SUMMARY
The Wnt/β-catenin signaling pathway plays a major role in tissue homeostasis, and its dysregulation can lead to various human diseases. Aberrant activation of β-catenin is oncogenic and is a critical driver in the development and progression of human cancers. Despite the significant potential of targeting the oncogenic β-catenin pathway for cancer therapy, development of specific inhibitors remains insufficient. Using a T-cell factor (TCF)-dependent luciferase-reporter system, we screen for small molecule compounds that act against Wnt/β-catenin signaling and identified MSAB (methyl 3-{[(4-methylphenyl)sulfonyl]amino}benzoate) as a selective inhibitor of Wnt/β-catenin signaling. MSAB shows potent anti-tumor effects selectively on Wnt-dependent cancer cells in vitro and in mouse cancer models. MSAB binds to β-catenin promoting its degradation, and specifically downregulates Wnt/β-catenin target genes. Our findings might represent an effective strategy for cancers addicted to the Wnt/β-catenin signaling pathway.
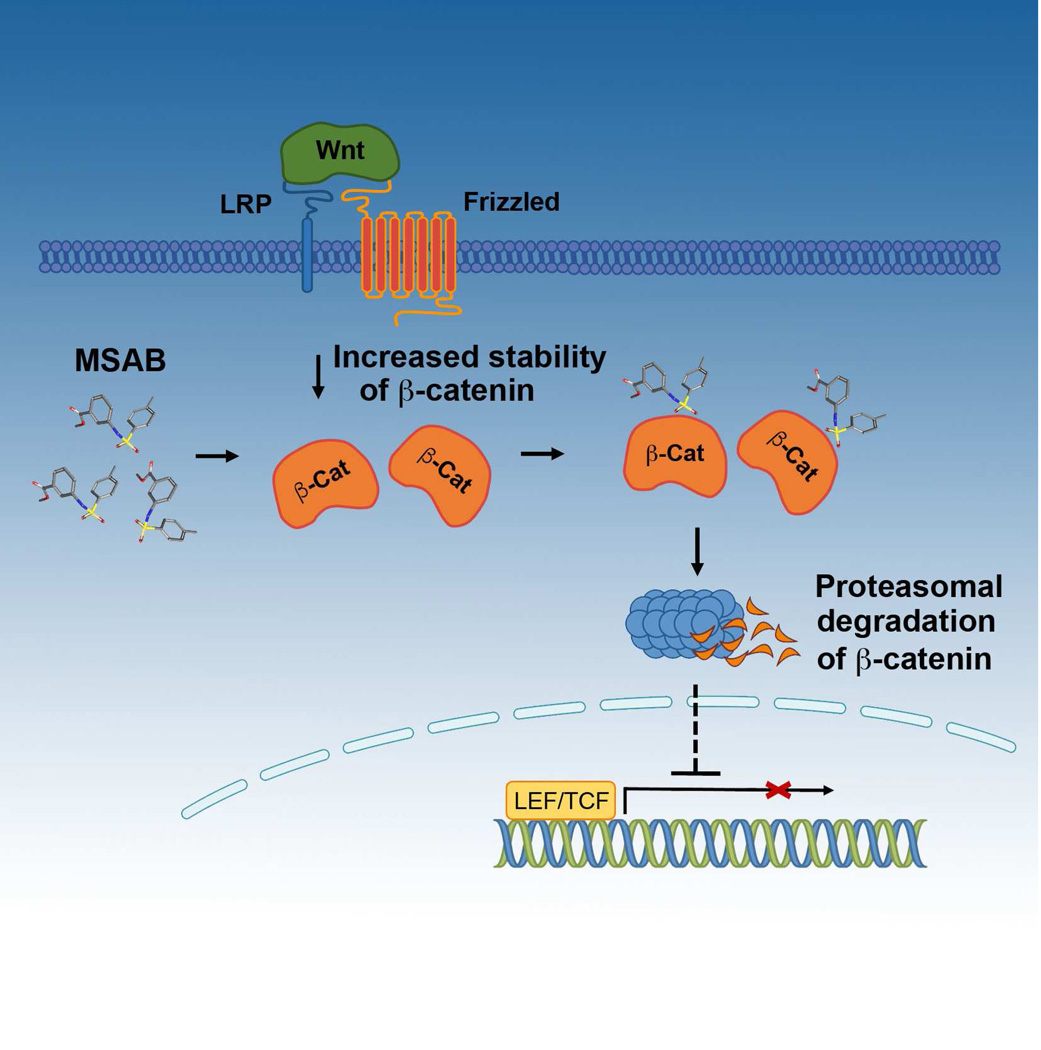
Graphical abstract
INTRODUCTION
Wnt signaling pathway plays crucial roles in multiple stages of development and tissue homeostasis (Clevers et al., 2014; Clevers and Nusse, 2012; Klaus and Birchmeier, 2008). In the absence of Wnt ligands, the level of cytoplasmic β-catenin is constantly in check through the action of the destruction complex, which consists of the scaffold protein Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and casein kinase 1 (CK1) (Behrens et al., 1998; MacDonald et al., 2009). Sequential phosphorylation by CK1 and GSK3β marks β-catenin for recognition by β-TrCP, an E3 ligase subunit, which subsequently causes ubiquitination and proteasomal degradation of β-catenin (Orford et al., 1997; Yost et al., 1996). When present, Wnt ligands interact with the receptor complex Frizzled/LRP5/LRP6 (low-density lipoprotein receptor-related protein), which then triggers a series of downstream events leading to stabilization and nuclear translocation of β-catenin (Bhanot et al., 1996; He et al., 2004; Huang and He, 2008). Once in the nucleus, β-catenin associates with members of T cell factor (TCF) family of transcription factors (Behrens et al., 1996; Molenaar et al., 1996) as well as with transcriptional co-activators such as CREB-binding protein (CBP), p300, Pygopus (PYGO), B-cell lymphoma 9 (BCL-9), and regulates transcription of a broad spectrum of downstream target genes involved in proliferation, fate specification, and differentiation (Hecht et al., 2000; Kramps et al., 2002; Mosimann et al., 2009; Takemaru and Moon, 2000).
Since the first discovery of proto-oncogene Int1, now known as Wnt1, misregulation of Wnt signaling has been closely linked to carcinogenesis (Nusse and Varmus, 1982). Mutations in the pathway components, including APC, Axin, and β-catenin, have been found to be closely associated with various types of cancers (Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997; Satoh et al., 2000). During the past decade, extensive efforts have focused on discovering small molecule inhibitors capable of downregulating such aberrant activation in cancer cells (Anastas and Moon, 2013; Fang et al., 2016; Hao et al., 2013; Kahn, 2014; Polakis, 2012). Compounds such as PKF115-584 and CGP049090 disrupt the interaction between β-catenin and TCF (Lepourcelet et al., 2004), ICG-001 interrupts binding between β-catenin and CBP (Emami et al., 2004) and carnosic acid interferes with β-catenin and BCL9 binding (de la Roche et al., 2012). Additionally, compounds that target more upstream of the pathway have been discovered. The Tankyrase inhibitors IWR-1 (Chen et al., 2009) and XAV939 (Huang et al., 2009), which stabilize Axin, and the CK1 activator Pyrvinium (Thorne et al., 2010) enhance the activity of the destruction complex leading to degradation of β-catenin. However, considering other multiple roles these binding partners or upstream regulators of β-catenin are involved in, it is conceptually attractive to directly target β- catenin as opposed to other factors in the Wnt signaling axis.
In this study, we report the discovery of a small molecule, methyl 3-{[(4-methyl phenyl) sulfonyl] amino} benzoate (MSAB) that selectively exerts anti-proliferative effects on Wnt-dependent cancer cells. MSAB stimulates the degradation of β-catenin, thus lowering high level of active β-catenin and suppressing its nuclear translocation. Furthermore, we present evidence suggesting its binding to β-catenin. We anticipate that these results will contribute to the rigorous search for tools capable of selectively suppressing oncogenic Wnt signaling, and to the understanding of β-catenin biology.
RESULTS
Identification of MSAB as a small molecule inhibitor of Wnt/β-catenin signaling pathway
To identify inhibitor compounds capable of modulating β-catenin-mediated Wnt signaling in cancer, we performed a cell-based screen in which luciferase is expressed in response to the signaling activity of Wnt. High-throughput screening was conducted utilizing HCT116 cells, which carries a mutant allele of β-catenin that lacks Ser45, the phosphorylation target of CK1α, resulting in aberrantly high level of active β-catenin. Using small molecule libraries, twenty two thousand compounds were screened against HCT116 cells stably transfected with TOP-Luc, a luciferase-based reporter containing TCF binding sites (Korinek et al., 1997) (Figure S1A). From the initial screen, we identified 56 potential Wnt pathway inhibitors that reduced the TOP-Luc activity to a level lower than 50% of the level observed in a DMSO-treated control (Figure S1A and B). These small molecules were validated by assessing the dose dependent inhibitory effects on TOP-Luc activity and by measuring compound-responsive levels of Wnt target gene expression. To control for false positives, we tested for inhibitory effects on constitutively expressed luciferase reporter (CMV-Luc) activity as well. Based on these criteria, 14 compounds advanced (Figure S1C), and were next tested for Wnt/β-catenin specificity by evaluating growth inhibitory effects on Wnt-dependent or -independent cancer cells (Akiri et al., 2009; Vijayakumar et al., 2011) (Figure S1A). A673 and H460 cells had been reported to carry very low levels of uncomplexed β-catenin and to exhibit low TCF-reporter activity. Additionally, expression of dnTCF4 had shown little effect on colony formation efficiency in these cell lines (Akiri et al., 2009; Vijayakumar et al., 2011). Thus, these two were tested as Wnt-independent cells in this study.
Based on these results, WI-21, hereafter referred to as MSAB (methyl 3-{[(4-methylphenyl)sulfonyl]amino}benzoate), was selected among the candidates as the strongest and most selective inhibitor of Wnt/β-catenin signaling activity (Figure 1A and Figure S1). MSAB treatment of HCT116 human colon cancer cells resulted in strong inhibition of the TOP-Luc activity in a dose dependent manner while displaying little effect on the expression of luciferase when driven by CMV-Luc, confirming its specificity (Figure 1B). In addition, MSAB showed little effect on FOP-Luc (a scrambled mutant of TCF promoter element), NF-κB-Luc, iNOS-Luc, or Notch-Luc reporter activities in the HCT116 cell line, further demonstrating high selectivity of MSAB against the Wnt/β-catenin pathway (Figure 1C).
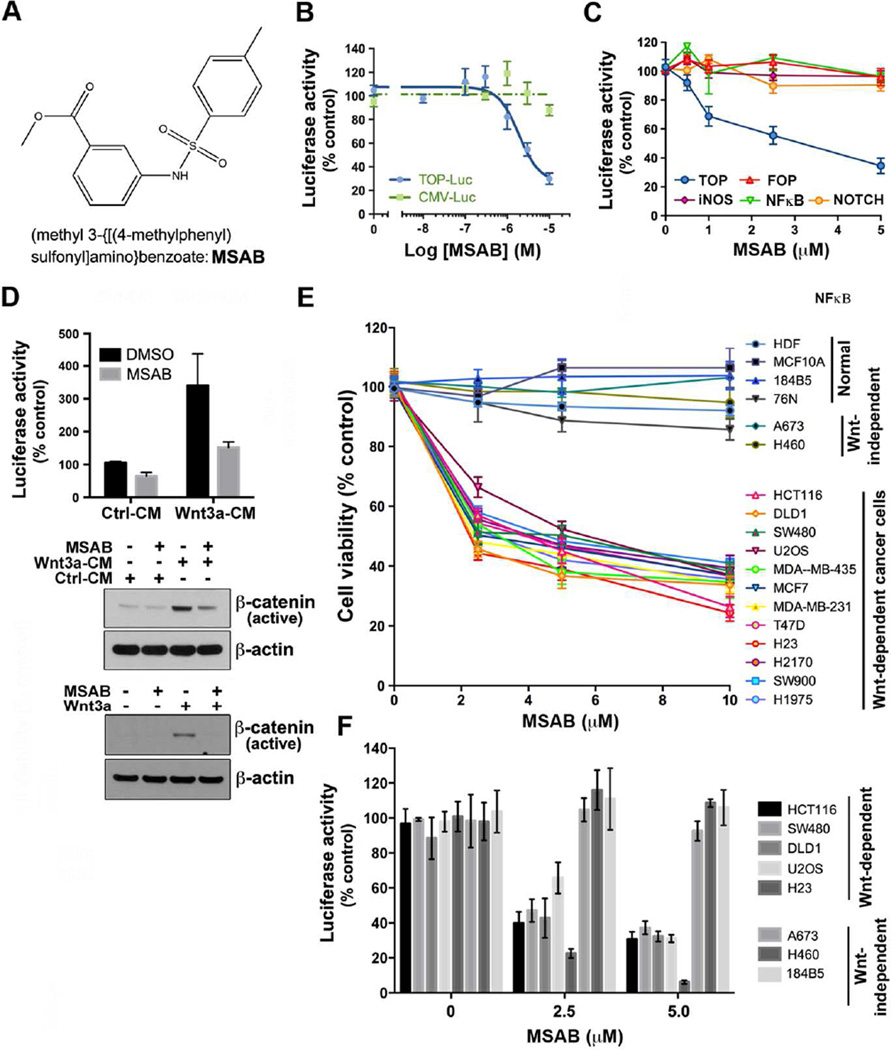
Figure 1. MSAB is a small molecule inhibitor of the Wnt/β-catenin signaling pathway.
(A) Chemical structure of methyl 3-{[(4-methylphenyl)sulfonyl]amino}benzoate (MSAB). (B) MSAB inhibits TCF luciferase reporter activity in HCT116 cells. HCT116 cells with luciferase-based reporter containing TCF binding sites (TOP-Luc) or a constitutively expressed luciferase (CMV-Luc) reporter were treated with MSAB as indicated. (C) The inhibitory effect of MSAB is specific to TOP-Luc reporter activity and shows little effect on FOP-Luc, iNOS-Luc, NF-κB-Luc, or NOTCH-Luc reporter activity in transiently transfected HCT116 cells. Renilla-luciferase was used as a control. (D) Wnt3a-induced TOP-Luc activation and increase of active β-catenin levels are suppressed by MSAB treatment. HEK293T cells transiently expressing TOP-Luc reporter were treated with control conditioned medium (control-CM), Wnt3a conditioned medium (Wnt3a-CM) or with recombinant Wnt3a in the presence of DMSO or MSAB, followed by luciferase assay or western blot analysis. (E) MSAB selectively decreases cell viability of Wnt-dependent cells while showing little effect on Wnt-independent cells and normal human cells. MSAB exhibited cytotoxicity preferentially in Wnt-dependent cancer cells but not in human normal breast epithelial cells (76N), human normal skin fibroblasts (HDF), or in immortalized breast epithelial cell lines (184B5 and MCF10A). Cell viability was measured by SRB (sulforhodamine B) assay. (F) MSAB selectively inhibited TCF luciferase reporter activity in Wnt-dependent cells while showing little effect in Wnt-independent cells. See also Figure S1.
In order to test if MSAB could inhibit Wnt signaling activity stimulated by Wnt3a, HEK293T cells were treated with Wnt3a-CM (conditioned medium) or Wnt3a recombinant protein, and the effect of MSAB on the activity of Wnt/β-catenin pathway was measured by TOP-Luc assay or through western blot analysis probing for active β-catenin (ABC). Both assays revealed dramatic inhibitory effect of MSAB on Wnt3a-mediated Wnt pathway activation (Figure 1D).
Next, the effects of MSAB on Wnt-mediated cell proliferation in Wnt-dependent or - independent cells were examined. As a result, MSAB inhibited proliferation of Wnt-dependent cancer cell lines, but had little effect on the viability of normal or immortalized breast epithelial cells including MCF10A, 184B5, and 76N, Human dermal fibroblasts (HDF), and Wnt-independent cancer cell lines including A673 and H460 at concentrations as high as 10 µM (Figure 1E). It was also confirmed through testing TCF-luciferase activity in these cell lines that Wnt-dependent cells demonstrate higher Wnt signaling activity than Wnt-independent cells (Figure S1D), and that MSAB can selectively inhibit this activity in Wnt-dependent cells while showing little effect in Wnt-independent cells and normal human cells (Figure 1F). Collectively, these results strongly suggest that MSAB is a potent and selective inhibitor of Wnt-dependent cancer cells.
MSAB inhibits growth of Wnt-dependent xenograft tumors
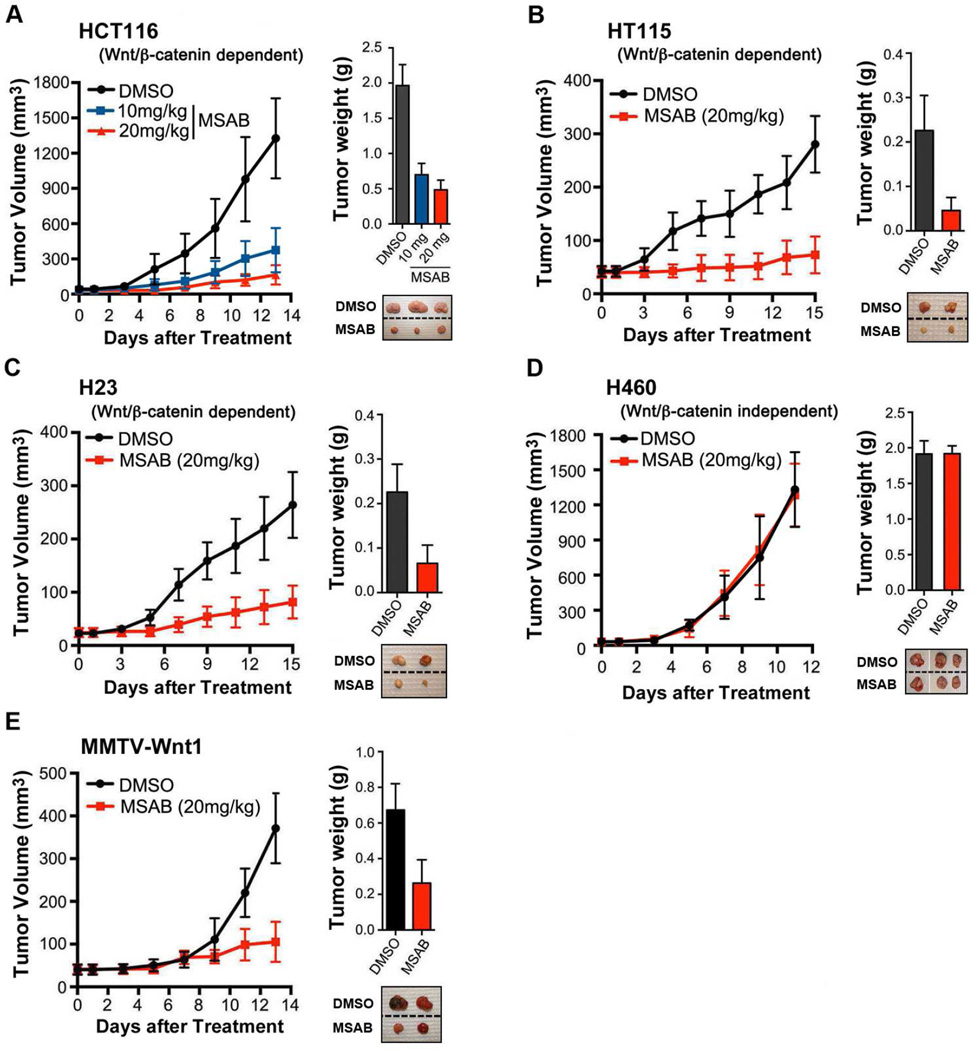
We next tested whether MSAB shows anti-tumor effects in human cancer cell driven xenograft tumors in nude mice. After the tumors reached a volume of 40 mm3, the mice were injected intraperitoneally daily with 10 or 20 mg/kg of MSAB or with the vehicle solution (DMSO) for up to 2 weeks. MSAB markedly reduced the size and weight of various types of Wnt-dependent HCT116, HT115, and H23 tumors in xenografted mouse models (Figure 2A, B and C). In order to test whether tumor reduction by MSAB is mediated by apoptosis of tumor cells, we carried out TUNEL staining and detected a significant increase in TUNEL positive apoptotic cells in HCT116 tumor xenografts treated by MSAB (Figure S2A). Moreover, western blot analysis showed an increase in the level of cleaved caspase 3 in HCT116 and H23 xenograft tumor tissues from mice treated with MSAB (Figure S2B), demonstrating that MSAB treatment resulted in apoptotic cell death of tumor xenograft cells leading to subsequent reduction in tumor size. Notably, there were no detectable signs of systemic toxicity, implying minimal offtarget or nonspecific effects of MSAB in vivo. Moreover, MSAB did not affect the growth of tumors driven by Wnt-independent H460 cancer cells in the xenograft model (Figure 2D). We also examined whether MSAB has an inhibitory effect on Wnt-initiated spontaneously developing mammary tumors (MMTV-Wnt1 mice). The average tumor growth rates in the treated and untreated groups showed significant difference (Figure 2E). We also tested several pharmacological metrics. MSAB showed a moderate bioavailability level at 30.5% absorption. To assess pharmacokinetics, Balb/c mice were treated with a single intravenous or oral dose of MSAB, which resulted in a moderate tissue distribution with a calculated volume distribution of 1.1 L/kg and moderate clearance of 41 mL/min/kg (Table S1). These results demonstrate that in addition to in vitro activity observed using cell lines, MSAB is capable of inhibiting Wnt-dependent tumor growth in vivo.
Figure 2. MSAB inhibits tumor growth of Wnt-dependent cancer cells in mouse xenograft model.
In mouse xenograft model, MSAB inhibited tumor growth of Wnt-dependent cancer cells (A) HCT116, (B) HT115, and (C) H23, but showed little effect on the growth of Wnt-independent cancer cell (D) H460. Cells from each cell-line (2×106 cells/mice) were injected subcutaneously into the flanks of nude mice. (E) MSAB also inhibited tumor growth of MMTV-Wnt1 transgenic mice. MSAB (10 or 20 mg/kg) was administered at the point when the tumor volume reached approximately 40 mm3, and was injected intraperitoneally once per day for 2 weeks. The control group was treated with an equal volume of vehicle. Tumor volumes were measured with a caliper every 2 days. The numbers of mice examined are as follows: HCT116 (DMSO control: n = 11, MSAB (10 mg/kg) - treated: n = 8 and MSAB (20 mg/kg) – treated: n = 7), HT115 (DMSO control: n = 8 and MSAB-treated: n = 10), H23 (DMSO control: n = 7 and MSAB-treated: n = 7), H460 (DMSO control: n = 7 and MSAB-treated: n = 7), MMTV-Wnt1 (DMSO control: n = 7 and CTM-treated: n = 7). See also Figure S2 and Table S1.
MSAB inhibits β-catenin activity and induces proteasome-dependent degradation of β-catenin
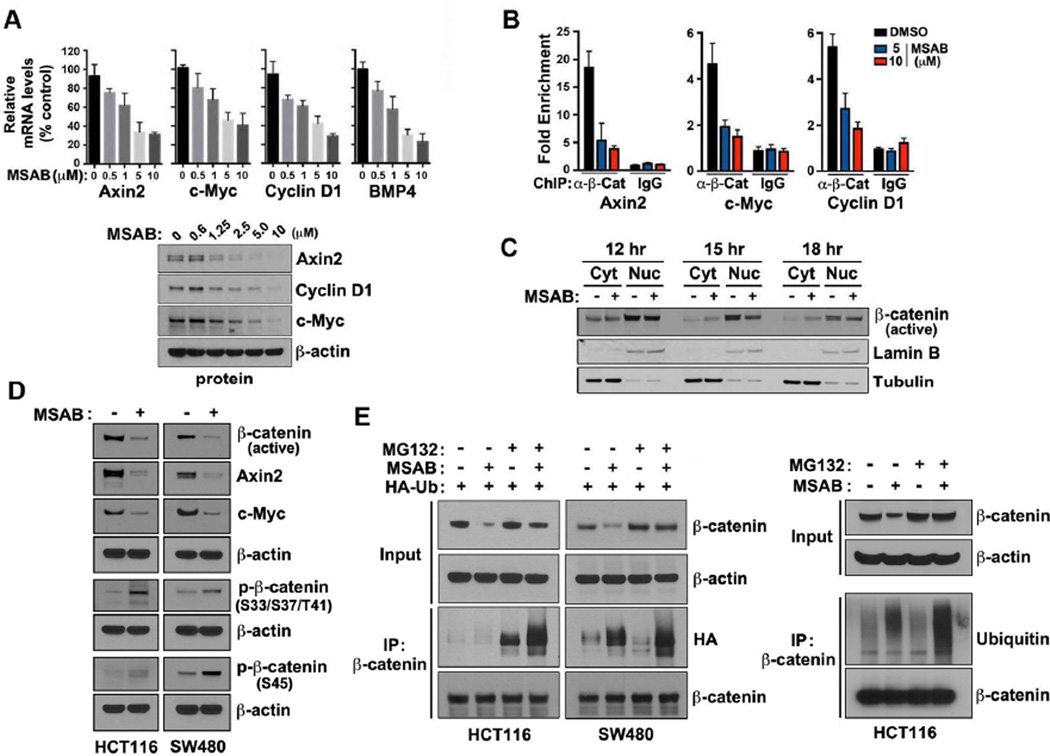
To further understand the inhibitory effects of MSAB on Wnt/β-catenin pathway, we first assessed the effects of MSAB treatment on the expression of Wnt/β-catenin downstream target genes. Expression level of Wnt/β-catenin downstream pathway genes including AXIN-2, c-MYC, Cyclin D1, and BMP4 was examined in HCT116 cells at the mRNA or protein level, which decreased in response to MSAB treatment in a dose-dependent manner (Figure 3A). Similar observations were made on DLD-1, SW480 and LS174T cells, showing decreased level of proteins encoded by target genes c-MYC and Cyclin D1 in response to MSAB (Figure S3A). Next, in order to check if MSAB disrupts the recruitment of β-catenin to the promoter region of its target genes, we carried out chromatin immunoprecipitation assays. The occupancy level of β-catenin in these promoter regions was significantly decreased by MSAB treatment (Figure 3B). To determine if this could be due to decreased levels of nuclear β-catenin, we examined the effects of MSAB on nuclear translocation of β-catenin. Cytoplasmic and nuclear fractions were extracted from HCT116 cells treated with MSAB over a time course and fractions were examined by western blot analysis. MSAB treatment resulted in the reduction of active β-catenin (ABC) level in the nuclear fraction, accompanied by an increase of ABC in cytoplasmic fractions (Figure 3C). However, the increase of cytoplasmic ABC did not appear sufficient to account for the magnitude of loss of nuclear ABC, leading to the hypothesis that MSAB downregulates the overall level of β- catenin. In order to test this possibility, we examined the effect of MSAB on ABC level in whole cell lysates and found that the total level of ABC decreased while the abundance of phospho-β-catenin (p-β-catenin) increased in response to MSAB treatment in HCT116 and SW480 cells (Figure 3D). Similar observations were made in DLD-1 and LS174T cells showing decreased ABC level in response to MSAB (Figure S3A). These results prompted the idea that MSAB might facilitate increased ubiquitination and proteasomal degradation of β-catenin. To test this possibility, HCT116 and SW480 cells expressing HA-tagged ubiquitin (HA-Ub) were treated with MSAB, followed by proteasome inhibitor MG132 (Figure 3E). Based on western blot analysis of whole cell lysate (upper panel), we found that MSAB-induced downregulation of β-catenin was markedly suppressed by proteasome inhibition. Furthermore, western blot analysis of immunoprecipitated β-catenin (lower panel) revealed that ubiquitination of β-catenin was significantly increased upon MSAB treatment, which became more evident when MG132 was treated in combination. Similar results were obtained when probing for endogenous ubiquitin (Figure 3E, right panels). Next, we tested whether MSAB affects β-catenin associated with E-cadherin. Results from co-immunoprecipitation assay demonstrated that the level of β-catenin interacting with E-cadherin remains unaffected after MSAB treatment (Figure S3B). Together, these results demonstrate that MSAB increases ubiquitination and proteasome-dependent degradation of β-catenin, which leads to its inhibitory effect on Wnt/β-catenin signaling pathway.
Figure 3. MSAB inhibits β-catenin activity and stimulates proteasomal degradation of β-catenin.
(A) MSAB decreases mRNA and protein levels of endogenous Wnt target genes in HCT116 cells. Cells were treated with MSAB for 20 h and mRNA levels of Wnt target genes were measured by qRT-PCR. Data shown represent the mean of three independent qRT-PCR reactions, graphed as relative expression level compared to that of DMSO-treated control. The effects of MSAB on Wnt target gene products in HCT116 cells were examined through western blot analyses. β-actin level was used as a loading control. (B) Chromatin Immunoprecipitation (ChIP) PCR results demonstrate that the occupancy level of β- catenin in Wnt target gene promoters decreased in response to MSAB treatment. HCT116 cells were treated with DMSO or MSAB (5 or 10 ΰM), followed by ChIP using IgG (negative control) or anti-β-catenin antibody. Fold-enrichment over IgG control of DMSO treated sample is shown for promoter regions of Axin2, c-Myc, and Cyclin D1. (C) MSAB decreases Wnt-mediated nuclear accumulation of β-catenin levels. HCT116 cells were treated with MSAB for 12, 15, or 18 h, and fractionated lysates were analyzed by western blotting for active β-catenin. Lamin B and tubulin serve as loading controls. (D) MSAB treatment downregulates the level of active β-catenin. HCT116 and SW480 cells were treated with DMSO or MSAB, and whole cell lysates were subjected to western blot analysis. (E) MSAB induces degradation of β-catenin in a proteasome-dependent manner. Left panels: HA-tagged ubiquitin was transiently overexpressed in HCT116 or SW480 cells which were treated with 5 ΰM of MSAB for 16 h, followed by addition of DMSO or 10 ΰM MG132 for 4 h. β-catenin was immunoprecipitated using anti-β-catenin antibody. Input and immunoprecipitated fractions were analyzed by western blot analysis to examine the protein level of HA-Ub, β-catenin, and β-actin. Right panels: HCT116 cells were treated with 5 ΰM of MSAB for 16 h, followed by addition of DMSO or 10 ΰM MG132 for 4 h. β-catenin was immunoprecipitated using anti-β-catenin antibody. Input and immunoprecipitated fractions were analyzed by western blot analysis to detect endogenous ubiquitin and β-catenin. See also Figure S3.
MSAB targets the β-catenin molecule
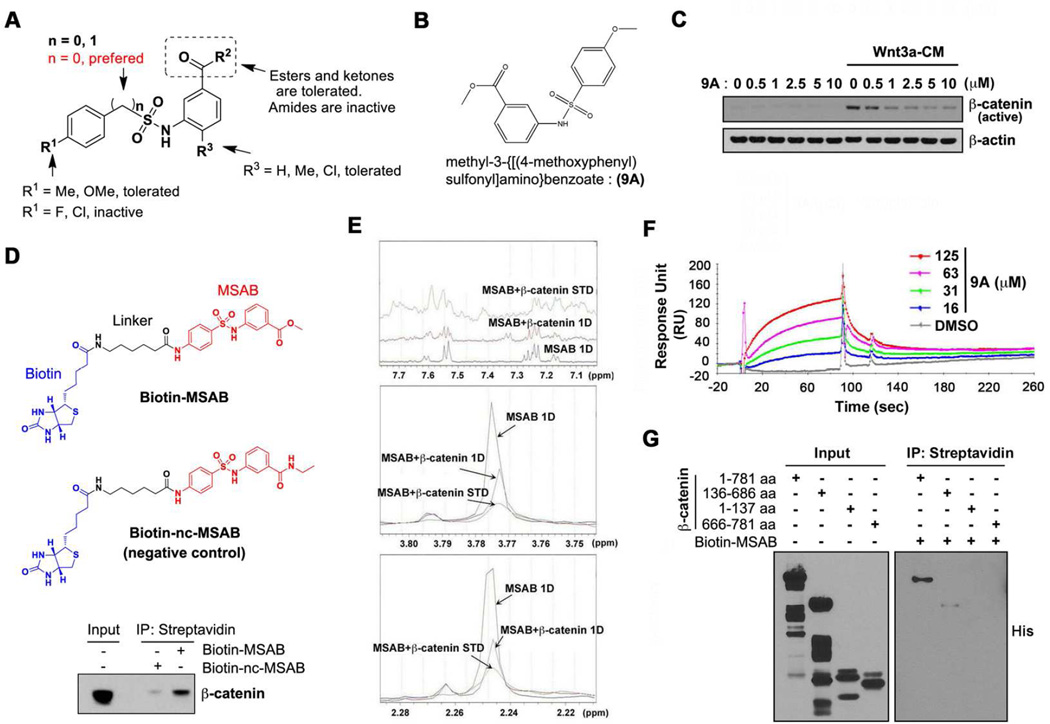
In order to understand structural elements of MSAB that are critically involved in β-catenin inhibition, we performed a structure activity relationship study using 82 structural analogs that were synthesized or purchased. Since MSAB was identified as an inhibitor of Wnt/β-catenin signaling pathway through TOP-Luc activity assay in HCT116 cells, these analogs were tested by the same assay and the IC50 of each compound was calculated. Representative analogs are shown in Table S2. Structure activity relationship study of phenylsulfonamido-benzoates provided information on the critical residues responsible for the inhibitory activity towards Wnt/β-catenin signaling pathway. The para-substitution of phenyl ring is important for maintaining the activity, and fluoroor chloro- substitutions result in loss of its inhibitory activity. Phenylsulfonamidobenzamides with amides in place of ester groups resulted in complete loss of activity (Figure 4A). The methoxyphenyl methylbenzoate analog, compound 9A (Figure 4B), was the most potent in the TOP-Luc reporter assay (Table S2). Hence, we tested whether 9A shows similar effects as MSAB, and found that it can suppress the increase of active β-catenin levels in HEK293T cells induced by Wnt3a-CM treatment (Figure 4C), and reduce the expression level of Wnt/β-catenin target genes AXIN-2, c-MYC, and Cyclin D1 in DLD-1 cells (Figure S4A). Analyses on cell viability of Wnt/β-catenin-dependent HCT116 cells and -independent A673 and H460 cancer cells demonstrated that 9A is also capable of inhibiting proliferation of HCT116 cells, while showing little effect on the viability of A673 and H460 cells (Figure S4B). These results demonstrate that compound 9A, a structural analog of MSAB, is also a potent and selective inhibitor of the Wnt/β-catenin signaling pathway, and underlines the potential of MSAB as a lead compound for Wnt/β-catenin inhibitor development.
Figure 4. MSAB binds to β-catenin.
(A) Summary of SAR (Structure Activity Relationship) for Phenylsulfonamidobenzoates as inhibitors of Wnt/β-catenin signaling pathway. (B) Chemical structure of methyl 3-{[(4-methoxyphenyl)sulfonyl]amino} benzoate (9A). (C) Active β-catenin level increased by Wnt3a-CM is suppressed by 9A treatment in HEK293T cells. Western blot analysis results probing for active β-catenin are shown where β-actin serves as a loading control. (D) The chemical structure of biotinylated MSAB (Biotin-MSAB, top panel) or biotinylated negative control MSAB (Biotin-nc-MSAB, middle panel). Bottom panel: β-catenin associates with Biotin-MSAB. Cell lysate was incubated with Biotin-MSAB or Biotin-nc-MSAB, followed by streptavidin-pull down assay. The level of β-catenin associated with Biotin-MSAB or Biotin-nc-MSAB was analyzed through western blot analysis (E) Identification of MSAB binding to β-catenin using 1D 1H NMR spectroscopy. Assessment of binding is based on the following experiments, blue: normal 1H spectrum of MSAB in the absence of protein (1D), red: normal 1H spectrum of MSAB and β-catenin protein (1D), green: 1H spectrum of MSAB and β-catenin protein Saturation Transfer Difference (STD). (F) Physical interaction between compound 9A, a MSAB analog, and β-catenin was tested using SPR. Various concentrations of MSAB ranging from 0 to 125 ΰM in 2-fold dilution series were tested in duplicate. Binding signal from a reference flow cell containing no protein was subtracted to account for detection of specific interaction between the compound and β-catenin. Compound 9A showed physical interaction with β-catenin in a concentration-dependent manner. (G) MSAB binds to the Armadillo repeat region of β-catenin. In vitro pull-down assays were carried out using Streptavidin beads, Biotin- MSAB, and histidine-tagged recombinant proteins of different regions of β-catenin: full length (amino acid residues: 1–781), N-terminal (1–137), Armadillo repeat (136–686), or C-terminal (666–781). Input and pull-down fractions were examined through western blot analysis using anti-His antibody. See also Figure S4, Table S2 and S3.
To identify the direct target(s) of MSAB, we used a pull-down approach using biotinylated MSAB analogs. Based on the structure activity relationship study results, a biotin moiety connected to a linker was attached to MSAB (Biotin-MSAB), as well as to a negative control, a structural analog of MSAB that lacks the inhibitory effect on TOP-Luc (Biotin-nc-MSAB) (Figure 4D; top and middle panel). TOP-Luc assay was carried out to check whether MSAB remains active following biotinylation, and as anticipated, TOP-Luc activity was inhibited by Biotin-MSAB, whereas, Biotin-nc-MSAB did not show any inhibitory effect (Figure S4C). We also checked the effects of Biotin-MSAB and Biotin-nc-MSAB on ABC level in HEK293T cell lines. Biotin-MSAB significantly suppressed the increase of ABC level induced by Wnt3a-CM, in a dose dependent manner (Figure S4D). Based on these results, we moved ahead and carried out pulldown assays using Biotin-MSAB and Biotin-nc-MSAB to identify binding targets of MSAB. Proteins that co-precipitated with Biotin-MSAB or Biotin-nc-MSAB were identified through tandem mass spectrometry (Table S3). Proteins detected with at least two unique peptides were selected for comparison, and interestingly, β-catenin ranked among the top candidates that showed stronger association with Biotin-MSAB than with Biotin-nc-MSAB. The specific enrichment of β-catenin in the Biotin-MSAB fraction was confirmed by western blot analysis (Figure 4D; bottom panel).
In order to check whether MSAB binds β-catenin, first, NMR analysis was carried out. Adding purified recombinant β-catenin protein to MSAB shifted the 1H peaks of MSAB, demonstrating an interaction between the two (Figure 4E). In addition, we conducted SPR spectroscopy to test whether compound 9A, an MSAB analog with comparable efficiency of inhibiting Wnt signaling activity to MSAB, can also bind β-catenin. Purified recombinant His-β-catenin proteins were captured on a CM5 chip, and then various concentrations of 9A were allowed to flow over, and as a result, 9A also showed a binding to β-catenin in a dose-dependent manner (Figure 4F). Furthermore, in order to identify the domain of β-catenin that interacts with MSAB, in vitro pull down assays were carried out using purified recombinant proteins of truncated forms of β-catenin. As a result, the Armadillo repeat region (amino acid residues 136–686), as opposed to Nterminal (residues 1–137) or C-terminal (residues 666–781) fragments of β-catenin, showed direct binding with immobilized Biotin-MSAB (Figure 4G). In order to validate the binding of MSAB to the Armadillo repeat region of β-catenin, we carried out NMR assays with MSAB and full-length or truncated forms of β-catenin. As a result, Armadillo repeat region 2 (residues 301–670) showed interaction with MSAB, in contrast to Armadillo repeat region 1 (residues 142–305), N-terminal, or C-terminal fragment (Figure S4E). These observations suggest that MSAB binds to β-catenin, most likely via its Armadillo repeat region. Altogether, our results corroborate the potential of MSAB as a lead compound for developing therapeutics that can specifically target Wnt/β-catenin dependent cancer cells.
DISCUSSION
Aberrant Wnt signaling is directly linked to multiple types of cancers, including colon cancer, sarcoma, melanoma, and hepatocellular carcinoma (Bafico et al., 2004; Clevers and Nusse, 2012; Rubinfeld et al., 1997; Vijayakumar et al., 2011). Previous reports have shown the crucial role of β-catenin in Wnt-dependent cancer cells and demonstrated that downregulation of β-catenin can suppress tumorigenesis (Kwong et al., 2002; Rosenbluh et al., 2012). Although the discovery of several previously reported Wnt/β-catenin pathway inhibitors (Anastas and Moon, 2013; Kahn, 2014) have advanced our knowledge on Wnt/β-catenin signaling, there is still a need for discovering novel inhibitors that can selectively suppress hyperactive β-catenin. In the current study, we have identified a class of small molecules that can specifically inhibit Wnt/β-catenin signaling pathway in multiple Wnt/β-catenin-dependent cancer cells, while showing minimal anti-proliferative effect on Wnt-independent cells, in vitro as well as in vivo. The effect of MSAB on activated β-catenin level in Wnt3a-stimulated HEK293T cells shows that MSAB is capable of targeting wild-type β-catenin when its level is elevated. Additionally, MSAB appears to target the oncogenic β-catenin mutant as well, based on the observation that MSAB inhibited the proliferation of cells such as LS174T, which harbor only the mutant form of β-catenin (Chan et al., 2002; Korinek et al., 1997). The observation that MSAB causes degradation of this oncogenic mutant isoform of β-catenin is noteworthy since this type of mutant β-catenins lack residues required for surveillance and degradation by the destruction complex. The functional consequence is that these mutants cannot be efficiently dealt with by approaches that target upstream members of the signaling pathway by activating the destruction complex. Altogether, these results clearly demonstrate the therapeutic potential of MSAB and its functional derivatives for targeting β-catenin-mediated cancers.
SAR analysis results not only corroborated the effectiveness of MSAB as a lead compound, but also provided us tools to carry out a search for proteins that interact with MSAB in cell extracts through affinity purification followed by tandem mass spectrometry. We were hoping to find clues about MSAB-interacting factors that would affect protein stability or nuclear translocation of β-catenin. However, rather unexpectedly, one of the top hits turned out to be β-catenin itself. The results from NMR and SPR assays demonstrating binding between β-catenin and MSAB or an MSAB analog 9A in vitro further strengthens the possibility that MSAB directly affects β-catenin function presumably through inducing allosteric conformational changes or affecting interactions between β-catenin and its regulators. Nevertheless, the precise mechanistic detail of MSAB action remains unclear and will require further investigation of β-catenin interacting proteins which are affected by MSAB, or in-depth characterization of how β- catenin is directly affected by MSAB. Such information on active β-catenin regulation is likely to provide additional perspectives on therapeutic strategies against Wnt/β-catenin-dependent cancer, as well as novel insights into Wnt/β-catenin biology.
EXPERIMENTAL PROCEDURES
Chemical Screen
High throughput chemical screening was performed as previously described (Raj et al., 2011; Stanton et al., 2009) with modifications. For screening of inhibitors targeting Wnt/β-catenin signaling pathway, HCT116-TOP cells (HCT116 cells stably transfected with TOPFLASH (TCF/LEF1-optimized promoter)-firefly luciferase reporter) were seeded using automated plate filler in 384-well plates and incubated at 37°C overnight. Next day, each small molecule compound (Compound library from Chembridge and Broad institute) was pin-transferred to each plate. Plates were covered with lids and incubated at 37°C for 20 h. The following day, assa y plates were allowed to equilibrate to room temperature for 10 min. Luciferase assay reagent (Steady-Glo Luciferase Assay System, Promega) was added to each well, incubated for 15 min, then read using a Perkin Elmer Envision luminometer to quantitate luciferase levels. Compounds that decrease luciferase activity were selected as hits. Luciferase values were normalized to the positive and negative controls to determine a normalized percent inhibition. Based on the normalized luciferase values, compounds showing >50% inhibition in both replicates compared to DMSO control were considered as active compounds. All small molecules were tested in duplicates.
Supplementary Material
Highlights.
Identification of MSAB, a β-catenin inhibitor, using cell-based chemical screening
MSAB inhibits growth of Wnt/β-catenin-dependent cancer cells in vitro and in vivo
MSAB disrupts β-catenin activity by prompting proteasomal degradation of β-catenin
MSAB binds to β-catenin
In Brief.
Hwang et al. identify a small molecule, MSAB, which can bind to β-catenin and specifically inhibit β-catenin signaling by promoting its proteasome-mediated degradation. MSAB also inhibits growth of Wnt/β-catenin-dependent cancer cells, suggesting that this small molecule could be an effective therapeutic strategy for Wnt/β-catenin-dependent cancers.
Acknowledgments
We thank N. Tolliday, V. Kolev for help with high throughput screening, B.H. Lee and S. Gondi for help with data analysis, and G. Heffron, W. Massefski from the NMR Facility at Harvard Medical School for NMR studies. S.Y.H. was supported in part by the Korean Government Research Foundation grant (KRF-2008-357-E00023). X.D. was supported by grants from the National Natural Science Foundation of China (81422045, U1405223 and 21272195). K.D.W. was supported by grant CPRIT RP140233. The research was supported by NIH/NCI grants (CA195534 & CA80058).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables.
AUTHOR CONTRIBUTIONS
S.Y.H. designed and performed experiments, and analyzed the data. X.D., Q.K. and T.Z. synthesized analog compounds and performed pharmacokinetic experiments. S.B. and C.L. performed western blot experiments. S.J.L. helped SPR analysis and H.S. helped mass-spec experiments and analyzed the data. J.Z. and A.M. helped high throughput screening and analyzed the data. K.D.W. helped with research design and analyzed the data. A.M. and S.W.L designed experiments and analyzed the data. S.Y.H. and S.W.L. wrote the manuscript, which was reviewed by all authors.
REFERENCES
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Chan TA, Wang Z, Dang LH, Vogelstein B, Kinzler KW. Targeted inactivation of CTNNB1 reveals unexpected effects of beta-catenin mutation. Proc Natl Acad Sci U S A. 2002;99:8265–8270. doi: 10.1073/pnas.082240999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- de la Roche M, Rutherford TJ, Gupta D, Veprintsev DB, Saxty B, Freund SM, Bienz M. An intrinsically labile alpha-helix abutting the BCL9-binding site of beta-catenin is required for its inhibition by carnosic acid. Nat Commun. 2012;3:680. doi: 10.1038/ncomms1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of betacatenin/ CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, von Kries JP, Birchmeier W. A Small-Molecule Antagonist of the beta- Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016;76:891–901. doi: 10.1158/0008-5472.CAN-15-1519. [DOI] [PubMed] [Google Scholar]
- Hao J, Ao A, Zhou L, Murphy CK, Frist AY, Keel JJ, Thorne CA, Kim K, Lee E, Hong CC. Selective small molecule targeting beta-catenin function discovered by in vivo chemical genetic screen. Cell Rep. 2013;4:898–904. doi: 10.1016/j.celrep.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. Embo J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Kwong KY, Zou Y, Day CP, Hung MC. The suppression of colon cancer cell growth in nude mice by targeting beta-catenin/TCF pathway. Oncogene. 2002;21:8340–8346. doi: 10.1038/sj.onc.1206050. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Polakis P. Drugging Wnt signalling in cancer. Embo J. 2012;31:2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, Taveras KM, Hyman JM, Lee SW, Koehler AN, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–156. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S, Liu G, Rus IA, Yao S, Chen Y, Akiri G, Grumolato L, Aaronson SA. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer Cell. 2011;19:601–612. doi: 10.1016/j.ccr.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.