Abstract
Skeletal muscle regeneration in normal and diseased muscle is regulated by multiple factors and cells present in the injured muscle micro-environment. In addition to muscle progenitor cells, several immunocytes participate in the regenerative response. Among them, macrophages are one of the most important components of the immune response that governs the step-wise progression of muscle regeneration. The initial role of macrophages is to phagocytose muscle cell debris and later, through their transition to an anti-inflammatory phenotype, they promote regeneration. However, in several genetic muscle disorders, continuous muscle injury disrupts the balance between pro-inflammatory and anti-inflammatory macrophages, leading to an overall inflammatory milieu and inhibition of muscle regeneration. Accumulating evidence suggests that Toll-like receptor (TLR)-mediated signalling plays an important role in the regulation of macrophage phenotypes during regenerative myogenesis in response to both acute and chronic muscle injury. Here, we discuss the role of TLR signalling in regulating macrophage phenotypes and skeletal muscle regeneration in healthy and diseased muscle.
Keywords: Skeletal muscle regeneration, Toll-like receptors, macrophages, muscular dystrophy
Introduction
Skeletal muscle is a postmitotic tissue that regenerates through the activation of satellite cells [1]. Although the intrinsic regulatory mechanisms that govern adult satellite cell proliferation and subsequent differentiation mimic those that occur during embryonic myogenesis, the extracellular events that ensue regenerative myogenesis vary dramatically. While immune cells are relatively scant in developing skeletal muscle, they abundantly invade the muscle micro-environment in response to injury [2]. Following acute `single-hit' injury, muscle degeneration begins with the necrosis of damaged muscle fibres, which results in discontinuity of the muscle sarcolemma, making it permeable. This disruption in myofibre integrity allows for the release of multiple normally muscle-compartmentalized factors into the circulating plasma [2, 3]. Toll-like receptors (TLRs) comprise a conserved family of receptors that play a major role in innate immunity. Their transmembrane structure allows for the recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), such as high mobility group box protein 1 (HMGB1), heat shock proteins, hyaluronic acid, and biglycans that are released from injured cells and tissues [4, 5]. TLR signalling cascades are mediated by four adapter proteins: myeloid differentiation factor 88 (MyD88); TIR domain-containing adapter, inducing interferon (TRIF); TRIF-related adapter molecule (TRAM); and TIR domain-containing adapter protein (TIRAP), all of which lead to downstream activation of transcription factors, including nuclear factor-κB, activator protein-1 and interferon regulatory factors (IRFs). In turn, these transcription factors induce the production of pro-inflammatory cytokines and chemokines that play a major role in the initiation and perpetuation of the inflammatory response [5]. Muscle-derived chemokines (myokines) facilitate the recruitment of immune cells into the injured muscle micro-environment [1, 2]. Neutrophils are the first to respond, within 1 h of muscle injury, peaking at 24 h. Following the onset of neutrophil invasion, phagocytic macrophages gradually invade the injured muscle, reaching peak levels at day 2 following injury. Monocytes expressing high Ly6C and chemokine receptor 2 (CCR2) exit the bone marrow and traffic to muscle injury sites, under the regulation of the monocyte chemoattractant protein (MCP) family of CC chemokines [2]. In injured muscle, monocytes differentiate into macrophages with different functional characteristics; iNOS+CD206− phagocytic M1 macrophages, which coincide with the activation and proliferation of satellite cells during early regeneration. Their invasion precedes the elevation of a population of non-phagocytic iNOS−CD206+ M2 macrophages, which peaks around day 4 post-injury, parallelling the stage of myoblast differentiation and muscle repair (Figure 1). Although not exclusively, a similar pattern of recruitment of macrophages may take place in chronic muscle injury common in various genetic muscle disorders [2, 3]. In mdx mice (a mouse model of Duchenne muscular dystrophy, DMD), pro-inflammatory M1 macrophages dominate the dystrophic muscle micro-environment at age 4–6 weeks, which corresponds to the acute inflammatory phase of muscle degeneration. Afterwards, macrophages are polarized to an anti-inflammatory M2c phenotype, which correlates with a phase of intense muscle regeneration (8–12 weeks) and a subsequent anti-inflammatory/pro-fibrotic M2a phenotype corresponding to a phase of halted muscle regeneration and continuous collagen accumulation [2, 3]. Accordingly, targeting the components of the immune system has been the subject of immense research for the development of therapies for various muscle diseases.
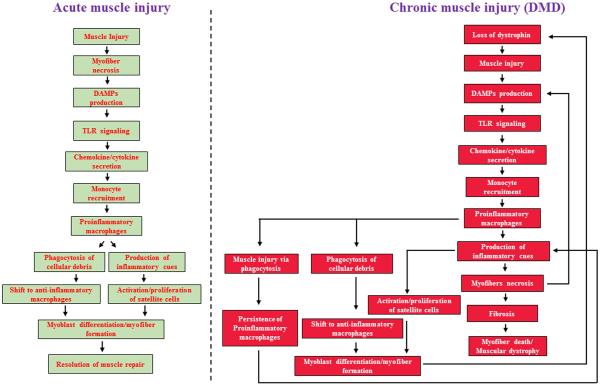
FIGURE 1. Schematic representation of potential role of TLRs and macrophages in the regulation of muscle repair in response to acute versus chronic injury.
(Left) Conventional activation of the inflammatory profile and subsequent muscle repair that follows single-hit injury in an orderly manner. Following acute injury, damaged muscle tissue secretes damage-associated molecular patterns (DMAPs) that activate toll-like receptor (TLR) signalling, which in turn induces the release of muscle cytokines and chemokines into the circulating plasma. Secreted cytokines and chemokines mediate the recruitment of monocytes from the bone marrow to the injury site. Upon entry into the muscle environment, monocytes differentiate into phagocytic pro-inflammatory M1 macrophages that are responsible for clearing of cellular debris and necrotic tissue. M1 macrophages also produce inflammatory cues that stimulate satellite cell activation and proliferation, after which they polarize to an anti-inflammatory M2 phenotype and promote myoblast differentiation and muscle formation, concluding the repair process. (Right) An atypical injury/regeneration programme characterized by a superimposed inflammatory response and recurring injury that perturbs the resolution of muscle repair in dystrophic muscle
TLR signalling in skeletal muscle regeneration
Macrophages constitute the predominant inflammatory cell type in most forms of skeletal muscle injury and they release multiple cytokines, chemokines and extracellular proteases that influence satellite cell behaviour, extracellular matrix remodelling and various aspects of skeletal muscle repair [2, 6]. In acutely damaged muscle, depletion of macrophages or interference with macrophage recruitment has been found to have detrimental consequences on muscle regeneration. A reduction in the number of macrophages delays the repair process in muscle following acute injury caused by freezing damage [7]. Furthermore, knockout (KO) of chemokine receptor 2 (CCR2) or its ligand, chemokine ligand 2 (CCL2), which is a macrophage chemoattractant, impedes skeletal muscle regeneration following cardiotoxin injection or ischaemia [8]. As proof of concept, transplantation of bone-marrow from wild-type mice rescued the defects in macrophage invasion and muscle regeneration in Ccr2-null mice [8, 9]. More recently, in work published in a recent issue of the Journal of Pathology, the role of TLR2 signalling has been investigated in muscle regeneration in response to traumatic injury. Deficiency of TLR2 reduced the number of macrophages in the injured muscle; however, it did not alter the expression pattern of macrophage polarization markers, such as iNOS and CD206. Consequently, regenerating muscle of TLR2-KO mice exhibited delayed clearance of necrotic tissue and impaired restoration of normal muscle architect [10]. In a related study, injection of Bothrops jararacussu snake venom in GA muscles of C3H/HeJ mice that expressed a non-functional TLR4 receptor resulted in persistent muscular inflammation, with lower numbers of F4/80+ macrophages but increased activity of matrix metalloproteinase-9 and collagen deposition during resolution of injury, compared to C3H/HeN mice, which expressed functional TLR4 receptor [11], leading to the conjecture that both TLR2 and TLR4 signalling are essential for skeletal muscle repair following acute injury.
TLR signalling and pathogenesis of muscular dystrophy
Duchenne muscular dystrophy (DMD) is a lethal muscle disorder caused by loss of functional dystrophin protein [6]. Chronic inflammation and fibrosis are hallmarks of muscle pathology in DMD. While eliminating macrophage signalling was deleterious for muscle regeneration in settings of acute injury, similar interventions have been shown to significantly improve muscle fibre pathology and strength in the models of DMD [6]. Loss of function of CCR2 in mdx mice reduced the invasion of CD11bhigh macrophages by impeding the recruitment of Ly6Chigh monocytes from the bone marrow. Moreover, lack of CCR2 helped to re-establish a more conventional macrophage polarization programme by preventing abnormal skewing of macrophages towards a pro-inflammatory phenotype, resulting in a proregenerative environment and improvement in diaphragm structure and function in mdx mice [12]. Similar results were obtained when CCR2 signalling was repressed by mutated CCL2 secreted from implanted mesenchymal stem cells [12]. In the recent study published in this journal, ablation of TLR2 in chronically diseased muscle of mdx mice likewise resulted in significantly reduced macrophage number and a prototypical shift that favored an anti-inflammatory (iNOS−CD206+) phenotype, and subsequent amelioration of muscle pathology [10]. A similar phenotype was also observed in mdx mice lacking TLR4 or one of its ligands, HGMB1. Decreased macrophage-mediated inflammation was also associated with reduced myopathy and increased force-generating capacity of dystrophic muscle [4]. Furthermore, deletion of adaptor protein Myd88 significantly improved skeletal and cardiac muscle function in 1 year-old mdx mice. This improvement was shown to be TLR-dependent, as treatment of young mdx mice with a TLR7/9 antagonist significantly reduced skeletal muscle inflammation and increased muscle strength [5]. Inactivation of Myd88 also improved myopathy in dysferlin-deficient A/J mice [13]. These findings, amongst many others, provided initial evidence of the extent of contribution of the micro-environment to disease progression and the promising beneficial effects of modifying the inflammatory milieu in dystrophic muscle.
Perspective and Future Direction
The studies summarized above emphasize the distinct roles of the inflammatory repertoire in the setting of acute versus chronic muscle injury. Following a single-hit injury, precise temporal and spatial activation of M1 and M2c macrophages, regulated by TLRs and various cytokines/chemokines, demarcate the stages of muscle regeneration. Therefore, it is conceivable that disruption in the sequential events of macrophage recruitment and function will result in diminished muscle regeneration in response to acute injury [7–11]. Pro-inflammatory macrophages persist for longer duration in dystrophic muscle, due to chronic cycles of muscle degeneration and regeneration, which further contributes to the dystrophic phenotype [3, 6]. Manipulation of upstream or downstream TLR signalling mitigated the extent of muscle damage in dystrophic muscle. This was a consequence of overall reduction in macrophage number and potential polarization towards M2 phenotype, favoring an anti-inflammatory/pro-regeneration phenotype [4, 5, 10, 12, 13]. The divergent outcomes of ablation of TLRs in acute versus chronic injury could also be attributed to a potential role of dystrophin as a signalling module. Disruption of the dystrophin–glycoprotein complex (DGC) leads to chronically increased activation of several inflammatory signalling pathways, such as NF-κB and AP1, which contribute to the pathogenesis of muscular dystrophy [6]. Aberrant regulation of such pathways does not occur in the settings of acute muscle injury in wild-type mice. Future studies will determine whether dystrophin-sufficient muscle subjected to recurring trauma will display similar phenotypic outcomes in response to TLR signalling deficiency, as observed in mdx mice. TLRs are also expressed by many other cell types present in the injured muscle micro-environment, including myoblasts, neutrophils and fibroblasts and fibro/adipocyte progenitors. Loss of TLR signalling in these cell types can also influence the skeletal muscle regenerative response. While published reports suggest that modulation of TLR signalling can improve muscle regeneration, it is noteworthy that most of these studies were performed in young or adult mice. Pro-inflammatory signalling is also critical for regulating satellite cell proliferation; therefore, it remains to be determined whether continued suppression of inflammatory cues, such as TLRs, can also have detrimental consequences on endogenous satellite cell function in dystrophic muscle. Moreover, anti-inflammatory M2 macrophages are also a major source of profibrotic cytokines and therefore their prolonged activation can lead to accumulation of fibrotic tissues in dystrophic muscle. Acquiring a holistic understanding of the specificity of various molecules has central implications upon developing target-based therapies for skeletal muscle disorders.
Acknowledgements
This work was supported by National Institute of Health grants AR059810 and AR068313 to AK.
Footnotes
Conflict of Interest Statement: Authors declare that no potential conflict of interest exist in the manuscript.
Author contribution statement: SMH and AK wrote this commentary.
References
- 1.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tidball JG, Dorshkind K, Wehling-Henricks M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development. 2014;141:1184–1196. doi: 10.1242/dev.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano C, Mojumdar K, Liang F, et al. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum Mol Genet. 2015;24:2147–2162. doi: 10.1093/hmg/ddu735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henriques-Pons A, Yu Q, Rayavarapu S, et al. Role of Toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle. Hum Mol Genet. 2014;23:2604–2617. doi: 10.1093/hmg/ddt656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J, Tajrishi MM, Ogura Y, et al. Wasting mechanisms in muscular dystrophy. Int J Biochem Cell Biol. 2013;45:2266–2279. doi: 10.1016/j.biocel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summan M, Warren GL, Mercer RR, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Huang D, Ransohoff RM, et al. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25:3344–3355. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren GL, Hulderman T, Mishra D, et al. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- 10.Mojumdar K, Giordano C, Lemaire C, et al. Divergent impact of Toll-like receptor 2 deficiency on repair mechanisms in healthy muscle versus Duchenne muscular dystrophy. J Pathol. 2016 doi: 10.1002/path.4689. doi: 10.1002/path.4689. [DOI] [PubMed] [Google Scholar]
- 11.Paiva-Oliveira EL, Ferreira da Silva R, Correa Leite PE, et al. TLR4 signaling protects from excessive muscular damage induced by Bothrops jararacussu snake venom. Toxicon. 2012;60:1396–1403. doi: 10.1016/j.toxicon.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Mojumdar K, Liang F, Giordano C, et al. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2. EMBO Mol Med. 2014;6:1476–1492. doi: 10.15252/emmm.201403967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uaesoontrachoon K, Cha HJ, Ampong B, et al. The effects of MyD88 deficiency on disease phenotype in dysferlin-deficient A/J mice: role of endogenous TLR ligands. J Pathol. 2013;231:199–209. doi: 10.1002/path.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]