Abstract
PI3K/Akt/mTOR signalling is dysregulated in many cancers, including renal cell carcinoma (RCC), and activation of this pathway has been suggested to correlate with aggressive behavior and poor prognosis in RCC tumors. mTOR inhibition plays a principal role in the targeted treatment of many cancer types, including RCC. Although mTOR inhibitors share the same mechanism of action, differences in metabolism, formulation and dosing schedule underpin distinct PK/PD profiles such that they may be differentiated for use in a variety of treatment niches. Approved mTOR inhibitors temsirolimus and everolimus serve as important therapeutic options within the current RCC treatment paradigm, although their recommended applications differ in setting and patient population characteristics. Clinical practice guidelines recommend temsirolimus for use in treatment-naive patients with poor-prognosis metastatic RCC of any histology (predominant clear cell or non-clear cell histology). Everolimus provides a standard-of-care therapy for patients with metastatic RCC whose disease has progressed after previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. As therapeutic failure impacts the vast majority of patients with RCC, sequencing strategies of available agents or simultaneous targeting of multiple members of the PI3K/Akt/mTOR pathway may provide additional clinical benefit. Various classes of agents targeting the PI3K/Akt/mTOR pathway are currently being investigated, including mTORC1/mTORC2 kinase domain inhibitors, mTOR/PI3K dual inhibitors, PI3K-selective inhibitors, and programmed cell death 6 modulators. Clinical trials of mTOR inhibitors in a variety of tumor types are ongoing, and the role of mTOR inhibitors continues to evolve across the RCC treatment landscape.
Keywords: mTOR inhibitors, renal cell carcinoma, temsirolimus, everolimus, treatment, PI3K/Akt/mTOR pathway
Introduction
Renal cell carcinoma (RCC) is the most common form of kidney cancer, representing up to 85% of cases.1 Patients often present with advanced disease; approximately 25–30% of patients have metastatic RCC (mRCC) at diagnosis.2,3 Whereas previous systemic treatment options were limited to cytokine therapy and investigational agents, in current practice targeted therapies are considered a standard of care in the mRCC setting.
Based on results from pivotal phase III clinical trials, seven targeted agents have received approval from the US Food and Drug Administration for the treatment of patients with mRCC.3–12 These include the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab in combination with interferon-α (IFN-α), the VEGF receptor-tyrosine kinase inhibitors (VEGFr-TKIs) sorafenib, sunitinib, pazopanib, and axitinib, and the mammalian target of rapamycin (mTOR) inhibitors everolimus and temsirolimus. In the first-line setting, current guidelines based on level 1 evidence recommend the use of sunitinib, bevacizumab plus IFN-α, and pazopanib in patients in the favorable or intermediate Memorial Sloan-Kettering Cancer Center (MSKCC) risk category13 and temsirolimus among patients of poor MSKCC risk.14–17 Unfortunately, patients ultimately become resistant to first-line agents and require further treatment. Second-line options include sorafenib, sunitinib and pazopanib for use in cytokine-refractory patients, and everolimus is a standard of care for patients who fail initial VEGFr-TKI therapy.
The focus of this review is to compare and contrast preclinical and clinical evidence supporting the use of mTOR inhibitors as class of agents in patients with mRCC.
The role of mTOR in RCC
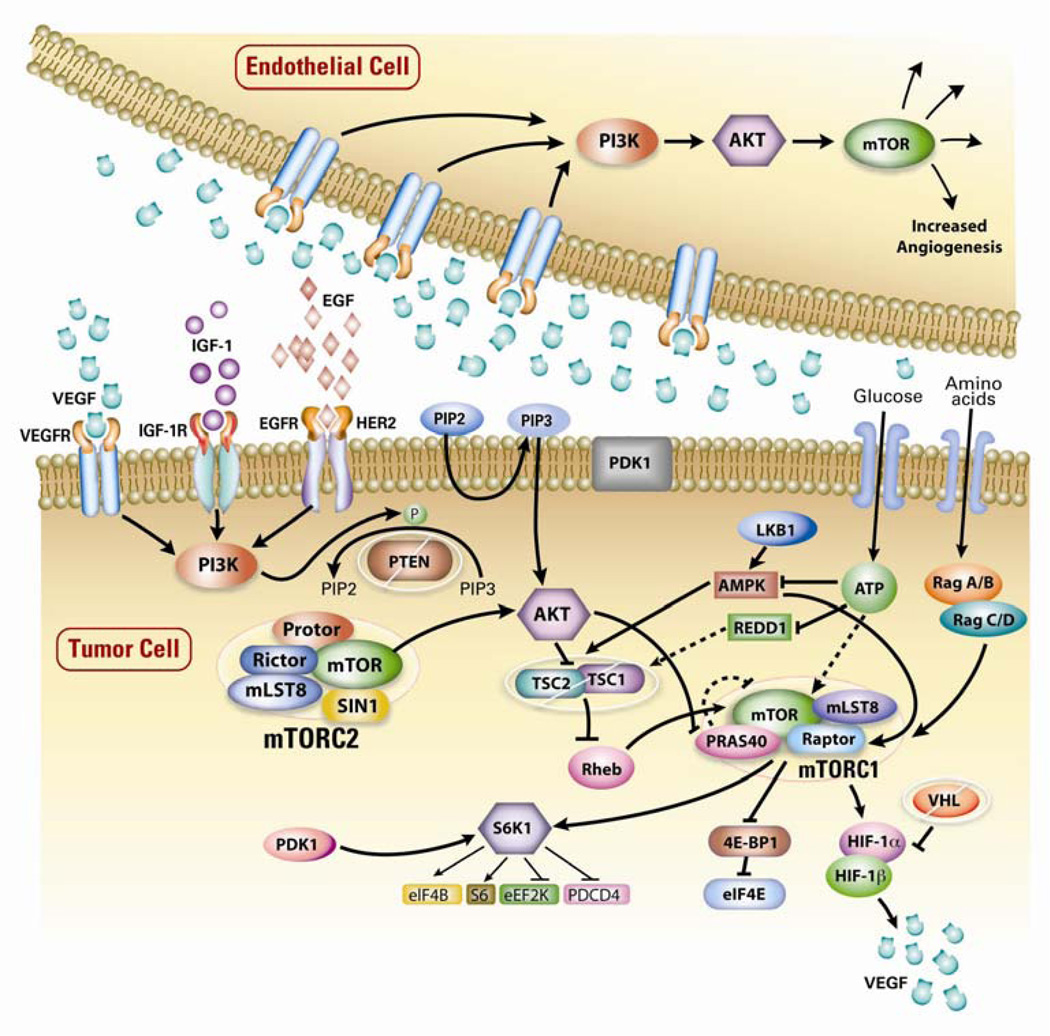
mTOR is an important component of the phosphoinositide 3-kinase (PI3K)/Akt signalling pathway that mediates eukaryotic cell growth and proliferation (Fig. 1).18–20 PI3K/Akt/mTOR signalling is dysregulated in many cancers, including RCC,21 and activation of this pathway has been suggested to correlate with aggressive behavior and poor prognosis in RCC tumors.22 Hyperactivity of mTOR signalling can occur via a number of mechanisms, including overexpression or activation of growth factor receptors, activation of mutations in PI3K/Akt, or decreased expression of tuberous sclerosis tumor suppressor genes TSC1/2, PTEN or Von Hippel-Lindau (VHL) tumor suppressor genes.18,23 Overproduction of growth factors such as VEGF in tumor cells in turn can result in activation of mTOR signalling in neighboring endothelial cells, leading to increased angiogenesis.23 mTOR also regulates the translation of mRNA for hypoxia inducible factors (HIF)-1α and HIF-2α, as well as p70S6 kinase (p70S6K) in cancer cells. Overexpression of HIF-1α and HIF-2α appears to be a critical step in the pathogenesis of RCC,21 while overexpression of p70S6K is observed in ~60% of patients with RCC and seems to be predictive of response and treatment outcomes.24,25
Figure 1. mTOR signalling pathway in cancer20.
mTOR is a serine/threonine kinase that regulates protein synthesis necessary for cell growth, proliferation, metabolism and angiogenesis. The binding of growth factors to cell surface receptors, such as VEGFr, IGF-receptor (IGF-1r) and EGFr, and by nutrients (amino acids and glucose) entering the cell, subsequently stimulate the mTOR signaling pathway. Key upstream elements include PI3K and AKT involved in the phosphorylation and activation of mTOR. Intracellular kinase PI3K synthesizes membrane phospholipids responsible for activation of the kinase AKT while negative regulator PTEN reverses PI3K activity and suppresses AKT activation. Activation of AKT facilitates phosphorylation of the tuberous sclerosis tumor suppressor gene TSC2 and leads to inactivation the TSC1-2 complex, a key regulator of mTORC1. mTORC1 controls essential signal transduction pathways via its downstream effectors S6K1 and 4E-BP1 and coordinates the production of the transcription factor HIF-1α. Promotion of transcription of angiogenic factors such as VEGF is regulated by HIF-1α in complex with HIF-1β. Dysregulation of the PI3K/AKT/mTOR pathway is observed in many cancers. A number of mechanisms are responsible for hyperactive mTOR signaling including overexpression or activation of growth factor receptors, activation of mutations in PI3K/AKT and decreased expression of PTEN, TSC1–2 or VHL. In this figure, activation is depicted as an arrow while inhibition is represented as a bar.
4E-BP1, 4E-binding protein 1; HIF-1α, hypoxia-inducible factor-1α; mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; PTEN, phosphatase and tensin homolog; S6K1, S6 kinase 1; TSC, tuberous sclerosis complex; VHL, von Hippel-Lindau. Republished with permission of Informa UK, Ltd., from Research and innovation in the development of everolimus for oncology, Lebwohl D, Thomas G, Lane HA, et al, Expert Opinions on Drug Discovery, Vol. 6, No. 3:323–338, 2011; permission conveyed through Copyright Clearance Center, Inc.
mTOR is a serine/threonine kinase that specifically binds to and is inhibited by the FK506 binding protein 12 (FKBP12)-rapamycin complex, a complex involved in the regulation of protein translation, cell growth, and metabolism.18,19,26 Subsequently, phosphorylation of downstream targets p70S6K and 4E binding protein (4E-BP1) is also inhibited.21,27 Structurally mTOR exists as two distinct protein complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2).18,19,28 mTORC1 is involved in rapamycin-sensitive temporal control of cell growth and is activated by Akt via direct phosphorylation of TSC2 and by regulation of cellular energy. mTOR2 is involved in rapamycin-insensitive spatial control of cell growth. Inhibition of these protein complexes ultimately results in decreased cell growth and proliferation, cellular metabolism and angiogenesis, leading to cell cycle block at the G1 phase.18 Dysregulation of mTOR signalling is apparent in many types of tumors; mTOR has presented itself as a valid target for the treatment of cancer in RCC.19
Rapamycin and its analogs
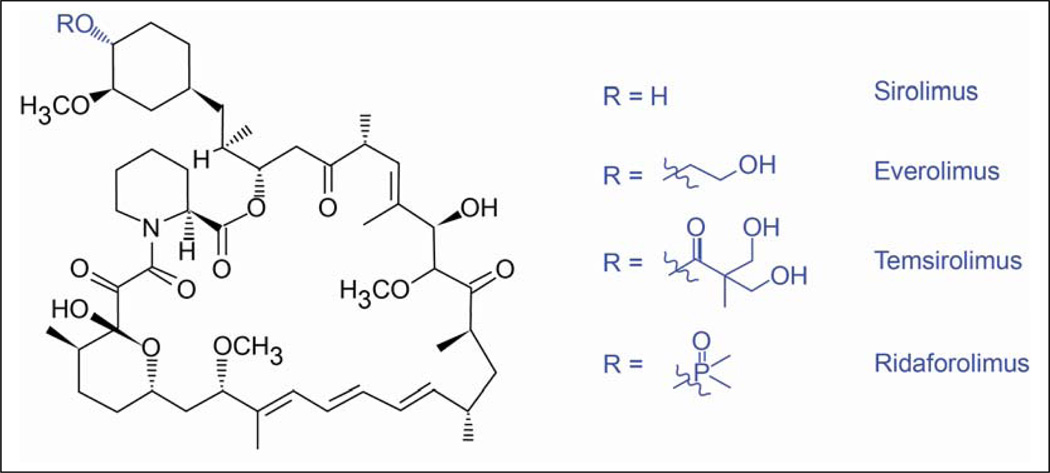
The mTOR inhibitors temsirolimus, everolimus and ridaforolimus are structural derivatives of the macrocyclic lactone rapamycin (also known as sirolimus, Fig. 2). Originally shown to possess fungicidal, immunosuppressive and antiproliferative properties, sirolimus was first approved as an immunosuppressant for patients with solid organ transplants, followed by usage in sirolimus-eluting stents for the prevention of coronary artery restenosis.29 Recent phase I and II trials have also shown sirolimus to reduce the size of angiomyolipomas in patients with tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM).30–32 Temsirolimus, everolimus and ridaforolimus inhibit mTOR by binding to the cytosolic protein FKBP-12. All three agents have been evaluated in clinical cancer trials.21,29 Temsirolimus has been investigated as a treatment for advanced cancer, including mRCC, locally advanced or metastatic breast cancer and mantle cell lymphoma.7,33–36 Everolimus has been assessed as a treatment for patients with advanced cancer, including pancreatic neuroendocrine tumors (pNET), metastatic breast cancer and mRCC.10,21,29,37 Ridaforolimus is being evaluated in patients with advanced solid malignancies, including metastatic sarcoma and RCC.38–40
Figure 2. Rapamycin and its analogs.
Structural derivatives of the macrocyclic lactone sirolimus (also termed rapamycin) include: temsirolimus (42-[2,2-bis (hydroxymethyl)] rapamycin, also known as CCI-779); everolimus (42-O-(2-hydroxyethyl) rapamycin, also known as RAD001); ridaforolimus (macrolide dimethylphophinic acid rapamycin-40-O-yl ester derivative of sirolimus, also known as deforolimus).
Development of mTOR inhibitors as novel therapies for mRCC and other cancers
Temsirolimus
In preclinical studies, temsirolimus exhibited antitumor activity (normalized p70S6K activity and reduced neoplastic proliferation) in a variety of cancers, including glioma, rhabdomyosarcoma, medulloblastoma and prostate and breast cancer.41–45 Results from a phase I study in patients with advanced solid tumors identified weekly temsirolimus IV 25, 75 and 250 mg/m2 to be appropriate doses for further clinical testing.46 Subsequent clinical studies demonstrated IV temsirolimus to have antitumor activity in patients with various types of cancer, including mRCC (Table 1).7,33–36,46–51
Table 1.
Completed Oncology Trials of Temsirolimus (IV administration)
| Study | Patient Population (N) |
Treatment | Efficacy | Safety |
|---|---|---|---|---|
| Phase I dose escalation46 | Advanced cancer (24) | Temsirolimus 7.5–220 mg/m2 weekly | PR: n = 2 | Treatment-related acne-like, maculopapular rashes and mucositis or stomatitis |
| Phase I34 | Advanced cancer (63) | Temsirolimus 0.75–24 mg/m2 | PR, n = 4 (3 unconfirmed) SD, n = 2 |
Treatment-related asthenia, mucositis, nausea and cutaneous toxicity |
| Phase II33 | Metastatic renal cell carcinoma (111) | Temsirolimus 25, 75 or 250 mg weekly | ORR: 7% CR: n = 1 PR: n = 7 Minor response: 26% TTP: 5.8 months OS: 15.0 months |
Grade 3/4 hyperglycemia (17%), hypophosphatemia (13%), anemia (9%), hypertriglyceridemia (6%) |
| Phase I/II48 | Metastatic renal cell carcinoma (39) | Temsirolimus 15 mg weekly plus interferon-α | PR: n = 3 SD: n = 14 PFS: 7.6 months |
Grade 3/4 leukopenia, hypophosphatemia, asthenia, anemia, hypertriglyceridemia |
| Phase III7 | Metastatic renal cell carcinoma (626) | Temsirolimus 25 mg weekly Interferon-α Temsirolimus 15 mg weekly + interferon-α |
ORR: 8.6%, 4.8%, 8.1% OS: 10.9, 7.3, 8.4 months PFS: 3.8, 1.9, 3.7 months (local) 5.5, 3.1, 4.7 months (independent) |
Temsirolimus: grade 3/4 anemia (20%), asthenia (11%), hyperglycemia (11%), dyspnea (9%), infection (5%) |
| Phase II35 | Locally advanced or metastatic breast cancer (109) | Temsirolimus 75 or 250 mg weekly | ORR: 9% PR: n = 10 TTP: 12.0 weeks |
Grade 3/4 mucositis (9%), leukopenia (7%), hyperglycemia (7%), somnolence (6%), thrombocytopenia (5%) and depression (5%) |
| Phase III51 | Postmenopausal locally advanced BC or mBC | Temsirolimus 30 mg daily for 5 days every 2 weeks + letrozole 2.5 mg daily (n = 556) Placebo + letrozole 2.5 mg daily (n = 556) |
Temsirolimus + letrozole, PFS: 8.8 months ORR: 27% CR: n = 11 PR: n = 139 SD: n = 100 Placebo + letrozole, PFS: 8.9 months ORR: 27% CR: n = 11 PR: n = 139 SD: n = 106 |
Temsirolimus + letrozole, grade 3/4 hyperglycemia (4%), dyspnea (3%), neutropenia (3%), asthenia (3%) Placebo + letrozole, grade 3/4 dyspnea (3%), asthenia (2%), hyperglycemia (1%), neutropenia (1%) |
| Phase II47 | Mantle cell lymphoma (35) | Temsirolimus 250 mg weekly | ORR: 38% CR: n = 1 PR: n = 12 TTP: 6.5 months DOR: 6.9 months |
Grade 3/4 thrombocytopenia (66%), neutropenia (29%), anemia (26%) |
| Phase II50 | Mantle cell lymphoma (29) | Temsirolimus 25 mg weekly | ORR: 41% CR: n = 1 PR: n = 10 TTP: 6 months DOR: 6 months |
Grade 3/4 thrombocytopenia (39%), fatigue (25%), neutropenia (18%), anemia (15%) |
| Phase III36 | Mantle cell lymphoma (162) | Temsirolimus 175 mg weekly for 3 weeks then: 75 mg, 25 mg or investigators choice | ORR, 22%, 6%, 2% PFS: 4.8, 3.4, 1.9 months OS: 12.8, 10.0, 9.7 months CR: n = 1, 0, 1 PR: n = 11, 3, 0 |
Grade 3/4 thrombocytopenia (59%, 52%, 36%), anemia (20%, 11%, 17%), neutropenia (15%, 22%, 26%), asthenia (13%, 19%, 8%) |
| Phase II49 | Recurrent or metastatic endometrial carcinoma, chemotherapy-naive (33, 29 evaluable for tumor response) or chemotherapy-treated (27, 25 evaluable for tumor response) | Temsirolimus 25 mg weekly | Chemotherapy-naïve PR: n = 7 SD: n = 20 Chemotherapy-treated: PR: n = 2 SD: n = 12 |
Chemotherapy-naive: grade 3 fatigue (12%), diarrhea (6%), pneumonitis (6%) Chemotherapy-treated: grade 3 fatigue (11%), diarrhea (11%), pneumonitis (11%), dyspnea (7%), grade 3/4 hypokalemia (11%) |
ORR, overall response rate; OS, overall survival; TTP, time-to-progression; DOR, duration of response; PR, partial response; CR, complete response.
In a phase II study, patients with advanced-refractory RCC (n = 111) treated with temsirolimus 25, 75 and 250 mg weekly IV displayed antitumor activity at all dosing levels and treatment was generally well tolerated.33 Since no major differences in terms of toxicity or measurable efficacy between the three dosing levels were observed, a 25-mg weekly dosage was selected for further clinical evaluation. Specifically, when categorized by MSKCC criteria,13 intermediate- and poor-risk patients demonstrated improved median survival compared with that predicted by the criteria, which were developed in IFN-α treated patients. A randomized phase III trial in treatment-naive patients with poor-prognosis mRCC demonstrated that temsirolimus 25 mg IV weekly prolonged progression-free survival (PFS) and overall survival (OS) compared with IFN-α (3.8 months vs 1.9 months for PFS; 10.9 months vs 7.3 months for OS, respectively).7 Based on these results, IV temsirolimus was approved in 2007 as a targeted therapy for patients with advanced RCC in the United States, and exclusively as a first-line treatment for patients with poor prognosis in Europe.52
An oral formulation of temsirolimus was developed to improve dosing convenience. A phase I study determined the maximum tolerated dose (MTD) of oral temsirolimus in patients with advanced cancer, starting at a dose of 25 mg administered on an intermittent schedule.53 Antitumor activity was observed at a MTD of 75 mg once daily for 5 days every 2 weeks. In a phase II study, patients with metastatic breast cancer (mBC) received oral temsirolimus (25 mg daily or intermittently using 75 mg for 5 days every 2 weeks), letrozole, or both. During the study, temsirolimus dose was amended to 10 mg daily or 30 mg intermittently, as 83% of patients required dose delays, reductions, or discontinuations. Overall, both modified doses combined with letrozole were tolerable and showed clinical activity.54
Following this, a randomized, placebo-controlled, phase III trial of intermittent oral temsirolimus 30 mg (daily for 5 days every 2 weeks) with 2.5 mg letrozole or letrozole alone was conducted in postmenopausal women with locally advanced or mBC.51 However, the study was terminated early due to a lack of efficacy and a poorer tolerability profile for the combination regimen compared with letrozole alone. Although phase II results of the combination regimen were encouraging, negative findings in the phase III setting may have resulted from the inability to identify patients with PI3k/Akt/mTOR pathway-dependent tumors and inclusion of patients with ER-positive mBC.55 Alternatively, intermittent dosing may not have been effective in inhibiting the PI3k/Akt/mTOR pathway. This in itself is interesting given the results of the phase III BOLERO-2 trial, in which everolimus plus exemestane improved PFS by 4.1 months over exemestane alone in patients with mBC who had progressed on letrozole.56 Due to these negative phase III temsirolimus data and the apparent lack of interest in developing a clinical biomarker to track target activity such as 4E-BP1 or p70S6K,42,57 development of an oral formulation of temsirolimus has stalled.
Everolimus
The antitumor activity of oral everolimus was initially demonstrated in a rat pancreatic tumor model at dosages of 0.5 or 2.5 mg/kg daily and 5 mg/kg once or twice weekly.58 A single dose of everolimus 5 mg/kg was shown to block phosphorylation of 4E-BP1 and inactivate S6K1 in human peripheral blood mononuclear cells (PBMCs).58 Dosing in humans was evaluated in a phase I dose escalation study of patients with advanced cancer who received oral everolimus 5, 10, 20 and 30 mg weekly.59 Everolimus 20 mg weekly dosing was determined to be the minimum dose to provide sustained mTOR inhibition over a 1-week period. Additionally, everolimus 50 and 70 mg weekly and 5 and 10 mg daily were also assessed and the higher dose further evaluated. Phase I PK/PD studies demonstrated that continuous daily dosing with everolimus 10 mg resulted in a more profound and sustained inhibition of mTOR than that achieved with a weekly dosage schedule.60,61 Specifically, treatment with 10 mg daily or ≥50 mg weekly dosing of everolimus resulted in almost complete inhibition of S6K1 (P < 0.001) and eukaryotic initiation factor 4G (eIF-4G, P < 0.001). However, a correlation between everolimus plasma trough concentrations and inhibition of peIF4G and p4E-BP1 was not evident with weekly dosing, only daily dosing.61 On that basis, a daily dose of 10 mg was selected for further trials with everolimus. The clinical benefit of oral everolimus was subsequently shown in patients with various cancers, including mRCC (Table 2).10,37,56,62–76
Table 2.
Completed Oncology Trials of Everolimus (Oral Administration).
| Study | Patient Population (N) |
Treatment | Efficacy | Safety |
|---|---|---|---|---|
| Phase I62 | Advanced NET (22, 21 evaluable for response/toxicity) | Everolimus 5–10 mg plus pasireotide sc 600–900 µg plus pasireotide 40–60 mg | PR: n = 1 SD: n = 19 |
Treatment related hyperglycemia, hypophosphatemia, thrombocytopenia, lymphopenia, elevated alkaline phosphatase, mucositis, prolonged QTc and joint pain |
| Phase II63 | Metastatic pNET (160) | Everolimus 10 mg (n = 115) or Everolimus 10 mg plus octreotide LAR (n = 45) | Everolimus, PR: n = 11 SD: n = 78 PFS: 9.7 months Everolimus plus octreotide LAR, PR: n = 2 SD: n = 36 PFS: 16.7 months |
Grade 3/4 asthenia and thrombocytopenia |
| Phase III37 | Advanced pNET (410) | Everolimus 10 mg daily continuous plus BSC (n = 207) Placebo plus BSC (n = 203) |
PFS: Everolimus, 11.0 months (73% cross over) Placebo, 4.6 months |
Grade 3/4 stomatitis (7%, 0%), anemia (6%, 0%), hyperglycemia (5%, 2%) |
| Phase III (RADIANT-2 substudy)I64 | Advanced NET (429) | Everolimus plus octreotide LAR (n = 216) Placebo plus octreotide LAR (n = 213) |
PFS: Everolimus plus octreotide LAR, 14.3 months (prior SSA), 25.2 months (no prior SSA) Placebo plus octreotide LAR, 11.1 months (prior SSA), 13.6 months (no prior SSA) |
NR |
| Phase II65 | Metastatic renal cell carcinoma (39) | Everolimus 10 mg daily continuous | PFS: 11.2 months OS: 22.1 months PR: n = 5 SD: n = 21 |
Grade 3/4 pneumonitis (18%); transaminase elevations (10%); thrombocytopenia, hyperglycemia and alkaline phosphatase elevations (8% each); and hyperlipidemia (5%) |
| Phase III10 | Metastatic renal cell carcinoma (416) | Everolimus 10 mg daily continuous (n = 277) Placebo (n = 139) |
PFS: Everolimus, 4.9 months Placebo, 1.9 months OS (80% cross over): Everolimus, 14.8 months Placebo, 14.4 months |
Grade 3/4 decreased lymphocytes (16%, 2%), increased glucose (15%, <1%), decreased hemoglobin (12%, 1%), infections (7%, 3%), dyspnea (6%, 1%), fatigue (5%, 0%), stomatitis (4%, <1%) |
| Phase Ib66 | HER-2 negative mBC (16, 13 evaluable for response) | Everolimus 20–30 mg weekly + cisplatin 25 mg/m2 with paclitaxel 80 mg/m2 once weekly for 3 weeks (4 week cycle) | TTP: 5.0 months CR: n = 1 PR: n = 2 SD: n = 7 |
Grade 3/4 neutropenia (8.5%) |
| Phase II67 | Recurrent mBC(49) | Everolimus 10 mg daily (n = 33) Everolimus 70 mg weekly (n = 16) |
CR: n = 1 PR: n = 3 SD: n = 5 |
Grade 3/4 fatigue, pneumonitis, infection and neutropenia |
| Phase Ib68 | Pretreated with trastuzumab mBC (33) | Everolimus 5–10 mg daily + paclitaxel/trastuzumab (n = 23) Everolimus 30 mg weekly + paclitaxel/trastuzumab (n = 10) |
Daily everolimus + paclitaxel/trastuzumab, PFS: 33 weeks CR: n = 2 PR: n = 7 SD: n = 8 Weekly everolimus + paclitaxel/trastuzumab, PFS: 40.7 weeks CR: n = 0 PR: n = 3 SD: n = 5 |
Grade 3/4 neutropenia (57%, 40%), leukopenia (35%, 20%), lymphopenia (30%, 40%), stomatitis (17%, 30%) |
| Phase II69 | Trastuzumab and taxane-refractory HER2-positive mBC (55) | Everolimus 10 mg daily + paclitaxel/trastuzumab | PFS: 26 weeks CR: n = 0 PR: n = 9 SD: n = 30 |
Grade 3/4 neutropenia (28%), stomatitis (20%), lymphopenia (14%) |
| Phase Ib70 | HER2-positive mBC (50) | Everolimus 5 mg daily + trastuzumab ± vinorelbine (n = 30) Everolimus 20–30 mg daily + trastuzumab ± vinorelbine (n = 20) |
PFS: Daily everolimus + trastuzumab ± vinorelbine, 30.7 weeks Weekly everolimus + trastuzumab ± vinorelbine, 27.1 weeks |
Daily everolimus + trastuzumab ± vinorelbine: Grade 3/4 neutropenia (83%), leukopenia (47%), stomatitis (17%) Weekly everolimus + trastuzumab ± vinorelbine: Grade 3/4 neutropenia (90%), leukopenia (40%), lymphopenia (15%) |
| Phase I/II71 | Trastuzumab-resistant HER2-positive advanced breast cancer (47) | Everolimus 10 mg daily + trastuzumab (8 mg/kg, then 6 mg/kg every 3 weeks) | PR: n = 7 SD: n = 9 |
Grade 3/4 hyperglycemia (13%), lymphopenia (13%), neutropenia (9%), diarrhea (9%), fatigue (9%), mucositis (9%), hypokalemia (6%) |
| Phase II72 | Postmenopausal ER-positive operable breast cancer (270) | Everolimus 10 mg daily + neoadjuvant letrozole 2.5 mg daily (n = 138) Placebo + neoadjuvant letrozole 2.5 mg daily (n = 132) |
Everolimus + letrozole, ORR: 68% CR: n = 18 PR: n = 76 Placebo + letrozole, ORR: 59% CR: n = 12 PR: n = 66 |
Everolimus + letrozole: Grade 3/4 hyperglycemia (5%), stomatitis (2%), pneumonitis (2%) Placebo + letrozole: Grade 3/4 asthenia (<1%), arthralgia (<1%), cellulitis (<1%) |
| Phase II73 | ER-positive AI refractory (11) | Everolimus 10 mg daily + fulvestrant 500 mg day 1, 250 mg day 14, 250 mg day 28, and monthly thereafter | CBR: 55% TTP: 8.6 months |
Mucositis (63.6%), rash (45.5%), infection (36.4%), fatigue (27.3%) |
| Phase II74 | Postmenopausal ER-positive advanced breast cancer (111) | Everolimus 10 mg daily + tamoxifen 20 mg daily (n = 54) Tamoxifen 20 mg daily (n = 57) |
Everolimus + tamoxifen, CBR at 6 months: 61% TTP: 8.6 months OS: NR Tamoxifen alone, CBR at 6 months: 42% TTP: 4.5 months OS: 32.9 months |
Grade 3/4 stomatitis (11%, 0%), pain (9%, 18%), anorexia (7%, 4%), infection (7%, 5%), fatigue (6%, 11%), nausea (4%, 0%), rash (4%, 0%), diarrhea (2%, 0%), pneumonitis (2%, 4%) |
| Phase III56,75 | Postmenopausal ER-positive advanced breast cancer (724) | Everolimus 10 mg daily + exemestane 25 mg daily (n = 485) Placebo + exemestane 25 mg daily (n = 239) |
Everolimus + exemestane, PFS: 7.4 months CR: n = 3 PR: n = 83 SD: n = 521 Placebo + exemestane, PFS: 3.2 months CR: n = 0 PR: n = 9 SD: n = 430 |
Everolimus + exemestane: Grade 3/4 stomatitis (8%, 0%), anemia (5%, 1%), hyperglycemia (4%, <1%), dyspnea (4%, 0%), fatigue (3%, <1%) Placebo + exemestane: Grade 3 3/4 increased alanine aminotransferase (2%, 0%), dyspnea (1%, <1%), stomatitis (1%, 0%), fatigue (1%, 0%), diarrhea (1%, 0%), back pain (1%, 0%) |
| Phase II76 | Recurrent endometrial carcinoma (35, 28 evaluable for efficacy) | Everolimus 10 mg daily for 28 day cycles | SD at 8 weeks: n = 12 CBR at 20 weeks: n = 6 |
Treatment-related fatigue, anemia, pain, lymphopenia and nausea |
AI, aromatase inhibitor; CR, complete response; CBR, clinical benefit rate; DOR, duration of response; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NET, neuroendocrine tumor; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease; SSA, long-acting somatostatin analog; TTP, time-to-progression.
The antitumor activity of 10 mg daily everolimus was demonstrated in a phase II study conducted in patients with mRCC of predominantly clear-cell histology who had received ≤1 prior therapy other than an mTOR inhibitor.65,77 Data from the pivotal phase III RECORD-1 study showed that among patients with clear-cell mRCC, everolimus 10 mg daily resulted in a median PFS of 4.9 months compared with 1.9 months with placebo and treatment was generally well tolerated.10 Pharmacodynamic modelling of tumor growth in patients from RECORD-1 demonstrated that compared with placebo, everolimus 5 mg and 10 mg daily significantly slowed growth of mRCC target lesions, nontarget lesions, and new metastases (P < 0.0001), with the 10-mg daily dosing more effective than 5 mg daily in reducing growth of target lesions. Based on results from RECORD-1, in 2009, oral everolimus was approved in the United States for patients with mRCC who failed treatment with sunitinib or sorafenib and in Europe for patients who progressed on or after treatment with VEGF-targeted therapy.78,79
Ridaforolimus
Preclinical investigations demonstrated that ridaforolimus inhibited proliferation of multiple tumor cell lines in vitro and in vivo, including tumors of breast, colon, lung, prostate, glial and pancreatic origin.80,81 During a phase I evaluation of ridaforolimus in patients with advanced solid malignancies (including RCC), dosages of 3 to 28 mg IV once daily for 5 days were investigated and the MTD was 18.75 mg daily.82 The antitumor activity of ridaforolimus was also observed in metastatic sarcoma and endometrial cancer cell lines and sensitivity was shown to correlate with the proportions of cells in G0/G1 phase of cell cycle proteins.83 Assessment of dosing schedules, 10 mg daily and 10 mg daily for 5 days every other week or weekly found intermittent dosing not to be associated with the immunosuppressive effects observed with daily dosing.84 Subsequently, intermittent dosing was recommended for optimal antitumor activity and minimal systemic effects. Phase I studies with ridaforolimus IV in combination with paclitaxel or capecitabine demonstrated antitumor activity in patients with solid tumors including RCC.85,86 Results from a phase II trial (NCT00093080) of ridaforolimus 12.5 mg IV once daily for 5 days every 2 weeks in patients with relapsed and/or refractory sarcomas reported a 29% clinical benefit response rate and a 2% partial response rate in patients with bone sarcoma, leiomyosarcoma and liposarcoma.38–40
An oral formulation is also being clinically evaluated in patients with soft-tissue and bone sarcomas. In the phase III SUCCEED (NCT00538239) trial, patients with metastatic sarcomas are receiving oral ridaforolimus 40 mg for 5 consecutive days each week or placebo. Interim results demonstrated a 3.2-week improvement in PFS in the maintenance setting following chemotherapy.87,88
PK/PD profiles and mTOR pathway inhibition
Although temsirolimus and everolimus inhibit mTOR via a similar mechanism of action (MoA), the metabolism, formulations, dosing schedules and routes of administration are distinctively different, resulting in varying PK/PD profiles. Temsirolimus is an inactive soluble ester with low oral bioavailability, yet as an IV formulation, temsirolimus acts as prodrug which is metabolized to the active compound sirolimus.34,89 The IV formulation of temsirolimus subsequently exploits the anticancer properties of sirolimus with improved pharmacokinetics without clinical evidence of immunosuppression.89 In contrast, everolimus is orally bioavailable with no active metabolites.59
Temsirolimus
In a phase I study of patients with advanced solid tumors who received temsirolimus 7.5- to 220-mg/m2 weekly IV infusions, the maximum plasma drug concentration (Cmax) and area under the plasma concentration curve (AUC) was shown to increase subproportionally with dose.46 The mean volume of distribution at steady state (VDss) ranged from 127 to 384 liters and the sirolimus-to-temsirolimus ratio ranged from 2.5 to 3.5. Total body clearance (Cl) was shown to be nonlinear, ranging from 19 to 51 L/hour (34 to 220 mg/m2). The PK parameters of temsirolimus were established in a randomized phase II study of patients with advanced RCC who received once-weekly IV doses of 25, 75 or 250 mg temsirolimus.90 Data revealed dose, single versus multiple dose and body surface area were significant PK covariates.90 AUC correlated with AE severity for thrombocytopenia, (P = 0.007), pruritus (P = 0.011) and hyperlipidemia (P = 0.40). Temsirolimus exposure also correlated with a subset of gene transcripts in PBMCs after 16 weeks of therapy (P < 0.001). Further results from a phase I PK study in patients with advanced cancer treated with IV temsirolimus 0.75 to 24 mg/m2 once daily for 5 days every 2 weeks demonstrated that exposure increased less than proportionally with dose.34 The elimination half-life (t½) was 13 to 25 hours, and sirolimus was shown to be the main metabolite.
Phase I PK data of treatment with oral temsirolimus in patients with advanced cancer demonstrated extensive first-pass metabolism resulting in low bioavailability (1.5% to 2.5%).53 However, when sirolimus concentration was also considered, relative exposure (AUCoral/AUCIV) ranged within the limits of oral sirolimus itself (from 8.8–26.5% compared with 18%, respectively). The MTD of the oral formulation was 75 mg for 5 days every 2 weeks, with 50% of patients requiring dose reductions.
In a PD evaluation of patients with mRCC (n = 9 from a subset of patients enrolled in a phase II study of temsirolimus33), a single dose of temsirolimus 25, 75 or 250 mg IV inhibited p70S6K activity in PBMCs, and inhibition was found to be independent of the administered dose.91 There was also a significant linear association between time to disease progression and inhibition of kinase activity 24 hours after treatment (P = 0.04). However, due to the limited sample size, firm conclusions cannot yet be made regarding the value of p70S6K as a biomarker towards the prediction outcomes of patients treated with temsirolimus. Additionally, data from a large retrospective analysis have shown a rise in cholesterol levels to be associated with prolonged survival in temsirolimus-treated patients (OS: hazard ratio [HR] 0.76 per mmol/L, P < 0.0001; PFS: HR 0.81 per mmol/L, P < 0.0001). Although further prospective biomarker studies are warranted, these results suggest cholesterol increase may potentially serve as an important biomarker with respect to temsirolimus therapy and survival outcomes.92
Everolimus
A phase I PK/PD study of oral everolimus in patients with advanced solid tumors demonstrated sustained inhibition of mTOR activity in tumor tissue at doses of ≥20 mg weekly or 5 to 10 mg daily.59 The t½ of oral everolimus was 30 hours (range 26 to 38 hours) and the AUC increased proportionally with dose while Cmax increased less than proportionally with doses ≥20 mg. Data from another phase I PK/PD tumor modelling study demonstrated time- and dose-dependent S6K1 inhibition in everolimus-treated PBMCs.60 S6K1 inhibition in both rat and human PBMCs was associated with an antitumor effect and assessment of rat and human PK/PD models suggested daily administration of everolimus exerts greater antitumor activity than weekly administration.
Results from a phase I PD study conducted in patients with advanced solid tumors treated with everolimus weekly (20, 50 or 70 mg) or daily (5 or 10 mg) reported dose- and schedule-dependent inhibition of the mTOR pathway with near-complete inhibition at 10 mg daily or ≥50 mg weekly.61 A comparison of these dosages in the tumor PD model demonstrated more profound and better maintained mTOR inhibition with the 10-mg daily dosage. Daily and weekly dose levels also resulted in maximal mTOR inhibition, as indicated by inhibition of peIF-4G and pS6 phosporylation. In the daily schedule, inhibition of peIF-4G was only complete at the 10-mg dose level, while in the weekly schedule, complete pS6 inhibition was observed at all dose levels. However, complete and prolonged inhibition of peIF-4G was observed only at doses ≥50 mg. Overall, 10 mg oral everolimus daily was considered the optimal dose, as it was shown to fully inhibit the phosphorylation of both markers.
Clinical use of mTOR inhibitors in mRCC
National guidelines recommend temsirolimus for use in treatment-naive patients with poor prognosis (high MSKCC risk) mRCC of any histology (predominant clear-cell or non-clear cell histology).14–17 This recommendation is based on results from the global trial for Advanced Renal Cell Carcinoma (ARCC), a randomized, phase III study of temsirolimus versus IFN-α.7 Patients enrolled in the trial were newly diagnosed (no previous systemic therapy was permitted) with primarily poor-prognosis mRCC (defined as individuals demonstrating at least 3 MSKCC predictors of short survival) of any histology type, including those with neurologically stable brain metastases. Patients were randomized to receive temsirolimus 25 mg IV weekly, IFN-α 3 times weekly or temsirolimus 15 mg IV weekly plus IFN-α 3 times weekly. For those who received temsirolimus only, median OS was 10.9 months compared with 7.3 months in those who received IFN-α. The combination of temsirolimus and IFN-α did not improve OS (8.4 months) over temsirolimus alone. Median PFS for patients treated with temsirolimus, IFN-α or both were 3.8, 1.9 and 3.7 months, respectively, as determined by site investigator’s assessments. Based on these data, temsirolimus has a category 1 level recommendation for first-line treatment of poor-prognosis patients with relapsed or unresectable advanced RCC.17
Everolimus is standard-of-care therapy for patients with mRCC whose disease has progressed after previous VEGFr-TKI therapy.14–17 This recommendation is based on evidence from Renal Cell Cancer treatment with Oral RAD001 given Daily (RECORD-1), a pivotal phase III trial of oral everolimus plus best supportive care (BSC) vs placebo plus BSC.10 Patients with mRCC whose disease had progressed during treatment with prior sunitinib and/or sorafenib were randomized 2:1 to receive either everolimus 10 mg once daily or placebo. Patients were stratified by previous therapy (1 or 2 VEGFr-TKIs) and by MSKCC risk (favorable, intermediate or poor). Overall median PFS by independent central review was 4.9 months for patients who received everolimus and 1.9 months for patients who received placebo (P < 0.001). A pre-planned, prospective subanalysis of RECORD-1 also found everolimus to provide clinical benefit over placebo in patients who had received treatment with either 1 previous VEGFr-TKI (n = 308) or 2 previous VEGFr-TKIs (n = 108).93 A trend toward longer PFS was observed in patients treated with 1 previous VEGFr-TKI (median PFS, 5.4 months) than in patients treated with 2 previous VEGFr-TKIs (median PFS, 4.0 months). Based on these results, everolimus has a category 1 level recommendation in patients with mRCC and predominant clear cell histology who have progressed on previous VEGFr-TKI therapy.17
Although no head-to-head studies comparing mTOR inhibitors in patients with mRCC have been conducted, a recent retrospective analysis evaluated effectiveness of second-line everolimus (n = 233), temsirolimus (n = 178) and sorafenib (n = 123) in VEGFr-TKI-refractory patients with mRCC.94 Most patients received first-line sunitinib (86%) and most of them experienced disease progression (86%). After adjusting for baseline characteristics, OS was significantly prolonged for everolimus compared with temsirolimus (HR 0.56; 95% CI 0.40–0.78; P < 0.001) and sorafenib (HR 0.65; 95% CI 0.42–0.99; P = 0.047). Median PFS was significantly longer for everolimus than for temsirolimus (HR 0.73; 95% CI 0.55–0.96; P = 0.025) and, although not statistically significant, longer than for sorafenib (HR 0.75; 95% CI 0.53–1.07; P = 0.110). Results of this analysis suggest that VEGFr-TKI-refractory patients with mRCC who receive second-line everolimus experience a greater survival benefit than patients who receive second-line temsirolimus or sorafenib.
Future directions
In the majority of patients with mRCC, targeted therapies do not produce complete responses and most individuals eventually become refractory to treatment. Additional novel agents are therefore warranted to provide further clinical benefit in this setting (Table 3).95–102 mTORC1/mTORC2 kinase domain inhibitors95,103–105: mTORC1 controls cell growth in response to nutrients and growth factors, and regulation is associated with oncogenic PI3K activity; mTORC2 mediates activity involved in cancer cell transformation and survival. By binding to the ATP binding site of the kinase domain of mTOR, these agents simultaneously inhibit both mTOR complexes, TORC1 (rapamycin sensitive) and TORC2 (rapamycin insensitive). mTOR/PI3K dual inhibitors: high PI3K and mTOR expression observed in patients with RCC is associated with decreased survival, providing the rationale to synergistically target coexpression of these two proteins.102 PI3K-selective inhibitors: another class of agents focusing on the PI3K pathway, a pathway which is constitutively activated in RCC cells regardless of VHL status and is associated with adverse clinical outcomes.102 Programmed cell death 6 (PDCD6) modulators: the pro-apoptotic protein PDCD6 has been shown to suppress phosphorylation of signalling regulators downstream from PI3K, including Akt, mTOR, and p70S6K. Binding of PDCD6 to VEGFr-2 plays a key role in the PI3K/mTOR/p70S6K signalling pathway and subsequently in modulating cellular angiogenesis.106
Table 3.
Targeted Agents in Development
| Study | Agent | Activity |
|---|---|---|
| Preclinical95 | PP242 and PP30 (TORC2 inhibitors) | Inhibits TORC2; blocks phosphorylation of Akt and S473; inhibits proliferation of primary cells more completely than rapamycin |
| Preclinical, in vitro and in vivo cancer models96 | WYE-125132 (TORC1 and TORC2 inhibitors) | Inhibits TORC1 and TORC2; inhibits cancer cell growth and survival, protein synthesis, cell size, bioenergetic metabolism and adaptation to hypoxia |
| Preclinical in vitro and in vivo97 | WAY-600, WYE-687, WYE-354 (pyrazolopyrimidine ATP-competitive mTOR inhibitors) | Block substrate phosphorylation by TORC1 and TORC2 in response to growth factor amino acids and hyperactive PI3K/Akt |
| Preclinical (RCC cell lines and xenografts)98 | NVP-BEZ235: dual PI3K/mTOR inhibitor | Inhibition of Akt and S6 phosphorylation, induction of apoptosis, and reduction in markers of tumor cell proliferation |
| Preclinical (renal cell carcinoma lines and RCC xenografts in nude mice)99 | NVP-BEZ235: dual PI3K/mTOR inhibitor; little antiangiogenic activity | Antitumor activity alone and increased when combined with sorafenib |
| Phase I, patients with advanced solid tumors100 | BEZ235: PI3K inhibitor | BEZ235 exhibited dose- and day-dependent PI3K inhibition as measured by elevation of plasma C-peptide levels; 14 of 51 evaluable patients had SD ≥4 months; tumors from 6 of these 14 patients carried dysregulations of the PI3K pathway; 18 of 35 evaluable patients had detectable decreases of 18FDG uptake |
| Preclinical (cancer cell line)101 | PF-04691502: ATP-competitive dual inhibitor of PI3K/mTOR | Reduced phosphorylation of Akt and inhibited cell proliferation; inhibited TORC1 activity and activation of PI3K and mTOR downstream effectors (including S6RP and Akt) |
| Preclinical (RCC cell lines)102 | LY294002: PI3K inhibitor | LY294002 plus rapamycin synergistically inhibited cell growth |
SD, stable disease.
Summary and Conclusions
mTOR inhibitors have similar mechanisms of action; however, because of differences in their metabolism (prodrug versus orally bioavailable), their formulations (IV versus oral) and their schedules of administration (weekly versus daily), they possess distinct PK/PD profiles, leading to their application for a variety of RCC treatment niches. To date, the effect of temsirolimus on mTOR pathway activity has been evaluated in only a limited number of patients, and the degree of mTOR pathway inhibition does not appear to correlate with administered dose. However, available evidence has shown 25-mg IV weekly dosing of temsirolimus has a significant antitumor effect in patients with poor-risk mRCC based on the results of the ARCC study.7 On the other hand, an oral dose of everolimus 10 mg daily provides sustained inhibition of mTOR signalling, and results from RECORD-1 have shown this dosage to correlate with significant antitumor effect in patients with mRCC.10,13
mTOR inhibitors as a class provide clinical benefit to patients with mRCC and other cancer types. Clinical trials of mTOR inhibitors in a variety of tumor types are ongoing, including evaluation of ridaforolimus, as a maintenance therapy in patients with metastatic sarcoma (NCT00538239). In the RCC setting, temsirolimus is recommended as first-line treatment for patients with mRCC who are of poor MSKCC risk.14–17 In contrast everolimus is recommended in patients with mRCC who have failed previous treatment with VEGFr-TKIs.14–17 While these agents form an intricate part of the mRCC targeted therapy toolbox, the majority of patients ultimately become refractory to treatment with mTOR inhibitors. For such individuals, simultaneous targeting of multiple members of the PI3K/Akt/mTOR pathway may provide additional clinical benefit. With respect to targeted therapies among the various cancer settings, the role of mTOR inhibitors continues to evolve across the mRCC treatment landscape.
Acknowledgments
Medical writing support in the preparation of this manuscript was provided by ApotheCom (Yardley, PA, USA) and was supported by Novartis Pharmaceuticals Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
S. K. Pal has served in a consultant/advisory role for Novartis, Allos Therapeutics, and Genentech and has received honoraria from Pfizer, Novartis, and Sanofi-Aventis. D. I. Quinn has served in a consultant/advisory role for Novartis and Pfizer and has received research funds from Novartis, Pfizer, and Merck
- Evan Yu, MD (University of Washington): evanyu@u.washington.edu
- Joanne Mortimer, MD (City of Hope): jmortimer@coh.org
- Tanya Dorff, MD (University of Southern California: dorff@usc.edu
References
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 14.Escudier B, Kataja V. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v137–v139. doi: 10.1093/annonc/mdq206. [DOI] [PubMed] [Google Scholar]
- 15.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 16.de Reijke TM, Bellmunt J, van Poppel H, Marreaud S, Aapro M. EORTC-GU group expert opinion on metastatic renal cell cancer. Eur J Cancer. 2009;45:765–773. doi: 10.1016/j.ejca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. [Accessed August 7, 2012];NCCN Clinical Practice Guidelines in Oncology™ Kidney Cancer (Version 2.2012) 2012 http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. [Google Scholar]
- 18.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl D, Thomas G, Lane HA, et al. Research and innovation in the development of everolimus for oncology. Expert Opin Drug Discov. 2011;6:323–338. doi: 10.1517/17460441.2011.558079. [DOI] [PubMed] [Google Scholar]
- 21.Husseinzadeh HD, Garcia JA. Therapeutic rationale for mTOR inhibition in advanced renal cell carcinoma. Curr Clin Pharmacol. 2011;6:214–221. doi: 10.2174/157488411797189433. [DOI] [PubMed] [Google Scholar]
- 22.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 23.Brugarolas J. Renal-cell carcinoma—molecular pathways and therapies. N Engl J Med. 2007;356:185–187. doi: 10.1056/NEJMe068263. [DOI] [PubMed] [Google Scholar]
- 24.Robb VA, Karbowniczek M, Klein-Szanto AJ, Henske EP. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007;177:346–352. doi: 10.1016/j.juro.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 25.Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–763s. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 26.Sabers CJ, Martin MM, Brunn GJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 27.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–1073. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 30.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies DM, de Vries PJ, Johnson SR, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17:4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 32.McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 34.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 35.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 36.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 37.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dancey JE, Monzon J. Ridaforolimus: a promising drug in the treatment of soft-tissue sarcoma and other malignancies. Future Oncol. 2011;7:827–839. doi: 10.2217/fon.11.57. [DOI] [PubMed] [Google Scholar]
- 39.Ridaforolimus. Drugs RD. 2010;10:165–178. doi: 10.2165/11586010-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chawla SP, Sankhala KK, Chua V, et al. A phase II study of AP23573 (an mTOR inhibitor) in patients (pts) with advanced sarcomas [abstract] J Clin Oncol. 2005;23(suppl 16 part I of II) Abstr 9068. [Google Scholar]
- 41.Grunwald V, DeGraffenried L, Russel D, et al. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- 42.Dudkin L, Dilling MB, Cheshire PJ, et al. Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res. 2001;7:1758–1764. [PubMed] [Google Scholar]
- 43.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- 44.Geoerger B, Kerr K, Tang CB, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. [PubMed] [Google Scholar]
- 45.Yu K, Toral-Barza L, Discafani C, et al. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 46.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 47.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–2356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 48.Motzer RJ, Hudes GR, Curti BD, et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 49.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow LWC, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer [abstract] Breast Cancer Res Treat. 2006;100(suppl 1):S286. [Google Scholar]
- 52.Torisel® (temsirolimus) 30 mg concentrate and diluent for solution for infusion [prescribing information] Sandwich, Kent, UK: Pfizer Limited; 2011. Sep, [Google Scholar]
- 53.Buckner JC, Forouzesh B, Erlichman C, et al. Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs. 2010;28:334–342. doi: 10.1007/s10637-009-9257-1. [DOI] [PubMed] [Google Scholar]
- 54.Carpenter J, Roche H, Campone M, et al. Randomized 3-arm, phase 2 study of temsirolimus (CCI-779) in combination with letrozole in postmenopausal women with locally advanced or metastatic breast cancer. Presented at: American Society of Clinical Oncology Annual Meeting; May 13–17, 2005; Orlando, FL. [Google Scholar]
- 55.Johnson SRD. 2009 Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2009. Role of the mTOR pathway in endocrine resistant breast cancer: opportunities for novel combination strategies. [Google Scholar]
- 56.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 58.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka C, O'Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 61.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 62.Chan JA, Ryan DP, Fuchs CS, et al. Updated results of a phase I study of pasireotide (SOM230) in combination with everolimus (RAD001) in patients (pts) with advanced neuroendocrine tumors (NET) [abstract] J Clin Oncol. 2011;29(suppl) Abstr 4120. [Google Scholar]
- 63.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anthony LB, Peeters M, Hainsworth JD, et al. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors (NET): Effect of prior somatostatin analog therapy on progression-free survival in the RADIANT-2 trial [abstract] J Clin Oncol. 2011;29(suppl) Abstr 4078. [Google Scholar]
- 65.Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer. 2009;115:2438–2446. doi: 10.1002/cncr.24280. [DOI] [PubMed] [Google Scholar]
- 66.Mayer IA, Burris H, Bendel J, et al. A phase Ib trial of RAD001, an mTOR inhibitor, with weekly cisplatin and paclitaxel in patients with HER2-negative metastatic breast cancer. Cancer Res. 2009;69(suppl 3):3093. [Google Scholar]
- 67.Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–4541. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 68.Andre F, Campone M, O'Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 69.Dalenc F, Campone M, Hupperets P, O'Regan R, Manlius C, Vittori L, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER-2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: a multicenter phase II clinical trial. J Clin Oncol. 2010;28(suppl) Abstr 1013. [Google Scholar]
- 70.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 71.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 73.Badin F, Romond E, Chambers M, Shelton B, Birk S, Kadamyan V, et al. A phase II trial of fulvestrant and RAD001 (everolimus) in patients with metastatic estrogen receptor positive breast cancer after aromatase inhibitor failure; a study in progress. Cancer Res. 2010;70(suppl 2) doi: 10.1007/s10549-013-2810-9. P4-02-05. [DOI] [PubMed] [Google Scholar]
- 74.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 75.Hortobagyi GN, Piccart M, Rugo H, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO 2 phase III trial. Cancer Res. 2011;71(suppl):S3–S7. [Google Scholar]
- 76.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jac J, Giessinger S, Khan M, Willis J, Chiang S, Amato R. A phase II trial of RAD001 in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2007;25(suppl) Abstr 5107. [Google Scholar]
- 78.Afinitor® (everolimus) tablets for oral administration [prescribing information] Stein, Switzerland: Novartis Pharma Stein; 2010. [Google Scholar]
- 79.Afinitor Summary of Product Characteristics [prescribing information] West Sussex, United Kingdom: Novartis Europharm Limited; 2011. Sep, [Google Scholar]
- 80.Clackson T, Metcalf C, Rivera V, Knowles HL, Tang H, Burns KD, et al. Broad anti-tumor activity of AP23573, an mTOR inhibitor in clinical development. Proc Am Soc Clin Oncol. 2003;22 Abstr 882. [Google Scholar]
- 81.Rivera V, Tang H, Metcalf C, III, Keenan TP, Sundaramoorthi R, Liu S, et al. Anti-proliferative activity of the mTOR inhibitor AP23573 in combination with cytotoxic and targeted agents. Proc Am Assoc Cancer Res. 2004;45 Abstr 3887. [Google Scholar]
- 82.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573;MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 83.Squillace RM, Miller D, Cookson M, et al. Antitumor activity of ridaforolimus and potential cell cycle determinants of sensitivity in sarcoma and endometrial cancer models. Mol Cancer Ther. 2011;10:1959–1968. doi: 10.1158/1535-7163.MCT-11-0273. [DOI] [PubMed] [Google Scholar]
- 84.Rivera VM, Squillace RM, Miller D, et al. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther. 2011;10:1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. [DOI] [PubMed] [Google Scholar]
- 85.Perotti A, Locatelli A, Sessa C, et al. Phase IB study of the mTOR inhibitor ridaforolimus with capecitabine. J Clin Oncol. 2010;28:4554–4561. doi: 10.1200/JCO.2009.27.5867. [DOI] [PubMed] [Google Scholar]
- 86.Sessa C, Tosi D, Vigano L, et al. Phase Ib study of weekly mammalian target of rapamycin inhibitor ridaforolimus (AP23573; MK-8669) with weekly paclitaxel. Ann Oncol. 2010;21:1315–1322. doi: 10.1093/annonc/mdp504. [DOI] [PubMed] [Google Scholar]
- 87.Chawla SP, Blay J, Ray-Coquard IL, Le Cesne A, Staddon AP, Milhem MM, et al. Results of the phase III, placebo-controlled trial (SUCCEED) evaluating the mTOR inhibitor ridaforolimus (R) as maintenance therapy in advanced sarcoma patients (pts) following clinical benefit from prior standard cytotoxic chemotherapy (CT) J Clin Oncol. 2011;29(suppl) Abstr 10005. [Google Scholar]
- 88.Ariad announces oral ridaforolimus achieved primary endpoint of improved progression-free survival in patients with metastatic soft-tissue or bone sarcomas in the phase 3 SUCCEED trial. Whitehouse Station, NJ: Merck.com, Merck & Co, Inc.; 2011. [Google Scholar]
- 89.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 90.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77:76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 91.Peralba JM, DeGraffenried L, Friedrichs W, et al. Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin Cancer Res. 2003;9:2887–2892. [PubMed] [Google Scholar]
- 92.Lee CK, Marschner I, Simes J, Voysey M, Egleston BL, Hudes G, de Souza PL. Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. Clin Cancer Res. 2012;18:3188–3196. doi: 10.1158/1078-0432.CCR-11-3137. [DOI] [PubMed] [Google Scholar]
- 93.Calvo E, Escudier B, Motzer RJ, et al. Everolimus in metastatic renal cell carcinoma: subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer. 2011;48:333–339. doi: 10.1016/j.ejca.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 94.Yang H, Wong MKK, Signorovitch JE, Wang X, Liu Z, Liu NS, et al. Overall and progression-free survival with everolimus, temsirolimus, or sorafenib as second targeted therapies for metastatic renal cell carcinoma: a retrospective US chart review. J Clin Oncol. 2012;30(suppl) Abstr 4612. [Google Scholar]
- 95.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu K, Shi C, Toral-Barza L, et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 97.Yu K, Toral-Barza L, Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 98.Cho DC, Cohen MB, Panka DJ, et al. The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin Cancer Res. 2010;16:3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roulin D, Waselle L, Dormond-Meuwly A, et al. Targeting renal cell carcinoma with NVP-BEZ235, a dual PI3K/mTOR inhibitor, in combination with sorafenib. Mol Cancer. 2011;10:90. doi: 10.1186/1476-4598-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28(suppl) Abstr 3005. [Google Scholar]
- 101.Yuan J, Mehta PP, Yin MJ, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol Cancer Ther. 2011;10:2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- 102.Elfiky AA, Aziz SA, Conrad PJ, et al. Characterization and targeting of phosphatidylinositol-3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in renal cell cancer. J Transl Med. 2011;9:133. doi: 10.1186/1479-5876-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feldman ME, Shokat KM. New inhibitors of the PI3K-Akt-mTOR pathway: insights into mTOR signaling from a new generation of Tor kinase domain inhibitors (TORKinibs) Curr Top Microbiol Immunol. 2010;347:241–262. doi: 10.1007/82_2010_64. [DOI] [PubMed] [Google Scholar]
- 105.Bracho-Valdes I, Moreno-Alvarez P, Valencia-Martinez I, et al. mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused. IUBMB Life. 2011;63:880–898. doi: 10.1002/iub.558. [DOI] [PubMed] [Google Scholar]
- 106.Rho SB, Song YJ, Lim MC, et al. Programmed cell death 6 (PDCD6) inhibits angiogenesis through PI3K/mTOR/p70S6K pathway by interacting of VEGFR-2. Cell Signal. 2012;24:131–139. doi: 10.1016/j.cellsig.2011.08.013. [DOI] [PubMed] [Google Scholar]