Abstract
AXL is a tyrosine kinase membrane receptor that signals via PI3K, MAPK, and protein kinase C (PKC), among other pathways. AXL has oncogenic potential and interacts with other membrane receptors, depending on their relative abundance and availability. The increased expression of AXL in cancer is often the result of pharmacologic selective pressure to a number of chemotherapies and targeted therapies and acts as a mechanism of acquired drug resistance. This resistance phenotype, frequently accompanied by epithelial-to-mesenchymal transition, can be reversed by AXL inhibition. In tumors with high levels of EGFR, including lung, head and neck, and triple-negative breast cancer, AXL dimerizes with this receptor and initiates signaling that circumvents the antitumor effects of anti-EGFR therapies. Likewise, AXL overexpression and dimerization with EGFR can overcome PI3K inhibition by activating the phospholipase C-γ-PKC cascade that, in turn, sustains mTORC1 activity. The causative role of AXL in inducing drug resistance is underscored by the fact that the suppression of AXL restores sensitivity to these agents. Hence, these observations indicate that AXL is selectively expressed in tumor cells refractory to therapy and that cotargeting AXL in this setting would potentially overcome drug resistance. The use of AXL inhibitors should be considered in the clinic.
Background
The gene AXL, a name derived from the Greek word anexelekto (“uncontrolled”) was first isolated from chronic myelogenous leukemia, and its overexpression was found to induce fibroblast transformation with simultaneous appearance of a 140-kDa tyrosine-phosphorylated protein (1). AXL is also known as adhesion-related kinase (2), Tyro7 (3), or unknown function (4). AXL belongs to the TAM family of receptor tyrosine kinases (RTK), which also includes Tyro3 and MERTK. TAM receptors have pleiotropic functions in many biologic processes, such as coagulation, immune response, and cancer progression (5). They share among their members 16% to 31% of their extracellular amino acid sequence and 54% to 59% of their intracellular domain (6). Autophosphorylation of the intracellular tyrosine kinase domain of AXL occurs following receptor activation and is mediated either by ligand-dependent or ligand-independent receptor dimerization. Growth arrest–specific protein 6 (Gas6) has been identified as the only ligand that binds the extracellular domain of AXL (7–9). Receptor homodimerization or heterodimerization with other RTKs, such as EGFR (10), results in rapid phosphorylation of AXL and the activation of a number of downstream effectors (see “AXL signaling pathway”).
AXL is ubiquitously expressed in a wide variety of tissues, such as brain (hippocampus and cerebellum), heart, liver, and bone marrow (monocytes and macrophages; reviewed in refs. 5, 11). Increased expression of AXL has been reported in several human cancers, including colon, esophageal, thyroid, breast, lung, liver, and astrocytoma–glioblastoma (reviewed in refs. 12, 13).
The AXL receptor regulates fundamental cellular processes, including proliferation, survival, and migration (13). Moreover, AXL was shown to play a pivotal role in enhancing motility and invasiveness of breast (14) and lung cancer cells (15).
AXL signaling pathway
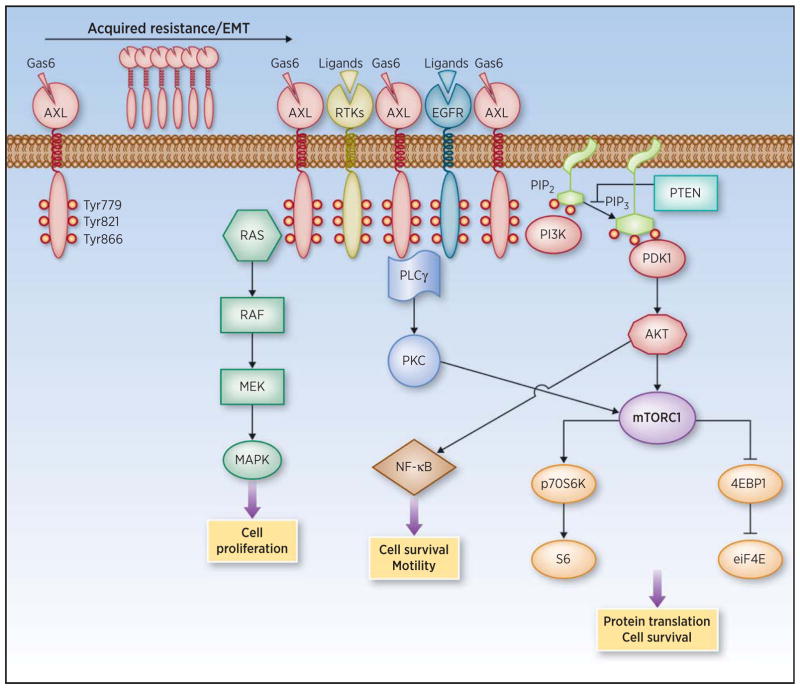
AXL activation initiates the signaling of a number of downstream pathways such as PI3K, MAPK, and PKC (Fig. 1; ref. 16). The phosphorylation of three specific tyrosine residues (Tyr) within the intracellular domain of AXL promotes the recruitment of p85 (the regulatory subunit of PI3K), phospholipase C-γ (PLCγ, the initiator of the PKC cascade), and growth factor receptor–bound protein 2 [Grb2, an adaptor molecule that allows the activation of the MAPK pathway (17)]. Although Grb2 binding seems to be specific for Tyr821, p85 can interact with both Tyr821 and Tyr779, whereas PLCγ can anchor to both Tyr821 and Tyr886 (Fig. 1; ref. 17).
Figure 1.
AXL overexpression and activation of downstream signaling pathways. AXL is overexpressed upon acquisition of therapy resistance and can induce epithelial-to-mesenchymal transition (EMT). It dimerizes with RTKs present in the membrane of tumor cells to initiate signaling cascades that ultimately lead to increased cell motility and survival.
Both ligand-dependent and -independent activation of AXL initiates downstream signaling in several cancer types, including prostate (18), ovarian (19), lung (mesothelioma; ref. 20), and head and neck (21). In turn, these signaling cascades can activate transcription factors regulating cell proliferation and survival. One example is the AKT-mediated destabilization of the IkBα–NF-κB complex, resulting in nuclear shuttling of NF-κB (18) and consequent transcription of antiapoptotic proteins such as cyclin D1, survivin, and focal adhesion kinase (22).
The activation of AXL is negatively regulated by a soluble form of the receptor that directly interacts with Gas6 and reduces ligand availability (23). Mechanistically, soluble AXL acts as a decoy receptor blocking Gas6 binding to membrane-bound TAM receptors and thus preventing AXL activation. A positive correlation between the levels of soluble AXL and membrane-bound AXL was observed in hepatocellular carcinoma (24), suggesting that the detection of soluble AXL could potentially be used as a biomarker to monitor increased AXL expression and emergence of drug resistance overtime. In addition, C1 domain–containing phosphatase and tensin homolog(C1-TEN), a focal adhesion molecule with phosphatase properties and highly similar to PTEN, has been described to interact directly with AXL and negatively regulate the downstream activation of AKT (25, 26). AXL activation and downstream signaling propagation results in enhanced cell motility and invasion by increasing filopodia formation and cell-to-cell interactions (27). This phenotype is mechanistically explained, at least in part, by the AXL-mediated phosphorylation of engulfment and cell motility scaffold protein that, in turn, promotes Rac-mediated cytoskeleton changes, resulting in increased cancer cell migration (28). Accordingly, this is reversed by both AXL and Rac inhibition (29).
AXL expression regulation
Although the regulation of AXL expression remains to be fully elucidated, it is not mediated by genomic amplification (30, 31). Likewise, no hotspot-activating mutations have been reported (30, 31). Overexpression of AXL may occur via alternative mechanisms, including activation of transcription factors, regulation of miRNAs, and posttranslational modifications. Specifically, transcriptional activation mediated by Fos/cJun/AP1 (16, 32), Sp1/Sp3 (33), and YAP1 (34) transcription factors results in increased AXL mRNA expression. AXL is also a direct transcriptional target of the Fos family member transcription factor Fos-related antigen 1 (Fra-1). Fra-1 was described to bind to four different regulatory regions of AXL-promoting gene expression (35). This was also confirmed by exogenous expression of Fra-1, which results in AXL upregulation (35). In imatinib-resistant chronic myeloid leukemia cells, the transcription factor activator protein 1 (AP-1) seems to be required for AXL overexpression, as the promoter activity of AXL is almost completely abolished when carrying a mutation on its AP1-binding site (16). AXL expression may also be regulated by miR-34a and miR-199a/b, which target the 3′-UTR of the AXL gene (36–38). In non–small cell lung (NSCLC), breast, and colorectal cancers, for example, high levels of AXL can result from low expression of these miRNAs, which are suppressed by promoter methylation (36).
AXL protein levels can also depend on its stability. Receptor ubiquitination mediated by the Casitas B-lineage lymphoma (Cbl) E3 ubiquitin ligases can regulate the abundance of AXL in several cells (39, 40). Likewise, increased AXL half-life by impaired degradation of the receptor can occur in lung cancer cell lines, resulting in the net increase of AXL levels (41).
Clinical–Translational Advances
Targeted therapy frequently results in a rapid increase of RTK expression that can compensate for the acute inhibition of a specific signaling pathway. In breast cancer, for example, HER3 is often overexpressed as a result of PI3K/AKT inhibition (42–44), whereas increased expression and activity of EGFR plays a pivotal role in limiting the efficacy of BRAF inhibition in colon cancer (45, 46). These occurrences do not require genomic amplification, are versatile (not specific for a tumor type or a treatment), and inevitably result in the activation of downstream effectors that can oppose the pharmacologic pressure. The net result is either activation of parallel signaling or reactivation of the suppressed pathway, both of which overcome the pharmacologic pressure.
Increased AXL expression has been correlated with resistance to both antimitotic drugs and targeted agents. In AML, AXL was the only RTK overexpressed in cells from 4 patients that progressed on chemotherapy. Consistently, cell lines intrinsically resistant to chemotherapy express higher levels of AXL, and the chemotherapy exposure is sufficient to induce the expression of AXL (47). A similar effect is observed in NSCLC cell lines, with acquired resistance to cisplatin in vitro. Refractoriness to cisplatin coincided with induction of AXL expression, transcriptional changes compatible with epithelial-to-mesenchymal transition (EMT), and partial resistance to the EGFR kinase inhibitor gefitinib (48). EMT is a conserved transdifferentiation process that many tumor cell types undergo during cancer evolution (49). It is caused by a complex transcription rewiring that results in the acquisition of mesenchymal properties and nonspecific drug resistance. A recent report confirmed the association between induction of EMT and increased AXL expression but concluded that EMT-associated drug resistance is independent of AXL function (50). Nonetheless, these data indicate that AXL inhibition sensitizes mesenchymal cells to antimitotic agents, such as docetaxel or aurora kinase and polo-like kinase 1 inhibitors, both in vitro and in vivo. This finding is in contrast with another report showing that the overexpression of AXL is sufficient to induce EMT directly in breast cancer cells and that AXL suppression can reverse this phenotype (51). Overall, there is consensus in ascribing to AXL a central role in leading to transcriptional changes related to EMT.
In terms of resistance to RTK inhibitors, although AXL can also interact with HER2 (52) and HER3 (53), EGFR seems to be the strongest dimerization partner of AXL in several tumor types. AXL interacts and dimerizes with EGFR in lung (54), triple-negative breast cancer (10), and head and neck squamous cell carcinomas (21, 32). In accordance, overexpression of AXL has been shown to be sufficient to limit the sensitivity to anti-EGFR therapy in several models, both in vitro and in vivo (10, 32, 38, 55, 56). In particular, AXL overexpression and activation, accompanied by EMT-associated transcriptional changes, was observed in EGFR-mutant lung cancer xenografts that acquired partial resistance to the EGFR kinase inhibitor erlotinib in vivo (54). The causative role of AXL in inducing this phenotype was demonstrated by the facts that exogenous expression of AXL was sufficient to induce partial resistance to erlotinib in parental erlotinib-sensitive cells and that AXL inhibition restored erlotinib sensitivity in the resistant xenografts. In head and neck cancer cells, overexpression of AXL and its dimerization with EGFR can maintain EGFR activation and signaling even in the presence of the EGFR blocking antibody cetuximab (32). In these cells, AXL overexpression and dimerization with EGFR also results in acquired resistance to α isoform–specific PI3K inhibition, both in vitro and in animal models (21). In this case, the mechanism of resistance involves the engagement of a parallel signaling cascade (PLCγ-PKC) that compensates for PI3K/AKT inhibition via downstream parallel mTORC1 activation.
As mentioned, AXL can also interact with HER2 in HER2+ breast cancer cells. In this context, AXL–HER3 dimerization bypassed HER2 signaling inhibition and provided the rationale to combine lapatinib, a small-molecule HER2 kinase inhibitor, with an AXL kinase inhibitor (53). Another plausible combinatorial strategy is the simultaneous suppression of AXL and the MAPK pathway in melanoma. In this case, AXL suppression seems to be important in cell lines/human tumors with low levels of microphthalmia-associated transcription factor and high levels of AXL, a cell state associated with acquired resistance to MAPK pathway inhibition (57, 58). These findings support the clinical development of AXL inhibitors in cancer in combination with targeted agents (EGFR, HER2, and PI3K inhibitors) at the time of acquired resistance and high AXL levels. Similarly, AXL inhibitors could be tested upfront if AXL overexpression is detected earlier in the course of the disease. In Table 1, we list the AXL inhibitors currently being developed in the laboratory, in animal models, and in the clinic.
Table 1.
Anti-AXL agents currently in preclinical or clinical development
| Company | Compound | Target(s) | Indication | Clinical status |
|---|---|---|---|---|
| Servier | S49076 (kinase inhibitor) | MET, AXL, FGFR1/2/3 | Advanced solid tumors | Phase I 2013-003079-37 |
| Mirati Therapeutics Inc. | MGCD516 (kinase inhibitor) | MET, AXL, and members of the VEGFR, PDGFR, DDR2, TRK, and Eph families | Advanced solid tumors | Phase I NCT02219711 |
| Mirati Therapeutics Inc. | MGCD265 (kinase inhibitor) | MET/AXL | Advanced malignancies | Phase I NTC00697632 |
| Betta Pharmaceuticals Co., Ltd | BPI-9016M (kinase inhibitor) | MET/AXL | Advanced solid tumors | Phase I NCT02478866 |
| BerGenBio AS | BGB324 (R428; kinase inhibitor) | AXL | NSCLC and AML | Phase I/II NCT02488408 NCT02424617 |
| Tolero Pharmaceuticals and Astex Pharmaceuticals | TP-0903 (kinase inhibitor) | AXL | Pancreatic cancer, lung cancer | Preclinical |
Abbreviations: AML, acute myelogenous leukemia; DDR, discoidin domain receptor; Eph, ephrin; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; TRK, tropomyosin receptor kinase.
In summary, the available data suggest that overexpression of AXL may be restricted to cells that are, or more frequently become, refractory to either chemotherapy or targeted therapy. Its suppression may revert the drug-resistant phenotype, either by reversing EMT or blunting the activation of a compensatory pathway that limits therapy effectiveness.
Acknowledgments
Given the space limitations of the review, the authors apologize for their inability to cite everyone who has contributed to this field of inquiry.
Grant Support
This work was funded by the Cycle for Survival (to M. Scaltriti and J. Baselga).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: M. Scaltriti, M. Elkabets, J. Baselga
Writing, review, and/or revision of the manuscript: M. Scaltriti, M. Elkabets, J. Baselga
References
- 1.O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–31. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rescigno J, Mansukhani A, Basilico C. A putative receptor tyrosine kinase with unique structural topology. Oncogene. 1991;6:1909–13. [PubMed] [Google Scholar]
- 3.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 4.Janssen JW, Schulz AS, Steenvoorden AC, Schmidberger M, Strehl S, Ambros PF, et al. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene. 1991;6:2113–20. [PubMed] [Google Scholar]
- 5.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–57. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 7.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–7. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 8.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 9.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–6. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 10.Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–73. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 13.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–85. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–15. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 15.Lay JD, Hong CC, Huang JS, Yang YY, Pao CY, Liu CH, et al. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer Res. 2007;67:3878–87. doi: 10.1158/0008-5472.CAN-06-3191. [DOI] [PubMed] [Google Scholar]
- 16.Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2:874–85. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, et al. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–31. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 18.Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, et al. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32:689–98. doi: 10.1038/onc.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70:7570–9. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou WB, Corson JM, Flynn DL, Lu WP, Wise SC, Bueno R, et al. AXL regulates mesothelioma proliferation and invasiveness. Oncogene. 2011;30:1643–52. doi: 10.1038/onc.2010.555. [DOI] [PubMed] [Google Scholar]
- 21.Elkabets M, Pazarentzos E, Juric D, Sheng Q, Pelossof RA, Brook S, et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27:533–46. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton DA, et al. Axl/Gas6/NFkappaB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2014;33:336–46. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- 23.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–33. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichl P, Fang M, Starlinger P, Staufer K, Nenutil R, Muller P, et al. Multicenter analysis of soluble Axl reveals diagnostic value for very early stage hepatocellular carcinoma. Int J Cancer. 2015;137:385–94. doi: 10.1002/ijc.29394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafizi S, Ibraimi F, Dahlback B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. FASEB J. 2005;19:971–3. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- 26.Hafizi S, Alindri F, Karlsson R, Dahlback B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun. 2002;299:793–800. doi: 10.1016/s0006-291x(02)02718-3. [DOI] [PubMed] [Google Scholar]
- 27.Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103:5799–804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Thuraia A, Gauthier R, Chidiac R, Fukui Y, Screaton RA, Gratton JP, et al. Axl phosphorylates Elmo scaffold proteins to promote Rac activation and cell invasion. Mol Cell Biol. 2015;35:76–87. doi: 10.1128/MCB.00764-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rea K, Pinciroli P, Sensi M, Alciato F, Bisaro B, Lozneanu L, et al. Novel Axl-driven signaling pathway and molecular signature characterize high-grade ovarian cancer patients with poor clinical outcome. Oncotarget. 2015;6:30859–75. doi: 10.18632/oncotarget.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL mediates resistance to cetuximab therapy. Cancer Res. 2014;74:5152–64. doi: 10.1158/0008-5472.CAN-14-0294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 33.Mudduluru G, Allgayer H. The human receptor tyrosine kinase Axl gene–promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation. Biosci Rep. 2008;28:161–76. doi: 10.1042/BSR20080046. [DOI] [PubMed] [Google Scholar]
- 34.Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–40. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayan AE, Stanford R, Vickery R, Grigorenko E, Diesch J, Kulbicki K, et al. Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL. Oncogene. 2012;31:1493–503. doi: 10.1038/onc.2011.336. [DOI] [PubMed] [Google Scholar]
- 36.Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene. 2011;30:2888–99. doi: 10.1038/onc.2011.13. [DOI] [PubMed] [Google Scholar]
- 37.Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH, Ambs S, Caplen NJ. Identification of the receptor tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat. 2011;130:663–79. doi: 10.1007/s10549-011-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giles KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD, et al. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther. 2013;12:2541–58. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- 39.Valverde P. Effects of Gas6 and hydrogen peroxide in Axl ubiquitination and downregulation. Biochem Biophys Res Commun. 2005;333:180–5. doi: 10.1016/j.bbrc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 40.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–12. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae SY, Hong JY, Lee HJ, Park HJ, Lee SK. Targeting the degradation of AXL receptor tyrosine kinase to overcome resistance in gefitinib-resistant non-small cell lung cancer. Oncotarget. 2015;6:10146–60. doi: 10.18632/oncotarget.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao JJ, Castel P, Radosevic-Robin N, Elkabets M, Auricchio N, Aceto N, et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci Signal. 2014;7:ra29. doi: 10.1126/scisignal.2005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118–22. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 46.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268:314–24. doi: 10.1016/j.canlet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Kurokawa M, Ise N, Omi K, Goishi K, Higashiyama S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 2013;104:904–11. doi: 10.1111/cas.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson C, Ye X, Pham T, Lin E, Chan S, McNamara E, et al. AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res. 2014;74:5878–90. doi: 10.1158/0008-5472.CAN-14-1009. [DOI] [PubMed] [Google Scholar]
- 51.Asiedu MK, Beauchamp-Perez FD, Ingle JN, Behrens MD, Radisky DC, Knutson KL. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene. 2014;33:1316–24. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bose R, Molina H, Patterson AS, Bitok JK, Periaswamy B, Bader JS, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci U S A. 2006;103:9773–8. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–8. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK, Yoon SJ, et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014;74:253–62. doi: 10.1158/0008-5472.CAN-13-1103. [DOI] [PubMed] [Google Scholar]
- 57.Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4:816–27. doi: 10.1158/2159-8290.CD-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller J, Krijgsman O, Tsoi J, Robert L, Hugo W, Song C, et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun. 2014;5:5712. doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]