Abstract
Objective:
To establish a reliable equation to predict hepatic venous pressure gradient (HVPG) using serological tests for surgical patients with hepatocellular carcinoma (HCC).
Background:
Accurate assessment of portal pressure for surgical patients with HCC is important for safe hepatic resection (HR). The HVPG is regarded as the most reliable method to detect portal hypertension. However, HVPG is not utilized in many medical centers due to invasiveness of procedure.
Methods:
Between 2006 and 2008, 171 patients (Correlation cohort), who underwent liver surgery in a tertiary hospital, were enrolled. Preoperative measurements of the HVPG and serological tests were performed simultaneously. Correlation between the HVPG and serological tests were analyzed to establish an equation for calculated HVPG (cHVPG). Between 2008 and 2013, 510 surgical patients (Application cohort) were evaluated, and HR recommended when cHVPG < 10 mm Hg. The outcomes of HR were analyzed to evaluate reliability of the cHVPG for HR.
Results:
In the correlation cohort, the equation for cHVPG was established using multivariate linear regression analysis; cHVPG (mm Hg) = 0.209 × [ICG-R15 (%)] − 1.646 × [albumin (g/dL)] − 0.01×[platelet count (103)] + 1.669 × [PT-INR] + 8.911. In the application cohort, 425 patients with cHVPG < 10 mm Hg underwent HR. Among them, 357 had favorable value of ICG-R15 < 20% (group A), and 68 had unfavorable value of ICG-R15 ≥ 20% (group B). There was no significant difference in patient demographics, tumor characteristics, operative outcome, and survival rates between group A and B.
Conclusions:
The equation for cHVPG of this study was established on statistical reliability. The cHVPG could be useful to predict portal pressure quantitatively for surgical patients with HCC using serological tests.
Keywords: hepatectomy, hepatic venous pressure gradient, hepatocellular carcinoma, liver function tests, portal hypertension
INTRODUCTION
Hepatic resection (HR) still stays as one of the main treatment modalities for the hepatocellular carcinoma (HCC) with or without liver cirrhosis. However, HR should be carefully selected among the patients with well-preserved liver function to avoid the postoperative complications related to liver failure. Also, well-preserved or compensated liver function has been defined by the absence of clinically relevant portal hypertension (PHT). Liver transplantation or nonsurgical treatments are recommended for the HCC patients with the evidence of PHT, rather than HR. The clinical evidence of PHT could be evaluated by various methods, such as hepatic venous pressure gradient (HVPG), dye-retention test, Child-Pugh scoring system, serum routine chemistry, and platelet counts.
Measuring HVPG has been known as the most reliable clinical method to estimate portal pressure, and HVPG exceeding 10 mm Hg is defined as decompensated PHT and associated with increased cirrhosis-related complications.1,2 Previous studies reported that HR for the patients with HVPG over 10 mm Hg is associated with serious postoperative complications of liver failure and mortality.3,4 Barcelona-Clinic Liver Cancer group treatment guideline for HCC recommended liver transplantation or nonsurgical modality for the patients with high HVPG ≥ 10 mm Hg.5,6 However, the measurement of HVPG has not been preferred in Asian countries because it requires more complicated procedures than other methods. Required procedures such as puncture of central vein and catheter insertion into vena cava may cause possible complications of bleeding, soft tissue hematoma, nerve injury, or arrhythmia.7,8
In the East Asian countries like Korea and Japan, the indocyanine green retention test (ICG-R15) has been commonly used for the surgical patients to determine PHT, and extent of hepatectomy.5,9 Measuring ICG-R15 has an advantage over HVPG or direct measurement of portal pressure because it is an easy and safe serological test without procedure-related complications. Previous studies reported that ICG-R15 was a valuable assessment tool to decide on the resectability and extent of hepatectomy.9–11 The value of ICG-R15 20% was supposed to be 10 mm Hg of HVPG, and ICG-R15 exceeding 20% has been considered as clinically relevant PHT.3,12,13 However, there was no study which tried to determine an accurate correlation between ICG-R15 and HVPG. Moreover, the value of ICG-R15 could be inaccurate under various patients’ conditions with jaundice, administration of H2 blocker, and genetic defect of ICG excretion.14–16
Abnormal liver function test (LFT) and complete blood cell count (CBC) can be seen in patients with liver dysfunction or cirrhosis. The value of serum bilirubin, serum albumin, prothrombin time (PT-INR), and platelet count are regarded to have correlation with liver cirrhosis.13,17–19 Those are simple and least invasive but have a limited value to stratify the degree of portal pressure.6,9,13 Furthermore, the values of those tests could be easily affected by conditions, such as hydration and nutritional status.
Authors of this study attempted to establish a reliable equation of calculated HVPG (cHVPG). First, we analyzed the serological tests, which had the significant correlation with measured HVPG and established a model of cHVPG using the values of those serological tests by the linear regression analysis. Then we prospectively assessed the feasibility of cHVPG for the surgical patients with borderline liver reserve.
PATIENTS AND METHODS
Correlation Between Hepatic Venous Pressure Gradient and Serological Tests
Between January 2006 and December 2008, 171 consecutive patients who underwent the liver surgery in our institute were included to establish the correlation between HVPG and the serological tests (correlation cohort). The patients with obstructive jaundice were excluded in this study. The 171 patients in the correlation cohort underwent preoperative measurements of HVPG, ICG-R15, CBC, and LFT simultaneously within 2 days before the scheduled liver surgery. A regression analysis was performed between the values of 171 patients’ HVPG and the serological tests to establish the equation of cHVPG. All procedures and tests were performed with the informed consent provided to the patients and their legal guardians. The study was conducted after approval of the institutional review board.
The reasons of liver surgery for 171 patients in the correlation cohort were described in Table 1. Among 171 patients, 73 (42.7%) had hepatitis B virus-related liver disease, 6 (3.5%) had alcoholic liver disease, 4 had hepatitis C virus-related liver disease, and 4 had cryptogenic liver cirrhosis. Eighty of 171 (46.8%) patients had biopsy-proven liver cirrhosis. The 171 patients underwent HR in 131, live donor liver transplantation (LDLT) in 38, and abdominal exploration in 2 due to peritoneal carcinomatosis. Among 131 patients who underwent HR, 101 (77.1%) underwent major HR (≥2 segment resection), and 30 (22.9%) received monosegmentectomy or wedge resection. Liver specimens of 169 patients who received liver surgery were analyzed histologically by a single pathologist (YB Kim). All 171 patients in the correlation cohort recovered from surgery and were discharged uneventfully.
TABLE 1.
Causes of Liver Surgery in the Correlation Cohort
| Diagnosis | N/171 (%) |
| Hepatocellular carcinoma | 57 (33.3) |
| Living liver donor | 38 (22.2) |
| End-stage liver cirrhosis | 29 (16.9) |
| Metastatic liver cancer | 17 (9.9) |
| Hepatolithiasis | 15 (8.8) |
| Cholangiocarcinoma | 9 (5.3) |
| Other* | 6 (3.5) |
*Other: 2 gallbladder cancer, 1 cystic adenoma, 1 hepatic adenoma, 1 benign inflammatory hepatic mass, and 1 chronic cholecystitis.
Preoperative Measurements of Hepatic Venous Pressure Gradient
After overnight fasting, the patients had been referred to the interventional radiologists (JW Kim and JH Won) who are exclusively responsible for the hepatic hemodynamic intervention. Under the local anesthesia, a 6-french venous introducer was inserted to the right internal jugular vein by the ultrasonography-guided Seldinger technique. A 5-french ballooning catheter with pressure sensor (C2 Cobra catheter; Torcon NB® Advantage catheter, Cook Medical Inc.) was advanced via the introducer into the right hepatic vein under fluoroscopic control. Free hepatic venous pressure (FHVP) was measured after the pressure was stabilized for 1 minute. Then the right hepatic vein was completely occluded by the catheter balloon to measure wedged hepatic venous pressure (WHVP). When the right hepatic vein was compressed or invaded by hepatic malignancy, the middle hepatic vein or the left hepatic vein was chosen for FHVP and WHVP. After 3 sets of measurements, alternating between FHVP and WHVP, the median value was recorded. HVPG was drawn by subtracting FHVP from WHVP.
Preoperative Measurement of Indocyanine Green-R15 and Laboratory Tests
A bolus of ICG was injected to the patients kept under overnight fasting with a dose of 0.5 mg/kg via the cephalic vein of 1 forearm. Fifteen minutes after the ICG injection, 8 mL of blood was sampled from the other forearm in a heparinized bottle. The injection of ICG and the blood sampling was exclusively conducted by a single technician (DH Kang). The concentration of ICG in the plasma was determined by the spectrophotometry at 805 nm (Libra S12 spectrophotometer, Bichrom Ltd.). The value of ICG-R15 was expressed as the percentage retention at 15 minutes.
Laboratory tests included CBC, serum electrolytes, serum bilirubin, serum albumin, aspartate transaminase, alanine transaminase, gamma-glutamyl transpeptidase, serum creatinine, and PT-INR. All blood samples for the laboratory tests were drawn after overnight fasting on the same day of ICG-R15 test.
Clinical Application of Calculated Hepatic Venous Pressure Gradient
Between January 2009 and December 2013, we applied the cHVPG by the K-equation for determination of PHT in the surgical patients with HCC (application cohort). In this period, the measurement of HVPG was not performed for preoperative evaluation of HCC, and the resectability of HCC was determined by the value of cHVPG by the K-equation, regardless of single value of serological test. The patient with cHVPG < 10 mm Hg was considered as having no PHT and regarded as a candidate of HR but cHVPG ≥ 10 mm Hg was considered as an evidence of PHT and unsuitable for HR. The patients with evident PHT with cHVPG ≥ 10 mm Hg were recommended to perform treatments other than HR such as liver transplantation, local ablation, or transcatheter arterial chemoembolization. To evaluate reliability of the cHVPG for HR, we divided the patients with cHVPG < 10 mm Hg (candidate of HR) into 2 groups by the value of ICG-R15 20%. Among the surgical patients with cHVPG < 10 mm Hg, the patients with ICG-R15 < 20% were regarded as group A and ICG-R15 ≥ 20% as group B. Operative outcomes and postoperative complications of group A and B patients were recorded. The short-term and long-term outcomes of HR in group A and B patients were analyzed and compared to determine the clinical feasibility of cHVPG in the assessment of PHT for surgical patients.
Statistical Analysis
Statistical analysis was performed using SPSS statistics 13.0. Data were expressed as mean or median values, ranges, and percentages. Univariate analysis was performed by the Student t test or χ2 test. In multivariate analysis, the value of HVPG was correlated to the serological tests using the linear regression analysis. The survival rates were analyzed by the Kaplan-Meier test. P values <0.05 were regarded as the valid significance in statistics. The value of R2, by the regression analysis, determined the reliability of the regression equation. The fitness of the regression model has been validated by the residual plots and analysis of variance (ANOVA) with F-statistics. Sensitivity and specificity for the potential diagnostic performance to predict PHT by cHVPG were assessed by receiver operating characteristic (ROC) curve.
RESULTS
In 171 patients of the correlation cohort, preoperative HVPG and the serological tests were measured without serious complications. However, 3 of 171 (1.8%) developed soft tissue hematoma related with central venous puncture of the right jugular vein. The cervical hematoma was managed conservatively and resolved without sequelae. The patients’ characteristics, measurement of HVPG, the serological tests, and liver histology of the correlation cohort were described in Table 2.
TABLE 2.
Patients’ Characteristics and Liver Function Tests of the Correlation Cohort
| Variables | n = 171 |
| Age (years) | 49 (16–84) |
| Male sex | 119 (69.6%) |
| Weight (kg) | 64.4 ± 9.9 |
| Height (cm) | 165.8 ± 7.8 |
| BMI (kg/m2) | 23.4 (17.1–33.1) |
| HVPG (mm Hg) | 6.72 ± 5.9 |
| ICG-R15% | 19.69 ± 13.8 |
| PT-INR | 1.24 ± 0.5 |
| Platelet count (×103) per μL | 207.1 ± 100 |
| Serum creatinine (mg/dL) | 0.85 ± 0.2 |
| BUN (mg/dL) | 24.61 ± 17.2 |
| Serum total bilirubin (mg/dL) | 1.83 ± 4.4 |
| Serum AST (U/L) | 43.90 ± 26.7 |
| Serum ALT (U/L) | 44.81 ± 36.1 |
| Serum γ-GT (U/L) | 81.3 ± 34.2 |
| Serum albumin (g/dL) | 3.84 ± 0.6 |
| Liver histology* | |
| Steatosis >10% | 32 (18.9%) |
| Inflammation 0, I, II/III, IV† | 159 (94.1%)/10 (5.9%) |
| Fibrosis 0, I, II, III/IV‡ | 89 (52.7%)/80 (47.3%) |
Data are number (%) or mean ± SD or median (range). ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; γ-GT, gamma-glutamyl transpeptidase; ICG-R15%, indocyanine green 15 minutes retention rate; PT-INR, prothrombin time-international normalized ratio.
*Liver histology was reviewed in 169 liver specimens.
†Inflammation grade: 0, normal; I, minimal; II, mild; III, moderate; and IV, severe.
‡Fibrosis stage: 0, normal; I, portal fibrosis; II, periportal fibrosis; III, septal fibrosis; and IV, cirrhosis.
Correlation of Hepatic Venous Pressure Gradient With Serological Test
The 171 patients in the correlation cohort showed mean 6.72 ± 5.9 mm Hg of HVPG (range from 0 to 31 mm Hg, 95% confidence interval of 0.8–12.6 mm Hg). Among them, 129 (75%) showed HVPG less than 10 mm Hg, and remaining 42 had HVPG ≥ 10 mm Hg. The 129 patients with HVPG < 10 mm Hg in the correlation cohort received right trisectionectomy in 2, right hemihepatectomy in 30, right anterior or posterior sectionectomy in 32, left hemihepatectomy in 29, left lateral sectionectomy in 8, monosegmentectomy in 10, wedge resection in 12, exploratory laparotomy only in 2, and LDLT in 4. The 42 patients with HVPG ≥ 10 mm Hg underwent LDLT (n = 34), and wedge resection (n = 8). The value of HVPG was significantly correlated with ICG-R15, platelet count, PT-INR, serum total bilirubin, and serum albumin in univariate analysis. Multivariate analysis revealed that the value of ICG-R15, platelet count, PT-INR, and serum albumin were independently correlated to HVPG; however, total bilirubin had no significant correlation (P = 0.652). The histological examination of the surgical specimens showed that only stage IV liver fibrosis was significantly correlated with HVPG ≥ 10 mm Hg (Table 3).
TABLE 3.
Correlation of Hepatic Venous Pressure Gradient With the Patients’ Characteristics and Serological Tests
| Variables | HVPG < 10 mm Hg (n = 129) | HVPG ≥ 10 mm Hg (n = 42) | Univariate Analysis, P | Multivariate Analysis, P |
| Age (years) | 49 (16–84) | 49 (36–69) | 0.281 | — |
| Male sex | 88 (68.75%) | 31 (72.1%) | 0.551 | — |
| Weight (kg) | 63.62 ± 9.2 | 66.98 ± 9.5 | 0.118 | — |
| Height (cm) | 165.66 ± 7.5 | 166.14 ± 8.7 | 0.767 | — |
| BMI (kg/m2) | 23.1 (17.1–33.1) | 24.1 (19.7–32.3) | 0.068 | — |
| ICG-R15% | 13.93 ± 13.9 | 39.11 ± 15.0 | <0.001 | <0.001 |
| Platelet count (×1000) | 241.18 ± 80.7 | 91.89 ± 72.4 | <0.001 | 0.003 |
| PT-INR | 1.06 ± 0.1 | 1.83 ± 0.7 | <0.001 | 0.014 |
| Serum creatinine (mg/dL) | 0.85 ± 0.1 | 0.83 ± 0.2 | 0.612 | — |
| BUN (mg/dL) | 24.61 ± 17.2 | 26.10 ± 18.0 | 0.701 | — |
| Serum total bilirubin (mg/dL) | 0.82 ± 0.3 | 5.24 ± 8.4 | 0.003 | 0.652 |
| Serum AST (U/L) | 43.43 ± 26.1 | 45.67 ± 28.9. | 0.367 | — |
| Serum ALT (U/L) | 45.52 ± 34.2 | 42.40 ± 41.8 | 0.277 | — |
| Serum γ-GT (U/L) | 81.30 ± 34.2 | 79.8 ± 39.8 | 0.432 | — |
| Serum albumin (g/dL) | 4.04 ± 0.4 | 3.12 ± 0.5 | <0.001 | 0.004 |
| Liver histology* | ||||
| Steatosis >10% | 24 (18.9%) | 8 (19%) | 0.797 | |
| Inflammation 0, I, II/III, IV† | 122 (96.1%)/5 (3.9%) | 37 (88.1%)/5 (11.9%) | 0.070 | |
| Fibrosis 0, I, II, III/IV‡ | 89 (70.1%)/38 (29.9%) | 0/42 (100%) | <0.001 |
Data are number (%) or mean ± SD or median (range). Significance was defined as P < 0.05.
*Liver histology was reviewed in 169 liver specimens.
†Inflammation grade: 0, normal; I, minimal; II, mild; III, moderate; and IV, severe.
‡Fibrosis stage: 0, normal; I, portal fibrosis; II, periportal fibrosis; III, septal fibrosis; and IV, cirrhosis.
ALT indicates alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; γ-GT, gamma-glutamyl transpeptidase; ICG-R15, indocyanine green 15 minutes retention rate; PT-INR, prothrombin time-international normalized ratio.
In the correlation cohort, ICG-R15 value was mean 19.7 ± 13.8 with ranged from 3.6% to 65.2%, serum albumin was mean 3.84 ± 0.62 (range from 2.3 to 5.2 g/dL), platelet count was mean 207,098 ± 100,235 per μL (range from 27,000 per to 575,000 per μL), and PT-INR was mean 1.24 ± 0.5 (range from 0.84 to 4.59). The individual correlation of HVPG with ICG-R15, serum albumin, platelet count, and PT-INR by a linear regression analysis was shown in Figure 1. The coefficient of determination (R2 value) was expressed for each correlation.
 |
FIGURE 1.

Correlation of HVPG with serological tests in the correlation cohort. Hepatic venous pressure gradient was significantly correlated with serological tests of ICG-R15, serum albumin, platelet count, and prothrombin time by multivariate analysis. Among them, the ICG-R15 had the most significant correlation with HVPG (R2 = 0.656). ICG-R15 (A) and prothrombin time-international normalized ratio (D) were positively correlated to HVPG (P < 0.05), and serum albumin level (B) and platelet count (C) are inversely correlated to HVPG (P < 0.05). A total of 95% prediction intervals are shown as dashed lines. Locally weighted scatter plot smooth for each serological test is shown as dotted line.
The equations showed that ICG-R15 and PT-INR were directly correlated to HVPG but platelet count and serum albumin level were inversely correlated. According to the R2 values of the equations, ICG-R15 had the best reliable correlation with HVPG among the serological tests (R2 = 0.656). According to the above equation of ICG-R15, 25.8% of ICG-R15 value corresponded to the value of 10 mm Hg in terms of HVPG. Additionally with the above equations, HVPG 10 mm Hg was equivalent to 3.3 g/dL of serum albumin 119,000 per μL of platelet count, and 1.67 of PT-INR. All 4 model assumptions were assessed by ANOVA test with F-statistics (P < 0.05).
By multivariate linear regression analysis, the cHVPG was established as following equation (K-equation).
 |
K-equation of multivariate analysis using significant correlation factors had coefficient of determination (0.707 of adjusted R2 value). The validity of K-equation has been assessed by the residual plots and ANOVA test (F = 98.278, P < 0.001). Cross-validation was also performed to estimate model (K-equation) performance. The 10-fold cross-validation performed allowing nonzero intercept term showed 0.661 of R2 value. The correlation plots between HVPG and cHVPG are shown in Figure 2. With the ROC curve for the correlation cohort, cHVPG values were plotted for their ability to predict the patients to be without PHT (HVPG < 10 mm Hg). The area under the curve of the ROC curve was 0.96 (95% confidence interval; 0.93–0.99, P < 0.001). At the point on the plot where a patient with cHVPG < 10 mm Hg, sensitivity was 98.4% and specificity was 76.2%, respectively, for identification of the patients without PHT. Also, the positive predictive value was 92.7% and the negative predictive value was 94.1% to predict the patients to be without PHT (Fig. 3).
FIGURE 2.
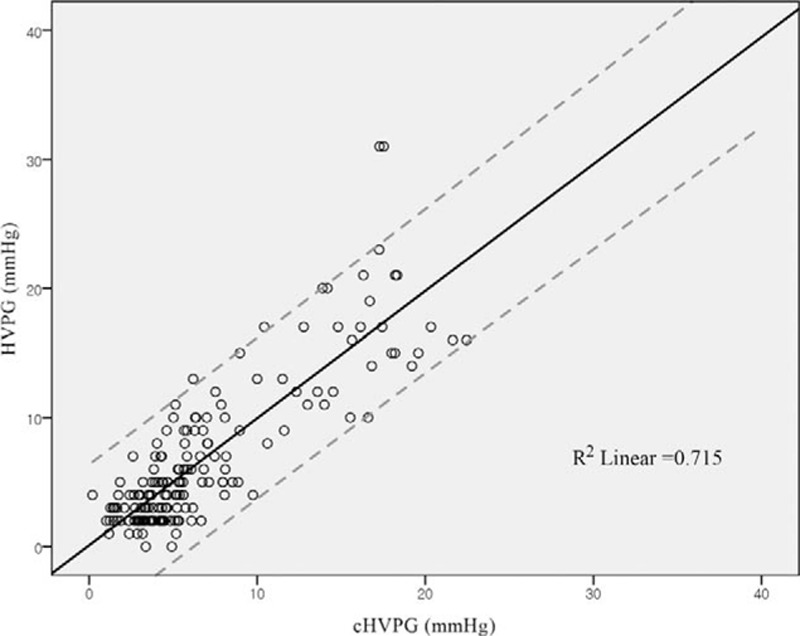
The scatterplots between hepatic venous pressure gradient and calculated hepatic venous pressure gradient. A total of 95% prediction intervals were shown as dashed lines.
FIGURE 3.

Receiver operating characteristics curve for calculated hepatic venous pressure gradient values in the correlation cohort to predict absence of portal hypertension (hepatic venous pressure gradient <10 mm Hg). A, The maximal value of Youden index. B, P < 0.001.
Clinical Application of Calculated Hepatic Venous Pressure Gradient by K-equation
Between January 2009 and December 2013, 510 consecutive HCC patients of the application cohort underwent the surgical evaluation in our institute. Among them, 17 patients were discovered that they had extra-hepatic metastasis of HCC during surgical evaluation, and they were converted to have palliative treatment. Remaining 493 surgical patients with HCC in the application cohort had further evaluation of resectability with the preoperative value of cHVPG. Among them, 452 patients had cHVPG < 10 mm Hg and were considered as candidates of HR, and finally 425 received HR and 27 received LDLT. These 27 LDLT patients were excluded from further analysis in the application cohort. Among the 425 patients who received HR, 357 patients had preoperative ICG-R15 < 20% (group A) and 68 had ICG-R15 ≥ 20% (group B). There was no patient with ICG-R15 < 20% who had cHVPG of more than 10 mm Hg. The remaining 41 patients, who had preoperative cHVPG ≥ 10 mm Hg, received nonresective treatments. The 41 patients with cHVPG ≥ 10 mm Hg showed 13.35 mm Hg of median cHVPG (ranged from 10.17 to 25.29 mm Hg) and 39.8% of median ICG-R15 (ranged from 24.7% to 64.4%). The treatment flow chart of 510 patients in the application cohort was shown in Figure 4.
FIGURE 4.

Treatment flow chart of the hepatocellular carcinoma patients in the application cohort by the cHVPG. The surgical patients with hepatocellular carcinoma in the application cohort were evaluated and selected for hepatic resection according to cHVPG. The hepatic resection was considered for the patients with cHVPG < 10 mm Hg. The hepatic resection was not recommended to the patient with cHVPG ≥ 10 mm Hg (n = 41). The hepatic resection was performed for the patients in group A and group B regardless of indocyanine green retention test.
The preoperative characteristics and operative outcomes of the 425 HR patients were analyzed and compared between group A (n = 357) and group B (n = 68) in Table 4. The mean value of cHVPG of group A patients was 4.76 ± 1.4 and that of group B was 7.80 ± 1.1. The patients’ characteristics showed no statistical difference between 2 groups. The values of serological tests were similar between 2 groups of patients except ICG-R15 (12.6 ± 4.1% in group A vs 24.6 ± 3.7% in group B, P < 0.001). The pathological characteristics of HCC and intraoperative outcomes of 2 groups were not statistically different (P > 0.05). The major HR (≥2 segment resection) was performed in 194 of group A (54%) and 32 of group B (47%), and the detailed type of HR was described and compared in Table 5. The posthepatectomy laboratory findings showed no significant difference between 2 groups in terms of serum bilirubin, PT-INR, serum creatinine, serum albumin, and platelet count. Also, the incidences of surgical complications after HR were similar between 2 groups (P > 0.05). There were 20 patients who drained more than 500 mL of ascites per day after posthepatectomy day 14. They were treated by dietary sodium restriction and diuretics. There was no patient who developed hepatic encephalopathy after HR in the application cohort. There was 1 case of in-hospital mortality among group B patients on posthepatectomy day 29 due to Acinetobacter pneumonia. She was 69 years old and had underlying chronic obstructive pulmonary disease. The length of hospital stay after HR was similar between 2 groups. The remaining 424 patients recovered from HR and were discharged with favorable liver function. There was no 3-month mortality among the 424 patients who were discharged from hospital uneventfully. However, 6 patients died within 6 months after HR due to recurrent HCC (n = 3), liver failure (n = 2), or variceal bleeding (n = 1). During median follow-up periods of 22 months after HR, 177 (41.6%) patients experienced HCC recurrence. However, there was no difference in recurrence of HCC, and 5-year survival rates were similar between 2 groups (P > 0.05).
TABLE 4.
Comparison of Perioperative Outcomes Between Group A (Indocyanine Green 15 Minute Retention Rate <20%) and Group B (Indocyanine Green 15 Minute Retention Rate >20%) in the Validation Cohort
| Outcomes | Group A (n = 357) | Group B (n = 68) | P |
| Preoperative outcomes | |||
| Age (years) | 53 (26–80) | 55 (29–77) | 0.820 |
| Male sex | 281 (78.7%) | 55 (80.8%) | 0.382 |
| Weight (kg) | 62.2 ± 10.2 | 64.8 ± 9.5 | 0.306 |
| Height (cm) | 167.54 ± 6.2 | 168.44 ± 7.3 | 0.901 |
| BMI (kg/m2) | 23.8 (16.5–31.3) | 24.1 (17.5–33.4) | 0.290 |
| ICG-R15 (%) | 12.9 (1.2–19.8) | 23.65 (20.1–37.7) | <0.001 |
| Platelet count (×1000) | 163.58 ± 65.1 | 140.82 ± 56.0 | 0.245 |
| PT-INR | 1.05 ± 0.1 | 1.07 ± 0.1 | 0.360 |
| Serum creatinine (mg/dL) | 1.08 ± 0.9 | 1.19 ± 1.3 | 0.537 |
| Serum total bilirubin (mg/dL) | 0.78 ± 0.4 | 0.87 ± 0.4 | 0.255 |
| Serum AST (U/L) | 43.85 ± 30.7 | 50.9 ± 37.7 | 0.427 |
| Serum ALT (U/L) | 40.76 ± 31.7 | 51.5 ± 57.2 | 0.188 |
| Serum albumin (g/dL) | 4.18 ± 0.3 | 4.03 ± 0.3 | 0.563 |
| Operative outcomes | |||
| Tumor size (cm) | 3.5 (0.4–23.0) | 3.4 (1.0–19.0) | 0.557 |
| Tumor number | 1 (1–8) | 1 (1–6) | 0.454 |
| Operation time (minutes) | 173.51 ± 81.9 | 165.04 ± 75.2 | 0.844 |
| Intraoperative blood loss (mL) | 428 ± 105 | 397 ± 153 | 0.341 |
| Major/minor resection* | 194/163 | 32/36 | 0.088 |
| Resection margin <1 cm | 143 (40%) | 26 (38.2%) | 0.927 |
| TNM stage I/II/III† | 242 (67.8%)/74 (20.7%)/30 (11%) | 46 (67.6%)/13 (19.1%)/8 (11.8%) | 0.754 |
| Postoperative outcomes | |||
| Peak creatinine (mg/dL) | 1.15 ± 1.2 | 1.09 ± 0.3 | 0.712 |
| Peak total bilirubin (mg/dL) | 2.26 ± 1.4 | 2.20 ± 0.9 | 0.764 |
| Peak PT-INR | 1.45 ± 0.3 | 1.44 ± 0.3 | 0.848 |
| Lowest albumin (g/dL) | 3.05 ± 0.4 | 2.95 ± 0.3 | 0.178 |
| Lowest platelet count (×1000) | 103.50 ± 42.8 | 101.46 ± 44.6 | 0.749 |
| Complications | |||
| Postoperative hemorrhage‡ | 9 (2.5%) | 1 (1.5%) | 0.580 |
| Lung complication§ | 40 (11.2%) | 8 (11.8%) | 0.959 |
| Prolonged ascites | 18 (4.4%) | 2 (2.9%) | 0.428 |
| Biliary complication** | 11 (3.1%) | 3 (4.4%) | 0.607 |
| Encephalopathy | 0 | 0 | 0.893 |
| Need of dialysis | 0 | 1 (1.5%) | 0.321 |
| In-hospital mortality | 0 | 1 (1.5%) | 0.321 |
| 3-month mortality | 0 | 0 | 0.893 |
| 6-month mortality | 5 (1.4%) | 1 (1.5%) | 0.672 |
| Length of hospital stay (day) | 12 (6–83) | 13 (8–30) | 0.190 |
| Tumor recurrence | 146 (40.9%) | 31 (45.6%) | 0.361 |
| 5-year survival rates (%) | 76.70% | 72.20% | 0.372 |
| Follow-up periods (months) | 22 (4–60) | 23.5 (1–60) | 0.655 |
Data are number (%) or mean ± SD or median (range). Significance was defined as P < 0.05.
*Major resection means resection of two or more segments.
**Biliary complication means bile leakage or bile duct stricture.
†TNM stage from AJCC 7th edition.
‡Postoperative hemorrhage includes wound or intraperitoneal hemorrhage.
§Lung complication includes pleural effusion or pneumonia.
ALT, alanine transaminase; AST, aspartate transaminase; ICG-R15, indocyanine green 15 minutes retention rate; PT-INR, prothrombin time-international normalized ratio.
TABLE 5.
Comparison in Type of Surgery Between Group A and Group B
| Type of Surgery | Group A (n = 357) | Group B (n = 68) | P |
| Major hepatectomy (n = 226) | 194 (54.3%) | 32 (47.1%) | 0.474 |
| Right trisectionectomy | 7 (2.0%) | 2 (2.9%) | |
| Right hemihepatectomy | 70 (19.6%) | 12 (17.7%) | |
| Central bisectionectomy | 17 (4.8%) | 3 (4.4%) | |
| Left hemihepatectomy | 31 (8.7%) | 5 (7.4%) | |
| Right anterior sectionectomy | 23 (6.4%) | 2 (2.9%) | |
| Right posterior sectionectomy | 19 (5.3%) | 3 (4.4%) | |
| Left lateral sectionectomy | 27 (7.6%) | 5 (7.4%) | |
| Minor hepatectomy (n = 199) | 163 (45.7%) | 36 (53.0%) | 0.398 |
| Monosegmentectomy | 111 (31.1%) | 26 (38.3%) | |
| Wedge resection | 52 (14.6%) | 10 (14.7%) |
DISCUSSION
HR is one of the major curative modality of treatments for HCC. An extensive resection of liver parenchyme up to 70% of total liver volume could be performed safely in the patients with normal background liver histology.20,21 However, HCC is usually developed from liver with chronic disease and cirrhosis. Thus, HR could be applied to the limited number of HCC patients with compensated or well-preserved liver cirrhosis defined by HVPG less than 10 mm Hg.1,2,22 HVPG was reported as the most reliable methods to assess PHT in many study.1,2,22–25 However, the measurement of HVPG has limitations of invasiveness, high cost, and need for skilled radiologist and high-technology facilities. Various serological tests, such as ICG-R15, LFT, and platelet count are widely used as surrogate methods to assess PHT despite of lower reliability than HVPG. Until now, there was no study which reported the relationship between HVPG and the serological tests for portal pressure assessment.
The present study is the first report of statistical analysis for the quantitative correlation between HVPG and the serological tests for assessment of PHT. In this study, the quantitative correlation between HVPG and the serological tests were derived by univariate and multivariate linear regression analysis of the correlation cohort of 171 surgical patients. The 171 surgical patients for the correlation analysis between HVPG and the serological tests had a spectrum of liver function and histology from live donor's liver to end-stage liver disease.
Authors of this study regarded that the wide distribution of portal pressure values due to various status of liver histology in the correlation cohort was suitable for making a good correlation equation to define clinically relevant PHT. The adjusted R2 value of the K-equation was 0.707, which was considered to be favorable reliability for the model, and the coefficient of the level-level regression between cHVPG and HVPG was close to 1 (0.984) as shown in Figure 2.
If the patients in the correlation cohort had normal distribution with mean value of 10 mm Hg of HVPG, the correlation cohort could have been statistically ideal, and the correlation between HVPG and serological tests could have been the most helpful for the patients to determine PHT, because 10 mm Hg of HVPG is well-known cut-off value for PHT. However, 171 consecutive patients of the correlation cohort in this study showed the mean HVPG value of 6.7 ± 5.9 mm Hg, and a relatively small portion (25%, n = 42) of patients had HVPG ≥ 10 mm Hg. The reason of this uneven distribution would be that the cohort was composed of surgical candidates, and the number of the patients was not modified intentionally to fit the mean value of HVPG to 10 mm Hg. However, the constructed ROC curve for the correlation cohort in this study showed that the absence or presence of PHT could be predicted by cut-off value, 10 mm Hg of cHVPG, with 92.7% of positive predictive value and 94.1% of negative predictive value (Fig. 3). It could be understood as the predictability of a patient with cHVPG less than 10 mm Hg to have an actual value of HVPG less than 10 mm Hg was 92.7%, which provided a safe selection criterion for HR in this study. In the ROC curve, 7.495 mm Hg of cHVPG was the maximal value of Youden index but an evaluation metrics of kappa statistics for agreement showed that the 10 mm Hg of cHVPG outperformed 7.495 mm Hg of cHVPG to predict PHT.26,27 Therefore, we considered that uneven distribution of the correlation cohort had little hindrance to reliability of cHVPG in predicting PHT.
In the correlation cohort of this study, the assessments of portal pressure were performed within 2 days before the elective surgery, which may strengthen the reliability of the tests by providing homogeneous status of hydration and nutritional support during hospitalization. This could calibrate patients’ factors to measure reliable values of HVPG and the serological tests for portal pressure. Also, we excluded the patients with obstructive jaundice or acute cholestasis such as hilar cholangiocarcinoma from the correlation cohort because those conditions could influence the value of indocyanine test to be abnormally high.28,29 In this study, the measurement of HVPG and ICG-R15 were performed by exclusively responsible experts in procedures. However, 3 patients (1.8%) developed cervical hematoma as a complication of HVPG measurement, managed conservatively with compression dressing. According to our experience, we also acknowledged the invasiveness of HVPG measurement and that it should be performed by skilled hands.
Many previous studies reported that the value of HVPG 10 mm Hg could be a cut-off value of clinical relevant PHT in chronic liver disease, related to decompensated symptoms of liver cirrhosis.4,13 The patients with decompensated liver cirrhosis may also present abnormal values of the serological tests, such as thrombocytopenia, hyperbilirubinemia, and hypoalbuminemia. Multivariate regression analysis of this study showed that the value of HVPG was significantly correlated with ICG-R15, serum albumin, PT-INR, and platelet count. The serum level of total bilirubin had a correlation with HVPG in the univariate analysis (P = 0.003) but had no significance in the multivariate regression (P = 0.65). However, it was difficult to interpret the reason that the serum bilirubin was significant in the univariate analysis only. Bilirubin alone is neither sensitive nor specific for intrinsic liver disease but serves as an indirect measure of the ability of the liver to take up and conjugate bilirubin and to secrete it eventually.30 This study developed the individual correlation equations between the value of HVPG and the 4 significant serological tests: ICG-R15, serum albumin, PT-INR, and platelet count. The reliability of each equation was determined by R2 value and ANOVA test with F-statistics, which would give information about the goodness of fit and statistical significance of our model. Authors of this study have assumed that linear relationship existed between HVPG and each test based on simplicity because those 4 serological tests have been well-known conventional parameters for predicting PHT. Also, there was no previous report, which suggested true model of their relationship. However, in this study, there seems to be nonlinear relationship between HVPG and some of serological tests, revealed by the local smoothers, especially in Figure 1C and 1D. We acknowledge that the weak linearity could be a potential limitation of this study.
ICG is a protein-binding anionic organic dye that is selectively taken up by hepatocytes and excreted unchanged via the bile. The removal of ICG reflects the capabilities of the liver to uptake and excrete, which can be extrapolated to reflect hepatocyte blood flow and functional hepatocytes mass. ICG elimination is, by far, the most widely used and published functional assessment of liver reserve worldwide and has also been useful in predicting short-term prognosis in liver transplant patients.30 Conventionally, ICG-R15 value of 20% was regarded as the cut-off value for clinically relevant PHT, and the value was suggested by clinical experience or recommendation of many centers. ICG-R15 value of 20% was supposed to be equivalent to HVPG of 10 mm Hg.9,31 However, the HVPG of 10 mm Hg was quantitatively equivalent to ICG-R15 value of 25.8% by the correlation equation of this study. The difference of cut-off value of ICG-R15 for clinical PHT between the quantitative value of this study and conventional value could have resulted in overestimation of clinical PHT to the patients with ICG-R15 values between 20% and 25.8%. Those patients with HCC could be deprived of the chance to have HR due to overestimated risk of postoperative complication related to liver failure, if the treatment modality for HCC were selected by the result of ICG-R15 test. The quantification of ICG-R15 for PHT in this study may help liver surgeons to assess liver function of surgical patients more precisely and decide the treatment modality more accurately. The serum albumin level, PT-INR, and platelet count were also significantly correlated to HVPG by multivariate analysis. Serum albumin and PT-INR represent synthetic function of the liver, and these have been used in Child-Pugh scoring system for stratification of chronic liver disease. Both serological parameters were produced exclusively by the liver. The serum albumin value of 3.3 g/dL and PT-INR value of 1.67 correspond to HVPG of 10 mm Hg by the individual equations in this study. The values of serum albumin and PT-INR for PHT were similar to the value of decompensated chronic liver disease in Child-Pugh scoring system. Platelet count of 119,000 per μL was correlated to 10 mm Hg of HVPG by the equation, and the value was similar to the results of previous studies.3,32 The median body mass index (BMI) of the correlation cohort of this study was 23.4 kg/m2, which might be lesser than those of the Western population. However, previous studies from the Western countries reported that the value of ICG-R15 or HVPG had not been significantly affected by BMI.4,33,34 Also, the result of this study was consistent with those of the previous reports. Thus, we consider that the equation of our study could be translated to population with a higher BMI.
We assessed the clinical feasibility of K-equation on a following prospective cohort of 510 surgical patients with HCC. Among the patients in the application cohort of this study, no one with favorable ICG-R15 < 20% showed cHVPG ≥ 10 mm Hg according to K-equation. This result could support the reliability of the correlation equation between HVPG and ICG-R15 of the correlation cohort, which suggested that 10 mm Hg of HVPG was equivalent to 25.8% of ICG-R15. Forty-one patients in the Application cohort who showed the unfavorable value of cHVPG ≥10 mm Hg were recommended to undergo non-HR treatment according to the treatment guideline of the previous study.3,6 Our decision to provide nonresective treatments for those patients with cHVPG ≥10 mm Hg was appropriately supported by their high values of ICG-R15 (median 39.8%, ranged from 24.7% to 64.4%). However, one of the limitations of our study is that clinical validation of K-equation was impossible in the strict sense because data for outcomes of HR for patients with high values of cHVPG (≥10 mm Hg) could not be collected.
In the application cohort of this study, HR was recommended and performed in patients with cHVPG < 10 mm Hg. There were 27 patients who underwent LDLT for HCC even with cHVPG < 10 mm Hg. In our institute, LDLT could be performed for HCC patients without vascular invasion or metastasis on the familial support of a live donor. The remaining patients with cHVPG < 10 mm Hg who underwent HR could be divided into 2 groups according to ICG-R15 value, either less than 20% (group A, n = 357) or more than or equal to 20% (group B, n = 68). Unless we applied K-equation for cHVPG, the patients of group B might have not been recommended for HR because of unfavorable ICG-R15. The Japanese surgical guideline for HCC recommended that the major HR including 2 or more segments should be performed in patients with ICG-R15 less than 20%, and the policy has been accepted widely in many center.9,35 However, in the application cohort in this study, 32 patients (47%) in group B received major HR successfully, and 22 of them underwent more extensive HR than hemihepatectomy on the basis of favorable results of cHVPG (<10 mm Hg). In this study, there was no significant difference in intraoperative outcomes, types of HR, operative complications, and survival rates after HR between group A and B. The successful surgical outcomes of group B patients in the application cohort of this study could imply that the cHVPG by K-equation should be a clinically reliable model to determine whether a surgical patient has preserved liver function or not. This study has drawn that the cHVPG had higher clinical reliability and accuracy than separate use of the serologic tests for assessment of liver reserve function of the surgical patients. Therefore, authors of this study suggested that K-equation for cHVPG was well established and its clinical feasibility was assessed by the correlation and the application cohorts in this study. The clinical application of K-equation for cHVPG could be useful to determine the evidence of PHT for the surgical patients with HCC. We expect that it is one of the strong merits that the cHVPG can be drawn without invasive procedures.
Assessment of the liver reserve function for the liver surgery using ICG-R15 has been widely performed in the most Eastern centers and some European centers, whereas ICG-R15 test might not be available in many centers in the United States and the West. This could be one of the limitations for wide application of the equation for cHVPG because the ICG-R15 is one of the significant variables in the equation. Besides, the value of ICG-R15 might be affected by some conditions such as jaundice and some genetic disorders.36–38 Uptake of ICG by hepatocytes is regulated by ATP-independent organic anion-transporting polypeptide (OATP) located at the basolateral membrane of the liver. Bilirubin competes with ICG for hepatocyte uptake via OATP. Thus, the HCC patients with jaundice due to common hepatic duct invasion might have abnormally high value of ICG-R15. Also, the patients with genetic defect in OATP like Rotor syndrome and constitutional ICG excretory defect should have unreliable value of ICG-R15. These conditions would be another limitation in application of the equation for the cHVPG, and direct measurement of the HVPG through interventional radiology could be selected to determine PHT for these patients.
In conclusion, the HVPG could be predicted with serological tests by the K-equation. The cHVPG by K-equation could provide a more feasible clinical reference to determine evidence of PHT than value of each serological test for the surgical patients with HCC. The authors of this study suggest that clinical application of cHVPG could help liver surgeons to assess portal pressure quantitatively and select HR appropriately for the surgical candidates with HCC.
Acknowledgments
The authors thank Da-Hye Kang for her excellent technical assistance in measurement of ICG-R15.
Footnotes
Authorship contribution: BWK and HJW designed the study. BWK, HJW, HYL, JHW, JK, XGH, JS, JBB, and YBK discussed and conducted the study and reviewed the manuscript. TK, BWK, HYL, and YBK collected and analyzed the data and wrote the manuscript.
The authors declare no conflicts of interest.
REFERENCES
- 1.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005; 353:2254–2261. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133:481–488. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 4.Boleslawski E, Petrovai G, Truant S, et al. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg 2012; 99:855–863. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Fuster J, Bruix J, et al. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 2004; 10:115–120. [DOI] [PubMed] [Google Scholar]
- 7.Huet PM, Pomier-Layrargues G. The hepatic venous pressure gradient: “remixed and revisited”. Hepatology 2004; 39:295–298. [DOI] [PubMed] [Google Scholar]
- 8.Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009; 6:573–582. [DOI] [PubMed] [Google Scholar]
- 9.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993; 9:298–304. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka N, Okamoto E, Kuwata K, et al. A multiple regression equation for prediction of posthepatectomy liver failure. Ann Surg 1984; 200:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg 1995; 169:589–594. [DOI] [PubMed] [Google Scholar]
- 12.Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer 1994; 74:2772–2780. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440. [DOI] [PubMed] [Google Scholar]
- 14.Dunk AA, Jenkins WJ, Burroughs AK, et al. The effect of ranitidine on the plasma clearance and hepatic extraction of indocyanine green in patients with chronic liver disease. Br J Clin Pharmacol 1983; 16:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laperche Y, Oudea MC, Lostanlen D. Toxic effects of indocyanine green on rat liver mitochondria. Toxicol Appl Pharmacol 1977; 41:377–387. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg 2005; 12:31–37. [DOI] [PubMed] [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 18.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964; 1:1–85. [PubMed] [Google Scholar]
- 19.Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg 2002; 236:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim S, Chen CL, Wang CC, et al. Small remnant liver volume after right lobe living donor hepatectomy. Surgery 2006; 140:749–755. [DOI] [PubMed] [Google Scholar]
- 21.Yigitler C, Farges O, Kianmanesh R, et al. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl 2003; 9:18–25. [DOI] [PubMed] [Google Scholar]
- 22.Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990; 99:1401–1407. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Bosch J, Blei A, et al. Portal hypertension and its complications. Gastroenterology 2008; 134:1715–1728. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44:217–231. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 2010; 51:1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 27.Carletta J. Assessing agreement on classification tasks: the kappa statistics. Comput Ling 1996; 22:249–254. [Google Scholar]
- 28.Stockmann M, Malinowski M, Lock JF, et al. Factors influencing the indocyanine green (ICG) test: additional impact of acute cholestasis. Hepatogastroenterology 2009; 56:734–738. [PubMed] [Google Scholar]
- 29.Suda K, Ohtsuka M, Ambiru S, et al. Risk factors of liver dysfunction after extended hepatic resection in biliary tract malignancies. Am J Surg 2009; 197:752–758. [DOI] [PubMed] [Google Scholar]
- 30.Cha C. Jarnagin WR, Belghiti J, Blumgart LH. Assessment of hepatic function: implications for the surgical patient. Blumgart's Surgery of the Liver, Biliary Tract, and Pancreas. Philadelphia, PA: Elsevier Saunders; 2012. 58–64. [Google Scholar]
- 31.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004; 240:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson PR, Hebbard GS, Gibson RN, et al. Percutaneous transhepatic measurement of the pressure gradient between the portal and hepatic veins. Aust N Z J Med 1993; 23:374–380. [DOI] [PubMed] [Google Scholar]
- 33.Eisenbrey JR, Dave JK, Halldorsdottir VG, et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology 2013; 268:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisotti A, Azzaroli F, Buonfiglioli F, et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology 2014; 59:643–650. [DOI] [PubMed] [Google Scholar]
- 35.Tsim NC, Frampton AE, Habib NA, et al. Surgical treatment for liver cancer. World J Gastroenterol 2010; 16:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chijiiwa K, Watanabe M, Nakano K, et al. Biliary indocyanine green excretion as a predictor of hepatic adenosine triphosphate levels in patients with obstructive jaundice. Am J Surg 2000; 179:161–166. [DOI] [PubMed] [Google Scholar]
- 37.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med 1998; 339:1217–1227. [DOI] [PubMed] [Google Scholar]
- 38.Nambu M, Namihisa T. Hepatic transport of serum bilirubin, bromsulfophthalein, and indocyanine green in patients with congenital non-hemolytic hyperbilirubinemia and patients with constitutional indocyanine green excretory defect. J Gastroenterol 1996; 31:228–236. [DOI] [PubMed] [Google Scholar]


