provided timed delivery of a visible light stimulus from a mounted high-power broad spectrum LED (MCWHL2; Thorlabs Inc., Newton, NJ) and concurrently recorded pupil images at 30 frames per second using an infrared-sensitive camera (PC164CEX-2; Supercircuits Inc., Austin, TX) under continuous infrared illumination (880 nm LED) for visualization of the eye. The direct PLR of the right eye was evaluated using a standardized protocol of 100 ms flashes of broad spectrum white light at each of 10 intensities between 8 and 15 log photons/cm2/s (approximately 0.07 mcd.s/m2 to 65 cd.s/m2). Pupil images were analyzed in Photoshop, and average PLR constriction amplitude was calculated for each flash intensity.
2.5. Histological analysis
All 3 dogs evaluated in this study were euthanized when they reached end-stage disease at 16–21 months of age (Katz et al., 2014). The eyes including at least the first 8 mm of the optic nerves were enucleated within 5 min after euthanasia, and the corneas were removed immediately. The samples were then fixed for either the best structural preservation or for immunohistochemistry, and areas of the retina adjacent to the optic nerve heads were dissected as described previously (Katz et al., 2005). Sections of the samples were cut at a thickness of 0.6 μm, mounted on glass slides, and stained with toluidine blue. Digital photographs of the sections were obtained using conventional transmitted light microscopy. Areas of the retinas from the posterior pole of an eye from each dog that were fixed for immunohistochemistry were embedded in paraffin. Sections of the paraffin-embedded retinas were immunostained with a mouse monoclonal anti-TPP1 antibody diluted 1:100 (Abcam ab54685). Immunostaining was performed essentially as described previously (Morgan et al., 2013).
2.6. Optic nerve axon number determination
Approximately 3 mm long segments of the EM-fixed optic nerves obtained from within 1 cm of the back of each globe were post-fixed in osmium tetroxide and embedded in epoxy resin. Cross sections of these samples were cut at a thickness of 0.4 μm, mounted on slides and stained with toluidine blue. Composite high resolution images of the entire cross section of each optic nerve were obtained as described previously (Morgan et al., 2014). Axon numbers were determined using Adobe Photoshop Touch (Adobe Systems, Inc., San Jose, CA) and Image J (http://rsb.info.nih.gov/ij/) (Whiting et al., 2014). Images were displayed on an iPad at a sufficient magnification that each axon could be clearly distinguished. Using a fine-tipped stylus, a dot was placed over each axon in a separate image layer in Photoshop. Once all of the axons had been marked, the image layer consisting of only the dot marks was imported into Image J, which was used to automatically count the marks.
2.7. Statistical analysis
All statistical tests were performed using SigmaPlot (Systat Software Inc., San Jose, CA). Data were subjected to the Shapiro-Wilk test to confirm normal distribution. Repeated measures 2-way ANOVA was used to compare data from CLN2-affected dogs treated with CNS gene therapy (n = 2) to those from normal TPP1 +/+ dogs (n = 7) and to those from untreated, CLN2-affected dogs (n = 5) to determine if the treatment was able to reduce or normalize deficits in ERG b-wave amplitude or PLR constriction amplitude related to CLN2 disease progression. Follow-up pairwise comparisons were performed with the Holm-Sidak correction (α = 0.05) to control family-wise error rate.
The numbers of axons in the optic nerves of the dogs that received the CNS gene therapy were compared to the number of axons from 5 normal Dachshunds of similar age from a previous study (Whiting et al., 2014). Statistical comparisons were performed using Student’s t-test.
3. Results
3.1. Retinal function
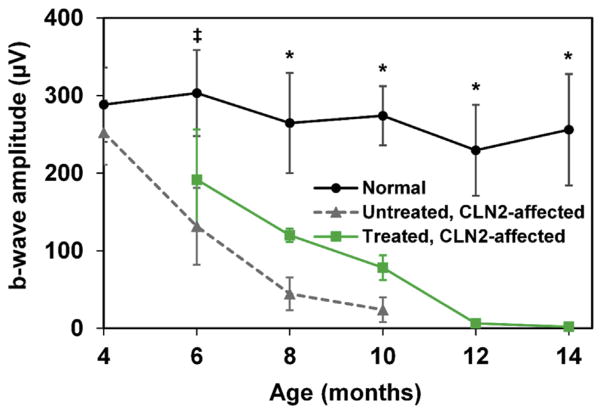
Dogs treated with CNS gene therapy exhibited progressive degeneration of ERG waveforms with age (Fig. 1), similar to the progression of deficits previously observed in untreated CLN2-affected dogs (Whiting et al., 2013a). By 12 months of age, the dogs had non-recordable ERGs under scotopic and photopic conditions. PLR constriction amplitudes in treated dogs were also similar to those of untreated, affected dogs (data not shown). At 10 months of age, neither dog responded to a flash intensity of 10 log photons/cm2/s, which is consistent with data collected in several of the untreated, CLN2-affected dogs (Whiting et al., 2013a). This is a clear contrast to age-matched normal dogs which have an average constriction amplitude of 41% and to CLN2-affected dogs that showed a preservation of the PLR after enzyme replacement therapy which had an average constriction amplitude of 45% (Whiting et al., 2014).
Fig. 1.
ERG b-wave amplitudes versus age in CLN2-affected dogs treated with CNS gene therapy (n = 2) compared with those of normal dogs (n = 7) and those of untreated, CLN2-affected dogs (n = 5); control data adapted from (Whiting et al., 2013a). Amplitudes reflect scotopic mixed rod and cone responses after high intensity light flashes of 13.2 log photons/cm2/s (10 cd.s/m2). Amplitudes from treated affected dogs were significantly reduced from normal by 6 months of age and continued to decline over time until 12 months of age when no response was detected even at the highest stimulus intensity (*p < 0.05; *p < 0.005). There were no significant differences between treated and untreated CLN2-affected dogs for any of the ages tested. Error bars represent standard deviation.
3.2. Retinal structure in vivo
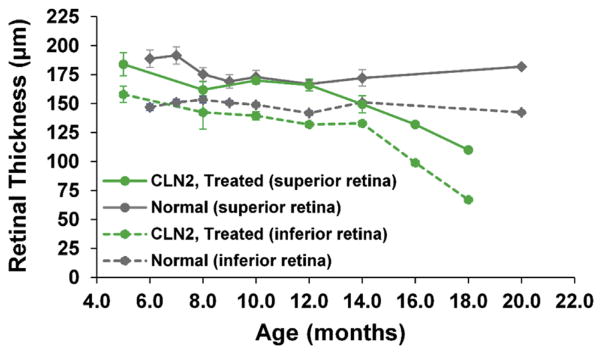
Between 6 and 12 months of age, both treated CLN2-affected dogs had total retinal thicknesses comparable to that of normal dogs in both the superior and inferior retina. However, as the disease progressed during the extended lifespan, superior and inferior retinal thickness in the treated CLN2-affected dogs decreased. No statistical comparison between the affected–treated and normal dogs could be made at 16 or 18 months since only one dog survived to these ages (Fig. 2). However, for this dog retinal thickness declined by 41% in the superior retina and 57% in the inferior retina between 4 and 18 months of age, whereas there was no decline in retinal thickness over this age span in the control dogs.
Fig. 2.
Total retinal thickness decreased progressively during the extended lifespan beyond 12 months of age in gene therapy treated CLN2-affected dogs. While superior and inferior retinal thickness appears be reduced from normal at 14 months, a statistically significant difference from the normal dogs could not be detected at this age due to the small sample sizes. Thickness measurements were taken 1 optic disc diameter (OD) superior to the ONH and 1 OD inferior to the ONH for each timepoint. Error bars represent standard error of the mean (SEM).
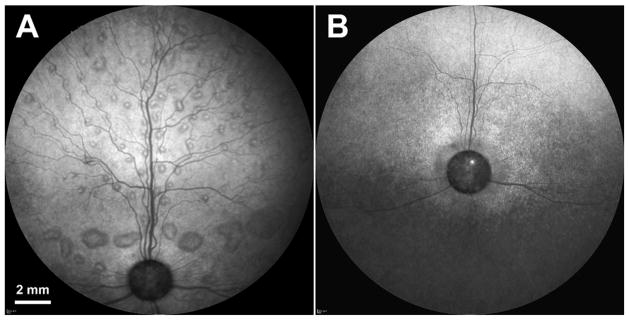
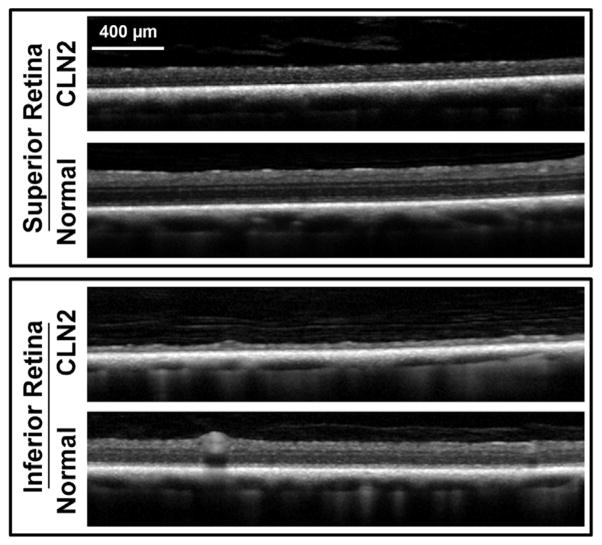
Two CLN2-affected dogs treated with CNS gene therapy developed disease-related multi-focal retinal detachments, dog A at 10 months of age and dog B at 8 months of age. The appearance of the lesions in both dogs was initially the same as that previously reported in Dachshunds with the CLN2 disease (Fig. 3A) (Whiting et al., 2015). These lesions remained grade 1 for dog A through end-stage disease. However, for dog B the lesions progressed to grade 2 at 10 months of age (Fig. 3A). At 14 months of age, lesions in dog B began to flatten as the retina showed thinning with cross-sectional OCT imaging (Fig. 2). As retinal thinning progressed with age, the previously observed retinal detachments were no longer visible by 17 months of age with SLO (Fig. 3B) or with OCT imaging (Fig. 4).
Fig. 3.
SLO fundus photos from the left eye of dog B at 10 months of age (A) and at 17 months of age (B). Retinal detachments that are present at the earlier time point are no longer visible at the later time point due to advanced degeneration and thinning of the retina.
Fig. 4.
OCT cross-sectional images showing thinning of the superior and inferior retina in CLN2-affected dog B at 17 months of age. OCT images from a normal dog of similar age, taken from comparable locations in the retina, are included for comparison.
3.3. Retinal histology
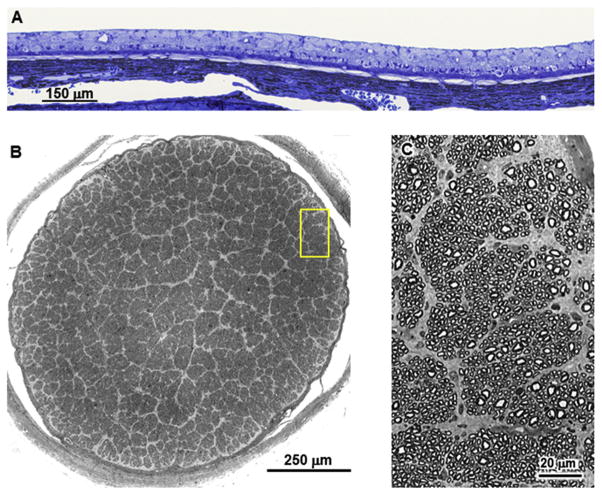
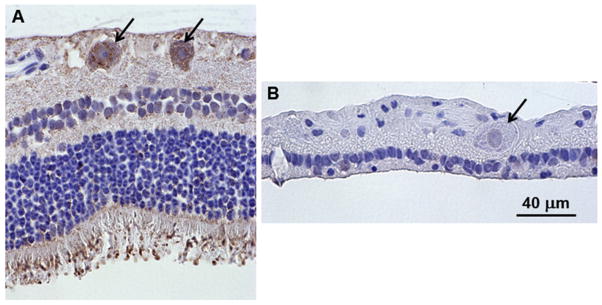
By end-stage disease, the retinas of the affected dogs were extremely thin with none of the normal retinal layers recognizable (Fig. 5A). Cells with large nuclei were scattered along the vitreal edge of what remained of the retina with a single row of cells with smaller, more compact nuclei just exterior to them adjacent to the underlying retinal pigment epithelium. In order to determine whether some these remaining cells could be ganglion cells, the numbers of ganglion cell axons were determined in cross-sections of the optic nerves from each of the dogs (Fig. 5B and C and Table 1). The numbers of axons were not significantly different from those of the optic nerves from normal Dachshunds of similar age, which were on average 158,617 ± 16,611 (mean ± SD) (Whiting et al., 2014). This indicates that despite the obvious dramatic loss of most cell types from the severely thinned retinas, there was no significant loss of ganglion cells. The selective preservation of the ganglion cells was not associated with the presence of TPP1 in the ganglion cell bodies, since TPP1 immunostaining, present in the ganglion cells of normal Dachshund retinas, could not be detected by immunostaining in sections of retinas of the treated, affected Dachshunds at end-stage disease (Fig. 6). In addition to punctate TPP1 immunostaining in the ganglion cell bodies of the normal dog retina, there was also substantial TPP1 immunostaining associated with the photoreceptor outer segments as well as diffuse immunostaining throughout the retina (Fig. 6A).
Fig. 5.
(A) Light micrograph of cross-section of the retina from Dog B from a region of the central retina. (B) A composite high resolution light micrograph of a cross-section of the optic nerve from Dog C. (C) A digitally magnified region of the area of the image in (B) indicated by the yellow rectangle demonstrating that the composite image had sufficient resolution to enable each individual axon in the composite image to be distinguished and counted.
Table 1.
Numbers of axons in optic nerve cross sections of 3 TPP1−/− dogs that received intracerbroventricular TPP1 gene therapy.
| Dog | Number of Axons at Euthanasia* | Age at Euthanasia (weeks) |
|---|---|---|
| A | 158,938 | 70.5 |
| B | 139,664 | 91.5 |
| C | 138,011 | 78 |
Normal: 158,617 ± 16,611 (n = 5; mean ± SD) (Whiting et al., 2014).
Fig. 6.
Light micrographs of retinal cross-sections immunostained with an anti-TPP1 antibody. The brown color indicates TPP1 antibody binding. (A) The retina from a normal Dachshund shows punctate immunostaining in the ganglion cells (arrows in A). (B) Ganglion cells from Dog B did not show any TPP1 immunolabeling (arrow in B). Intense TPP1 immunostaining was also present around the photoreceptor outer segments in the normal dog and diffuse immunostaining was present throughout most of the rest of the retina. Both retinas were artifactually detached from the retinal pigment epithelium during processing for paraffin embedding. Bar in (B) indicates the magnification of both micrographs.
4. Discussion
In the Dachshund model of CLN2 disease, dogs lack the lysosomal enzyme TPP1 due to a null TPP1 mutation, which results in progressive degeneration of the CNS and retina (Katz et al., 2008, 2014; Whiting et al., 2015). Untreated dogs with CLN2 disease must be euthanized by 10–11 months of age due to the severity of the neurological signs. At this age there is significant loss of retinal function and thinning of the inner retina but no significant loss of retinal ganglion cells (Katz et al., 2008; Whiting et al., 2015). TPP1 supplied to the CNS via the CSF, either by periodic infusion of the recombinant enzyme or by administration of a TPP1 gene therapy vector into the CSF, significantly delays progression of neurological signs and results in substantial lifespan extension in the Dachshund model. However, we found that this treatment does not delay retinal degeneration or loss of retinal function, which continue over the extended lifespans of the dogs until the ERG becomes non-recordable and the retina becomes only a very thin layer with none of the normal retina cell layers morphologically recognizable.
One might not expect TPP1 enzyme present in the CSF at therapeutic levels for the brain to reach the majority of the retinal cell layers. However, the sheath around the optic nerve is continuous with the brain dura, and the space between this sheath and the optic nerve is continuous with the subarachnoid space through which the CSF flows. Therefore, it is possible that TPP1 present in the CSF could be taken up by the retinal ganglion cell axons, of which the optic nerve is comprised, and thereby preserve the structure and function of these cells. Indeed, in a previous study we found that periodic infusion of recombinant TPP1 into the CSF selectively inhibited the disease-related decline in the pupillary light reflex, suggesting a potential preservation of intrinsically photosensitive retinal ganglion cells (Whiting et al., 2014).
Therefore, analyses were performed to determine whether ganglion cells were present in the extremely thin retinas of CLN2-affected dogs whose life spans had been extended by administration of the TPP1 gene therapy vector to the CSF which resulted in sustained elevated CSF levels of TPP1. Since each ganglion cell contributes one axon to the optic nerve, we determined the numbers of ganglion cells remaining in the retina at disease end-stage of the treated dogs by counting the numbers of axons remaining in cross-sections of the optic nerve. Rather surprisingly, there was no significant difference between the numbers of axons in the optic nerves of the treated dogs between 71 and 92 weeks of age and untreated normal Dachshunds that were euthanized at 42–44 weeks of age (Table 1).
We hypothesized that this selective preservation of the ganglion cells was due to TPP1 uptake from the CSF via their axons. However, we were unable to detect any TPP1 immunolabeling in the ganglion cell bodies from the treated dogs at end-stage disease. In the retinas from normal dogs, intense punctate TPP1 immunostaining was present in the retinal ganglion cells, suggesting that the enzyme is normally present in the lysosomes of these cells. Diffuse TPP1 immunolabeling throughout the retinas from normal dogs most likely represents the protein within the endoplasmic reticulum where it is synthesized and in transport vesicles that normally deliver the enzyme to lysosomes and to the cell surface. It is possible that TPP1 was present in the ganglion cells of the affected dogs, but at levels below the threshold of detection for IHC. If this is the case, the TPP1 levels would have been much lower than normal since TPP1 immunolabeling was easily detectable in the ganglion cell bodies of normal dog retinas. It is well established that in a variety of lysosomal storage diseases, even very low levels of lysosomal enzyme function can be therapeutic (Parenti et al., 2015). It is also possible that the ganglion cells were able to take up significant amounts of TPP1 from the CSF for quite some time after the CNS gene therapy treatment, but that the amount of enzyme available from the CSF waned over time as the dogs approached a belated end-stage disease status. Indeed, CSF levels of TPP1 waned over time in the dogs that received the TPP1 gene therapy treatment (Katz et al., 2015). If this was the case, retinal ganglion cell levels of TPP1 may have declined only late in the disease process, thus explaining the lack of immunolabeling despite preservation of the cells.
An alternative hypothesis to explain the selective survival of the ganglion cells is that these cells are simply less sensitive to the absence of TPP1 than are other retinal neurons. Although TPP1 is present and involved in protein turnover in most cell types in the body, CLN2 disease is primarily a neurological disorder with little evidence of dysfunction of non-neuronal cells which apparently tolerate the absence of TPP1 fairly well. Likewise, different neural cell types may differ in their sensitivity to a lack of functional TPP1. The selective preservation of retinal ganglion cells could reflect a better ability of these cells to survive without any TPP1 enzyme compared to other neuronal cells.
Most normal retinal ganglion cells have relatively large cell bodies and nuclei compared to those of other retinal cell types (e.g. see Fig. 6). Cell bodies with this type of appearance were rare in the eyes of the affected dogs (Fig. 5A). This suggests that although these cells survived until the dogs reached end stage neurological disease, the morphology of the ganglion cell bodies was significantly altered.
Substantial TPP1 immunolabeling was associated with the photoreceptor outer segments of the normal dog retina (Fig. 6A) and also with the underlying retinal pigment epithelium (RPE) (not shown). The RPE is known to release substantial amounts of lysosomal enzymes into the interphotoreceptor matrix that ensheaths the photoreceptor outer segments (Adler, 1989; Adler and Evans, 1985; Adler and Martin, 1983). Therefore, it appears likely that the TPP1 associated with the outer segments was secreted from the RPE.
We previously reported that localized retinal detachments are associated with CLN2 disease in the Dachshund model (Whiting et al., 2015). In untreated dogs, these lesions increase progressively in size and number as the disease progresses to end stage at 10–11 months of age. In the present study, we found that TPP1 gene therapy to the CSF did not prevent the development of these retinal detachment lesions. However, we found that after 11 months of age in the treated dogs, as the retina continued to thin and the photoreceptor cells eventually completely disappeared, the localized separations between the neural retina and the RPE also disappeared, so that the retina again became closely apposed to the RPE (Figs. 3–5). This suggests that the development of these lesions in affected dogs is due to abnormal interactions between the photoreceptor cells and the RPE due to the lack of functional TPP1 enzyme. Since TPP1 protein appears to be present in the inter-photoreceptor matrix, this enzyme may play a role in mediating the adhesion of the normal retina to the apical surface of the RPE.
Because administration of a TPP1 gene therapy vector to the CSF did not appear to inhibit degeneration of any retinal cell types other than the ganglion cells, it will be necessary to find means of more directly delivering functional TPP1 to the retina in order to prevent blindness in CLN2 disease. Numerous studies suggest that intravitreal administration would be effective in providing TPP1 enzyme to all cell layers of the retina, thereby delaying or preventing retinal degeneration (Meyer et al., 2016). Studies are currently under way to assess whether several alternative means of sustained administration of TPP1 to the vitreous will be effective in treating the retinal pathology. If these studies are successful, a combined ocular and CNS treatment may be effective in preserving both neurological function and vision in this disease.
Acknowledgments
Our thanks to Lani Castaner for her assistance with all aspects of this study, particularly for her oversight of the care of the dogs and with the performance of the various procedures that the dogs had to undergo. Drs. Whitney Young and Baye Williamson made significant contributions to overseeing the care and monitoring the health status of the dogs used in this study. We also thank Drs. Dawna Voelkl and Dietrich Volkmann for their supervision of the breeding of the dogs employed in this work. This research was supported in part by grants EY018815 and EY023968 from the U.S. National Institutes of Health.
Contributor Information
Rebecca E.H. Whiting, Email: whitingre@health.missouri.edu.
Cheryl A. Jensen, Email: jensenc@health.missouri.edu.
Jacqueline W. Pearce, Email: pearcej@missouri.edu.
Lauren E. Gillespie, Email: gillespiel@health.missouri.edu.
Daniel E. Bristow, Email: debdn2@mail.missouri.edu.
Martin L. Katz, Email: katzm@health.missouri.edu.
References
- Adler AJ. Selective presence of acid hydrolases in the interphotoreceptor matrix. Exp Eye Res. 1989;49:1067–1077. doi: 10.1016/s0014-4835(89)80027-2. [DOI] [PubMed] [Google Scholar]
- Adler AJ, Evans CD. Proteins of the bovine interphotoreceptor matrix: retinoid binding and other functions. Prog Clin Biol Res. 1985;190:65–88. [PubMed] [Google Scholar]
- Adler AJ, Martin KJ. Lysosomal enzymes in the interphotoreceptor matrix: acid protease. Curr Eye Res. 1983;2:359–366. doi: 10.3109/02713688209000781. [DOI] [PubMed] [Google Scholar]
- Awano T, Katz ML, O’Brien DP, Sohar I, Lobel P, Coates JR, Khan S, Johnson GC, Giger U, Johnson GS. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metabolism. 2006;89:254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Karaa A, Staropoli JF, Sims KB. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurology Neurosci Rep. 2013;13:366. doi: 10.1007/s11910-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekesten B, Komaromy AM, Ofri R, Petersen-Jones SM, Narfstrom K. Guidelines for clinical electroretinography in the dog: 2012 update. Doc Ophthalmol. 2013;127:79–87. doi: 10.1007/s10633-013-9388-8. [DOI] [PubMed] [Google Scholar]
- Gilliam D, Kolicheski A, Johnson GS, Mhlanga-Mutangadura T, Taylor JF, Schnabel RD, Katz M. Golden Retriever dogs with neuronal ceroid lipofuscinosis have a two-base-pair deletion and frameshift in CLN5. Mol Genet Metabolism. 2015;115:101–109. doi: 10.1016/j.ymgme.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O’Brien DP, Jeong M, Narfstrom K. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Investigative Ophthalmol Vis Sci. 2008;49:2686–2695. doi: 10.1167/iovs.08-1712. [DOI] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Sibigtroth CM, Taylor JD, Carpentier M, Young WM, Wininger FA, Kennedy D, Vuillemenot BR, O’Neill CA. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease) J Neurosci Res. 2014;92:1591–1598. doi: 10.1002/jnr.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Narfstrom K, Johnson GS, O’Brien DP. Assessment of retinal function and characterization of lysosomal storage body accumulation in the retinas and brains of Tibetan Terriers with ceroid-lipofuscinosis. Am J Veterinary Res. 2005;66:67–76. doi: 10.2460/ajvr.2005.66.67. [DOI] [PubMed] [Google Scholar]
- Katz ML, Tecedor L, Chen Y, Williamson BG, Lysenko E, Wininger FA, Young WM, Johnson GC, Whiting REH, Coates JR, Davidson BL. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci Transl Med. 2015;7:313ra180. doi: 10.1126/scitranslmed.aac6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CH, Krohne TU, Charbel Issa P, Liu Z, Holz FG. Routes for drug delivery to the eye and retina: intravitreal injections. Dev Ophthalmol. 2016;55:63–70. doi: 10.1159/000431143. [DOI] [PubMed] [Google Scholar]
- Mole SE, Cotman SL. Genetics of the neuronal ceroid lipofuscinoses (Batten disease) Biochim Biophys Acta. 2015;1825:2237–2241. doi: 10.1016/j.bbadis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole SE, Williams RE, Goebel HH. The Neuronal Ceroid Lipofuscinoses (Batten Disease) 2. Oxford University Press; Oxford: 2011. [Google Scholar]
- Morgan BR, Coates JR, Johnson GC, Bujnak AC, Katz ML. Characterization of intercostal muscle pathology in canine degenerative myelopathy: a disease model for amyotrophic lateral sclerosis. J Neurosci Res. 2013;91:1639–1650. doi: 10.1002/jnr.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BR, Coates JR, Johnson GC, Shelton GD, Katz ML. Characterization of thoracic motor and sensory neurons and spinal nerve roots in canine degenerative myelopathy, a potential disease model of amyotrophic lateral sclerosis. J Neurosci Res. 2014;92:531–541. doi: 10.1002/jnr.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti G, Andria G, Ballabio A. Lysosomal storage diseases: from pathophysiology to therapy. Annu Rev Med. 2015;66:471–486. doi: 10.1146/annurev-med-122313-085916. [DOI] [PubMed] [Google Scholar]
- Vuillemenot BR, Kennedy D, Cooper JD, Wong AM, Sri S, Doeleman T, Katz ML, Coates JR, Johnson GC, Reed RP, Adams EL, Butt MT, Musson DG, Henshaw J, Keve S, Cahayag R, Tsuruda LS, O’Neill CA. Nonclinical evaluation of CNS-administered TPP1 enzyme replacement in canine CLN2 neuronal ceroid lipofuscinosis. Mol Genet Metabolism. 2015;114:281–293. doi: 10.1016/j.ymgme.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochimica Biophysica Acta. 2013;1832:1827–1830. doi: 10.1016/j.bbadis.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Whiting RE, Narfstrom K, Yao G, Pearce JW, Coates JR, Castaner LJ, Jensen CA, Dougherty BN, Vuillemenot BR, Kennedy D, O’Neill CA, Katz ML. Enzyme replacement therapy delays pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis. Exp Eye Res. 2014;125:164–172. doi: 10.1016/j.exer.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Whiting RE, Narfstrom K, Yao G, Pearce JW, Coates JR, Castaner LJ, Katz ML. Pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis. Exp Eye Res. 2013a;116:402–410. doi: 10.1016/j.exer.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting RE, Pearce JW, Castaner LJ, Jensen CA, Katz RJ, Gilliam DH, Katz ML. Multifocal retinopathy in Dachshunds with CLN2 neuronal ceroid lipofuscinosis. Exp Eye Res. 2015;134:123–132. doi: 10.1016/j.exer.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting RE, Yao G, Narfstrom K, Pearce JW, Coates JR, Dodam JR, Castaner LJ, Katz ML. Quantitative assessment of the canine pupillary light reflex. Investigative Ophthalmol Vis Sci. 2013b;54:5432–5440. doi: 10.1167/iovs.13-12012. [DOI] [PMC free article] [PubMed] [Google Scholar]