Abstract
Background:
Bronchial occlusion therapy using silicon spigots is effective for intractable pneumothorax. However, sometimes the pneumothorax is refractory to bronchial occlusion because of collateral ventilation. For such difficult pneumothoraces, we attempted an intrabronchial infusion of autologous blood plus thrombin to control collateral ventilation and stop air leaks.
Methods:
We performed bronchial occlusions using silicon spigots in patients with spontaneous pneumothorax secondary to emphysema and refractory to chest drainage, but which was inoperable owing to each patient’s poor surgical candidacy and poor overall health condition. When bronchial occlusion proved ineffective, we undertook intrabronchial infusion of autologous blood plus thrombin, 2 to 4 days after bronchial occlusion. A catheter was inserted into the subpleural area, through a gap between the silicon spigot and the bronchial wall, using a flexible bronchoscope under fluoroscopic guidance. Autologous blood, followed by a thrombin solution, was infused using the catheter. We repeated the same infusion a total of 4 to 6 times while changing the target bronchi. All interventions were performed under local anesthesia.
Results:
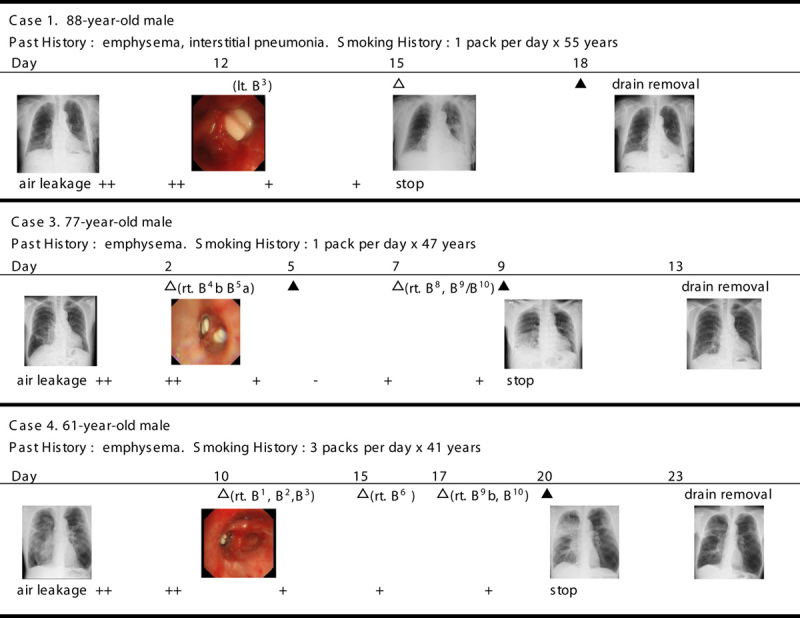
The subjects were 9 men, aged from 61 to 88 years, with smoking histories. Three patients also had interstitial pneumonia, and 6 patients had undergone pleurodesis in vain before bronchial occlusion. For 4of the 9 patients, autologous blood plus thrombin infusions successfully stopped air leaks, and in 3 patients, intrabronchial infusions and pleurodesis halted leaks altogether.
Conclusion:
Intrabronchial infusion of autologous blood plus thrombin was effective for intractable pneumothoraces that could not be clinically managed, even by bronchial occlusion using silicon spigots.
Key Words: autologous blood, bronchial occlusion, emphysema, silicon spigot, pneumothorax
The development of a pneumothorax is one of the most serious complications of emphysema. From August 2014 to July 2015, 68 emphysema patients presented at our hospital with spontaneous secondary pneumothorax that required a chest tube placement. Of these, 46 patients had a persistent air leak after chest tube placement that required further intervention (an operation in 35 cases and bronchial occlusion using silicon spigots in 11 cases).
Bronchial occlusion using silicon spigots (EWS, Novatech, La Ciotat, France) has proven effective for intractable pneumothorax that is refractory to chest drainage, and that is inoperable owing to the patient’s poor surgical candidacy and poor overall health condition.1–3 However, in some cases, bronchial occlusion cannot stop air leaks because of the existence of collateral ventilation, seen especially in patients with emphysema. For such intractable cases, we attempted the intrabronchial infusion of autologous blood plus thrombin after bronchial occlusion. Here, we report the outcomes of this treatment and discuss the effectiveness and problems associated with this procedure.
PATIENTS AND METHODS
Patients
The subjects of this study were 9 patients with emphysema and who had also developed a secondary, spontaneous pneumothorax. Each patient’s pneumothorax was refractory to chest drainage, with or without pleurodesis, and inoperable owing to the patient’s poor surgical candidacy and poor overall health condition. In addition, each pneumothorax could not be clinically managed, even by bronchial occlusion using silicon spigots.
Methods
The bronchi responsible for each air leak were identified using chest computed tomography, and/or a balloon occlusion test. In patients for whom bronchial occlusion using silicon spigots was ineffective, we subsequently performed an intrabronchial infusion of autologous blood plus thrombin, 2 to 4 days after the bronchial occlusion.
Intervention
Each patient was placed under local anesthesia and mild sedation with 1.5 to 3 mg intravenous midazolam, and a bronchoscopy was then performed whereby silicon spigots (EWS) were inserted through an endotracheal tube. If a satisfactory decrease in each air leak was not obtained within 2 to 4 days, autologous blood plus thrombin were infused in an intrabronchial manner. The infusion was performed as follows: a catheter (PW-1L-1 or PW-5L-1; maximum external diameter: 2.45 mm; working length: 1650 mm; Olympus, Tokyo, Japan) was inserted through a gap between the silicon spigot and the bronchial wall into the subpleural area using a flexible bronchoscope (BF-1T260 or BF-1TQ290; Olympus) under fluoroscopic guidance. Autologous blood was drawn from a venous cannula that had been placed in the patient’s median cubital vein, and the blood (3 to 4 mL) was immediately infused using a catheter, followed by 2500 U of thrombin solution (Mochida, Tokyo, Japan), and flushed by air (10 mL). We repeated the same infusion a total of 4 to 6 times while changing target bronchi.
We repeated the intrabronchial infusion at intervals of 3 to 5 days (mean 3.8 d) until the air leak resolved. However, if the air leak did not stop completely, additional insertions of spigots or even pleurodesis were performed as necessary.
We obtained authorization to perform this study in advance from the Ethics Committee of the National Organization Himeji Medical Center, Japan (registration number 26-11, approval date: August 11, 2014). We obtained informed consent from each enrolled individual, allowing us to provide treatment using the specific procedure outlined above and to use the data obtained for research purposes.
RESULTS
The subjects studied were 9 men aged from 61 to 88 years and all with a history of smoking. Three patients also had interstitial pneumonia as well as emphysema. Before the onset of a pneumothorax, all patients had severe dyspnea (Modified Medical Research Council dyspnea scale: MMRC grade 4) and had undergone long-term oxygen therapy. Six of the 9 patients had undergone pleurodesis between 3 and 19 days (mean 10.7 d) before the bronchial occlusion, but air leaks had not stopped in any patients. The remaining 3 patients did not undergo pleurodesis before the bronchial occlusion because of a collapsed lung with massive air leaks.
The outcomes of combination therapy for the 9 cases examined are summarized in Table 1. Figure 1 shows 3 representative cases (cases 1, 3, and 4). In all 9 cases, the reduction in air leaks after bronchial occlusion using silicon spigots was a partial one and, consequently, an intrabronchial infusion of autologous blood plus thrombin was attempted.
TABLE 1.
Outcomes of Combination Therapy for Bronchial Occlusion Using Silicon Spigots With IBI-ABT
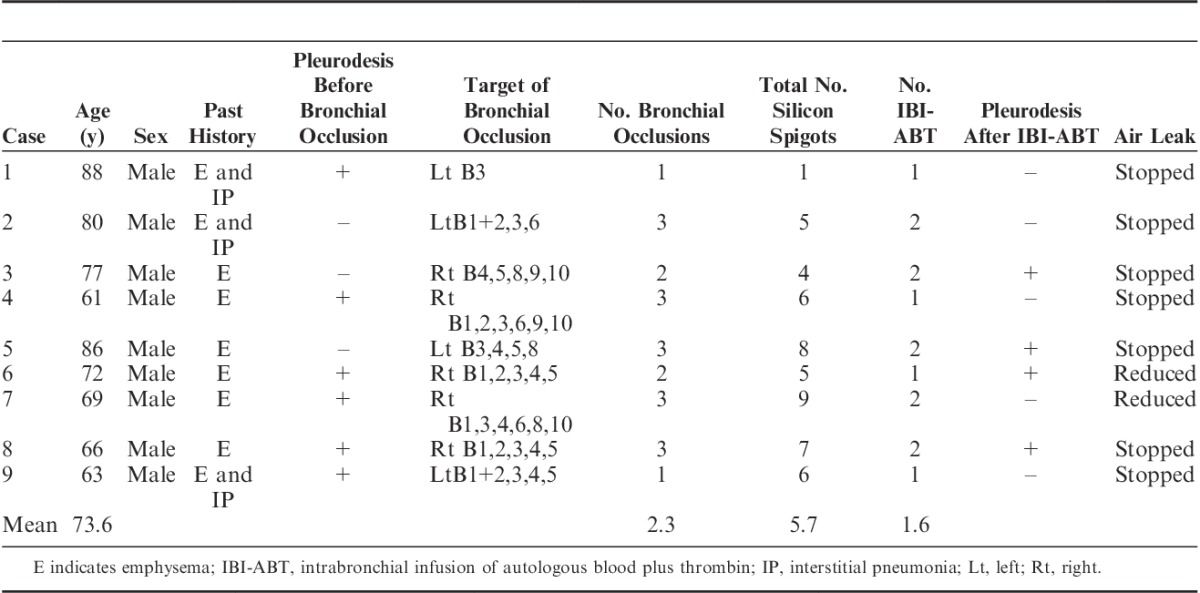
FIGURE 1.
Clinical courses for 3 representative patients (cases 1, 3, and 4). A white triangle indicates a bronchial occlusion with silicon spigots. A black triangle indicates an intrabronchial infusion of autologous blood and thrombin.
The total number of bronchoscopies performed per patient was between 2 and 5 times (mean 3.9 times). Of these, intrabronchial infusions of autologous blood and thrombin were performed 1 to 2 times (mean 1.6 times) per patient. The total volume of blood that entered each patient’s bronchi during each bronchoscopy was between 12 and 24 mL (mean 18.2 mL). Despite severe emphysema, complications secondary to repeated bronchoscopy and sedation were not seen.
In 4 patients, air leaks stopped within a few days of an infusion. In 3 patients, the effects of the intrabronchial infusions were incomplete and air leaks were finally stopped after an additional pleurodesis from between 2 and 6 days (mean 3.8 d) after the last intrabronchial infusion. Finally, air leaks did not stop for 2 patients, although a reduction in air leaks and reexpansion of the lung were achieved. These 2 cases eventually died due to an exacerbation of chronic obstructive pulmonary disease (COPD) during hospitalization for 13 (case 6) and 11 days (case 7), respectively, after intrabronchial infusion.
For case 1, we removed a silicon spigot 3 months after recovery from a pneumothorax. However, for the other 8 patients, spigots were not removed because of the patients’ poor overall health condition; but the expectation of a sustained therapeutic effect remained. No adverse effects were seen in relation to the remaining spigots.
In all cases, a consolidation shadow was transiently seen in the lung field of the infused side, a day after the intrabronchial infusion of autologous blood plus thrombin. Low-grade fever (4 cases) and hypoxemia (3 cases) were also transiently seen. Patients recovered from hypoxemia (SpO2 85% to 93% on oxygen) within 3 days and without the need for positive pressure ventilation. Because of the difficulty in differentiating inflammation caused by an intrabronchial infusion from bacterial pneumonia based on clinical presentation or imaging findings, penicillin antibiotics were given to all patients, with a consequent relief of symptoms within 3 days. Antibiotics were given for 3 to 10 days (mean 7 d).
DISCUSSION
Bronchial occlusion therapy using silicone-based plugs as a treatment for intractable pneumothorax was originally developed by Watanabe et al.4,5 In Japan, the silicon spigot (EWS) has been available and covered by health insurance since 2013; this technique is now recognized as a well-established treatment for intractable pneumothorax.
The effectiveness of combined therapy for intractable pneumothorax using both bronchial occlusion and chemical pleurodesis has also recently been reported.3,6 However, in several severe cases, bronchial occlusion and/or pleurodesis have been completely inadequate for various reasons. For example, high collateral ventilation may have developed,7,8 with collateral pathways traversing lobar fissures.9,10 This explains the difficulty in controlling air leaks from the pneumothorax by bronchial occlusion in severely emphysematous lungs. For the same reason, the outcome of lung volume reduction therapy with endobronchial valves depends largely on the presence of fissure completeness in the treated lobe.11
As for the identification of the responsible bronchus, in only 1 case (case 1) could we identify the culpable bronchus using a balloon occlusion test. For the other cases, the responsible bronchi were inferred from computed tomographic scans. In other words, it appeared difficult to identify responsible bronchi using the balloon occlusion test in patients with emphysema because of collateral ventilation.
Ingenito et al,12 in a study of biological lung volume reduction upon the instillation of a fibrin hydrogel and thrombin into the emphysematous area of a sheep model, reported that this procedure promoted fibroblast attachment and collagen synthesis. Their intention was to remodel the hyperinflated emphysematous lung into contracted scar tissue. On the basis of this study, Kanoh et al13,14 reported the intrabronchial infusion of autologous blood in lung volume reduction therapy.
Kanoh and Kobayashi used autologous blood as the injection material, mainly because of its bioadhesive properties but also because it is relatively safe, as it has few side effects, and is inexpensive. They also used thrombin to promote blood clotting in the lung to enhance a reduction in lung volume.15 We hypothesize that this technique may seal collateral pathways and thus enhance the effectiveness of bronchial occlusion with silicon spigots. However, it is unclear how such a small volume of instilled blood of approximately 12 to 24 mL could have such effects, but we believe inflammation evoked by blood and thrombin in emphysematous regions may have had some role.
Wiaterek et al16 reported a case of intractable pneumothorax that was successfully treated with intrabronchial, oxidized regenerated cellulose (Surgicel Absorbable Hemostat; Ethicon), and 3 mL of the patient’s blood delivered through a cut Fogarty balloon onto the absorbable hemostat. Compared with the absorbable hemostat, however, silicon spigots were thought to be fixed more firmly to the target bronchus.
The complications of bronchial occlusion by silicon spigot were thought to be acceptable, even after long-term placement in patients with a risk from their removal because of their poor overall health condition or with the necessity of long-term placement to prevent recurrence.2,6,17 However, when the benefit of the long-term placement of silicon spigots ends, these need to be removed within 3 months to a year of placement.17,18
Watanabe18 reported that 4 of 22 cases of bronchial occlusion showed subsequent obstructive pneumonia that was relieved by antibiotic agents during a follow-up of >1 year (median 24.5 mo). However, in all our intractable cases except for 1, in which bronchial occlusion treatment, with or without pleurodesis, was inadequate, >4 silicon spigots per patient were needed to reduce air leaks. Therefore, the risk of obstructive pneumonia was thought to be high. In addition, as the intrabronchial injection of autologous blood may carry a certain risk of infection, we recommend that antibiotics be used during such a procedure and that patients be placed under careful observation.
Careful analysis is also required of the circumstances surrounding the deaths of 2 patients after intrabronchial injection of autologous blood plus thrombin. The cause of death for the 2 was thought to be an exacerbation of COPD. Both patients seemed to have had particularly bad prognoses due to poor baseline conditions, such as hypercapnia (Pco2>50 Torr), and had shown a poor performance status (PS4). A decline in the overall health conditions of the 2 cases was seen >1 week after the resolution of lung consolidation caused by intrabronchial infusion. Therefore, we believe that the intrabronchial injection of autologous blood plus thrombin was not directly related to their deaths, although any relationship between the procedure and the late-onset exacerbation of COPD is unclear. We believe that a reduction in air leaks and a reexpansion of the lung after the procedure may have had a beneficial impact, at least, temporarily.
In 4 cases, pleurodesis was performed after the intrabronchial infusion of autologous blood plus thrombin. We, therefore, could not assess the benefit of the intrabronchial infusion on its own. Further study is necessary to evaluate the effectiveness of the proposed intervention in other settings.
CONCLUSIONS
This study suggests that the intrabronchial infusion of autologous blood plus thrombin may be effective in cases of intractable pneumothorax, in which air leaks are partially reduced but not completely stopped by bronchial occlusion using silicon spigots. However, our report of this procedure does have a major limitation in that it still represents a small case series with multiple interventions. In addition, the deaths of 2 patients, in response to what was thought to be an exacerbation of COPD, is problematic as we cannot completely exclude a relationship between these deaths and the procedure. Further investigations with a larger patient population are required.
ACKNOWLEDGMENT
The authors are indebted to Professor Yukihiko Sugiyama in the Department of Respiratory Medicine, Jichi Medical University, for his valuable advice.
Footnotes
Disclosure: There is no conflict of interest or other disclosures.
REFERENCES
- 1.Watanabe Y, Matsuo K, Tamaoki A, et al. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchology Interv Pulmonol. 2003;10:264–267. [Google Scholar]
- 2.Sasada S, Tamura K, Chang YS, et al. Clinical evaluation of endoscopic bronchial occlusion with silicone spigots for the management of persistent pulmonary air leaks. Intern Med. 2011;50:1169–1173. [DOI] [PubMed] [Google Scholar]
- 3.Ishida A, Kida H, Muraoka H, et al. Intractable pneumothorax managed by talc pleurodesis and bronchial occlusion with spigots. Respirol Case Rep. 2015;3:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe Y, Hiraki S, Araki M, et al. Bronchial embolization using dental impression material in a case of pyelobronchial fistula with Candida Fungemia. J Jpn Soc Respir Endoscopy. 1991;13:607–610. [Google Scholar]
- 5.Watanabe Y, Komoto R, Tamaoki A, et al. Bronchial occlusion. J Jpn Soc Respir Endoscopy. 2003;25:704–708. [Google Scholar]
- 6.Kaneda H, Minami K, Nakano T, et al. Efficacy and long-term clinical outcome of bronchial occlusion with endobronchial Watanabe spigots for persistent air leaks. Respir Investig. 2015;53:30–36. [DOI] [PubMed] [Google Scholar]
- 7.Morrell NW, Wignall BK, Biggs T, et al. Collateral ventilation and gas exchange in emphysema. Am J Respir Crit Care Med. 1994;150:635–641. [DOI] [PubMed] [Google Scholar]
- 8.Cetti E, Moore A, Geddes D. Collateral ventilation. Thorax. 2006;61:371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higuchi T, Reed A, Oto T, et al. Relation of interlobar collaterals to radiological heterogeneity in severe emphysema. Thorax. 2006;61:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choong CK, Macklem PT, Pierce JA, et al. Airway bypass improves the mechanical properties of explanted emphysematous lungs. Am J Respir Crit Care Med. 2008;178:902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. [DOI] [PubMed] [Google Scholar]
- 12.Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med. 2003;167:771–778. [DOI] [PubMed] [Google Scholar]
- 13.Kanoh S, Kobayashi H, Motoyoshi K. Intrabullous blood injection for lung volume reduction. Thorax. 2008;63:564–565. [DOI] [PubMed] [Google Scholar]
- 14.Kanoh S, Kobayashi H, Motoyoshi K. Bronchoscopic blood injection reducing lung volume in lymphangioleiomyomatosis. Ann Thorac Surg. 2009;87:1266–1268. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Kanoh S. Bronchoscopic autologous blood injection for lung volume reduction. Nihon Kokyuki Gakkai zasshi. 2009;47:765–771. [PubMed] [Google Scholar]
- 16.Wiaterek G, Lee H, Malhotra R, et al. Bronchoscopic blood patch for treatment of persistent alveolar-pleural fistula. J Bronchology Interv Pulmonol. 2013;20:171–174. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Sakiyama S, Toba H, et al. Therapeutic experience with endobronchial Watanabe spigot in our hospital: the potential for long-term placement. J Jpn Soc Respir Endoscopy. 2009;31:5–9. [Google Scholar]
- 18.Watanabe Y. Bronchial OcclusionThe Best Techniques for Performing Bronchoscopy. Tokyo: Chugai-Igaku; 2012:222–230. [Google Scholar]


