SUMMARY
Hypoxia inducible factors (HIFs) are critical regulators of the cellular response to hypoxia. Despite their established roles in normal physiology and numerous pathologies, the molecular mechanisms by which they control gene expression remain poorly understood. We report here a conserved role for the TIP60 complex as a HIF1 transcriptional cofactor in Drosophila and human cells. TIP60 (KAT5) is required for HIF1-dependent gene expression in fly cells and embryos, and colorectal cancer cells. HIF1A interacts with and recruits TIP60 to chromatin. TIP60 is dispensable for HIF1A association with its target genes but is required for HIF1A-dependent chromatin modification and RNA polymerase II activation in hypoxia. In human cells, global analysis of HIF1A-dependent gene activity reveals that most HIF1A targets require either TIP60, the CDK8-Mediator complex, or both as co-activators for full expression in hypoxia. Thus, HIF1A employs functionally diverse cofactors to regulate different subsets of genes within its transcriptional program.
ETOC Blurb
Hypoxia inducible factors (HIFs) are critical regulators of the cellular response to hypoxia. In this study, Perez-Perri el al uncover a conserved role for the TIP60 complex in HIF-dependent gene expression in flies and human cancer cells. Further work demonstrates that HIF1A interacts with and recruits TIP60 to chromatin. Global transcriptome analysis reveals that most HIF1A targets require either TIP60, the CDK8-Mediator complex, or both as co-activators for full expression in hypoxia
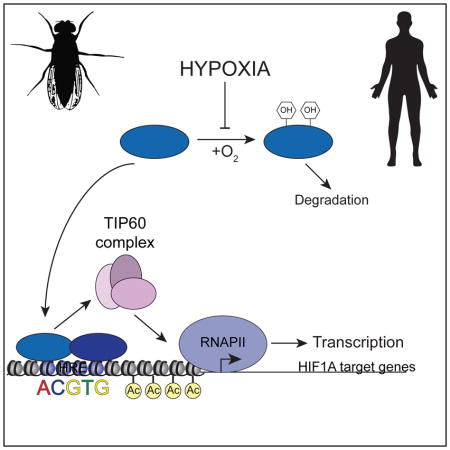
INTRODUCTION
The cellular response to hypoxia is essential for normal physiological processes, such as embryonic development and stem cell maintenance (Dunwoodie, 2009; Mazumdar et al., 2009), but is also involved in diverse human pathologies including cancer, stroke and heart failure (Majmundar et al., 2010; Semenza, 2012a). At the transcriptional level, the response to hypoxia is largely governed by Hypoxia-Inducible Factors (HIFs) (Dengler et al., 2014; Semenza, 2009). In human cells, numerous studies have delineated how the oxygen-sensitive subunits HIF1A and HIF2A are stabilized and activated in hypoxia and have identified hundreds of their target genes, but less is known about the mechanisms employed by HIFs to stimulate RNAPII activity.
It is generally accepted that the lysine (K) acetyl-transferases (KATs) p300/CBP are key HIF transcriptional coactivators (Arany et al., 1996; Ebert and Bunn, 1998; Ruas et al., 2002; Ruas et al., 2005). However, abrogation of the interaction between HIF1A and p300/CBP affects the expression of only a few HIF-target genes (Kasper et al., 2005). Here, we report the identification of a conserved role for the TIP60 chromatin-modifying complex as a HIF1A transcriptional cofactor. We show that HIF1A utilizes TIP60 (KAT5) for full induction of specific target genes, and for histone acetylation and RNAPII activation upon hypoxia at these loci. We find that HIF1A physically associates with components of the TIP60 complex and is required for TIP60 recruitment to chromatin. Global analyses of gene expression in human cells depleted of HIF1A, TIP60 or CDK8 revealed that, across much of its transcriptional program, HIF1A employs TIP60, CDK8-Mediator, or both as gene-specific coactivators. Altogether, our results illuminate the orchestrated action of functionally diverse cofactors during the transcriptional response to hypoxia.
RESULTS
Components of the TIP60 complex modulate HIF target gene activation in Drosophila
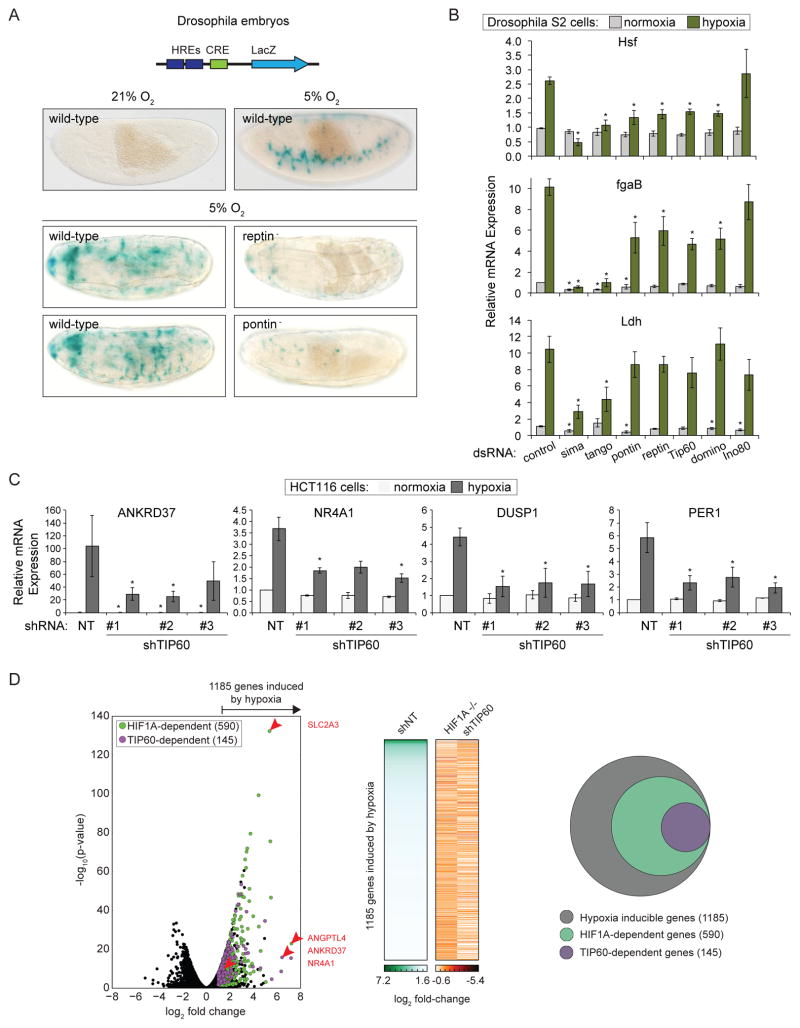
We previously carried out a genome-wide screen in Drosophila S2 cells and identified Pontin and Reptin as two of the strongest regulators of HIF-dependent transcription using a HIF reporter system (Dekanty et al., 2010). Pontin (RUVBL1, TIP49) and Reptin (RUVBL2, TIP48) are AAA+ ATPases with diverse cellular functions, including transcriptional regulation (Gallant, 2007). Here, we analyzed their requirement in vivo using Drosophila transgenic lines bearing a HIF-dependent LacZ reporter (Lavista-Llanos et al., 2002) and null mutations at the pontin or reptin loci. While the reporter is highly induced in wild-type Drosophila embryos subjected to hypoxia (5% O2 4 hr), its activity is severely compromised in Pontin and Reptin mutants (Figure 1A).
Figure 1. Subunits of the TIP60 complex modulate HIF target gene expression in Drosophila and Human Cells.
(A) Schematic diagram of the murine LDHA enhancer-derived hypoxia-response element (HRE)-LacZ reporter construct inserted into the fly genome. CRE, cAMP Response Element. Wild-type and pontin− or reptin− mutant embryos subjected to normoxia or hypoxia (5% O2 4 hr) were stained with X-gal (blue) to visualize reporter activity. (B) Relative mRNA levels for HIF target genes, as assessed by qRT-PCR, in Drosophila S2 cells treated with the indicated dsRNAs and maintained under normoxia or hypoxia (1% O2 20 hr). Expression values were normalized to Rpl29 RNA and are expressed relative to the control normoxia value. Data are represented as mean ± SEM from at least three independent replicates. Asterisks indicate p-values ≤0.05 by one-way ANOVA. (C) Relative mRNA levels for HIF1 target genes, as assessed by qRT-PCR, for HCT116 cells stably expressing shRNAs targeting TIP60 and subjected to normoxia or hypoxia (1% O2 24 hr). Expression values were normalized to 18S ribosomal RNA (rRNA) and are expressed relative to the control normoxia value. Data are represented as mean ± SEM from at least three independent replicates. Asterisks indicate p-values ≤0.05 by one-way ANOVA. (D) RNA-seq analysis of global mRNA levels in shNT control, HIF1A−/−, and shTIP60 HCT116 cells subjected to hypoxia. Left: Volcano plot of log2 fold change against -log10 p-value for all genes in normoxia versus hypoxia. Green circles, HIF1A-dependent hypoxia-inducible genes; purple circles, HIF1A-dependent genes that are also TIP60-dependent. Selected genes of interest are indicated by red arrows, with labels on right. Middle: Heat map of mRNA log2 fold change values. Right: Venn diagram representing the genes induced by hypoxia (gray), and subsets that require HIF1A (green), or both HIF1A and TIP60 (purple) for hypoxic induction. In all cases hypoxic induction is defined as ratio (shNT normoxia/shNT hypoxia) ≥2, FDR adjusted p-value <10%; and requirement for HIF1A or TIP60 is defined as ratio (HIF1A−/− or shTIP60 hypoxia/shNT hypoxia) ≤0.9, FDR adjusted p-value <10%. See also Figure S1.
Pontin and Reptin are components of multiple complexes with roles in transcription, including the TIP60 and INO80 complexes (Jha et al., 2013; Jonsson et al., 2004; Sapountzi et al., 2006). To determine if these complexes are involved in HIF-dependent transcription we tested the effect of depleting shared and specific subunits on expression of known HIF targets in S2 cells under normoxia and hypoxia (Hsf, fgaB and Ldh, Figure 1B, S1A). Depletion of Pontin, Reptin, or two different subunits specific to the TIP60 complex, Tip60 (KAT5) and Domino (p400), impaired the induction of two of three genes tested (Figure 1B). In contrast, depletion of Ino80 had a lesser effect. Depletion of the Drosophila homologs of HIF1A (sima) and HIF1B (tango) confirmed HIF1-dependent induction of these genes. Together these results suggest a role for the Pontin- and Reptin-containing Drosophila TIP60 complex as a gene-specific HIF transcriptional coactivator.
TIP60 depletion impairs expression of specific HIF1A target genes in human cells
We next asked whether this role of the TIP60 complex is conserved in human cells. We first depleted the catalytic subunit KAT5 using three independent shRNAs in HCT116 colorectal carcinoma cells (shTIP60) and confirmed that TIP60 knockdown did not affect HIF1A stabilization in hypoxia (1% O2 24 hr, Figure S1B, C). Examination of the effect on expression of a panel of HIF1A-dependent genes revealed gene-specific TIP60 requirements (Figure 1C, S1D–F). For example, whereas induced expression of ANKRD37 and NR4A1 is reduced by >50% (Figure 1C), expression of ALDOA and JMJD1A remains unaffected (Figure S1F). A similar gene-specific requirement for TIP60 was also observed in a different colon cancer-derived cell type, SW480 (Figure S1G).
To define the contribution of TIP60 to the global HIF1A-driven transcriptional response we used RNA-seq to measure mRNA levels in HIF1A−/− and shTIP60 HCT116 cells under normoxic and hypoxic conditions. From ~14,000 expressed genes, our analysis identified 1185 genes significantly induced by hypoxia, of which half (590, 49.7%) showed significantly reduced expression during hypoxia in HIF1A−/− cells (Figure 1D, Table S1). In turn, ~25% (145) of these HIF1A-dependent genes relied on TIP60 for full expression during hypoxia, demonstrating that TIP60 makes a substantial contribution to the HIF1A-driven expression program.
TIP60 complex subunits are recruited to HIF1A target genes
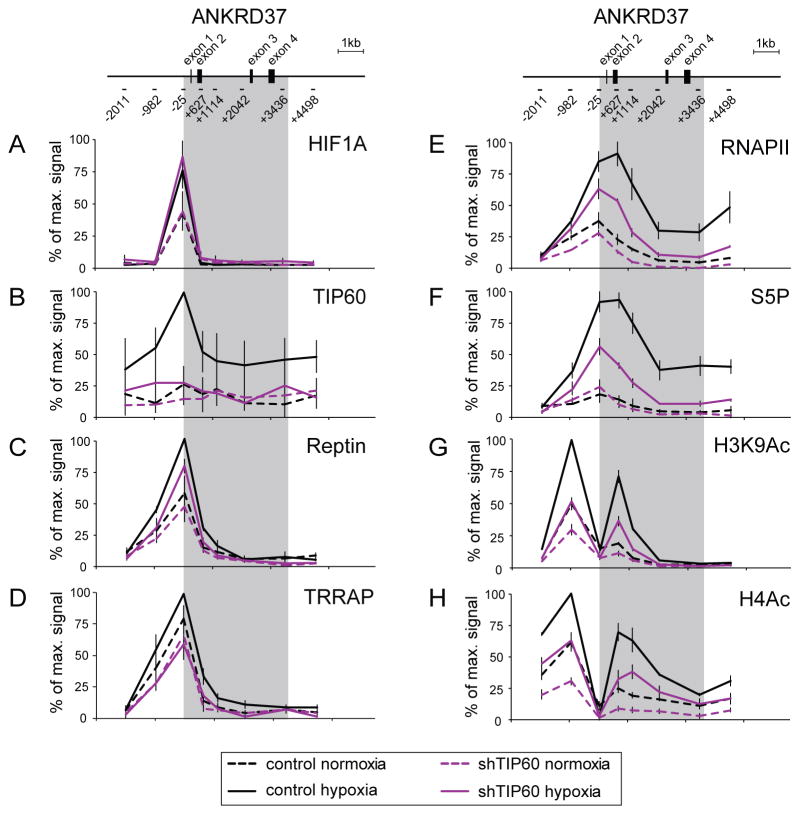
To explore the mechanistic basis for TIP60 requirement in HIF1A-driven gene expression we performed quantitative chromatin immunoprecipitation (ChIP) analysis of the ANKRD37 locus, a hypoxia-responsive gene strongly affected by TIP60 depletion. Analysis of HIF1A binding to the ANKRD37 locus demonstrated that TIP60 knockdown does not alter HIF1A association with chromatin upon hypoxia (Figure 2A).
Figure 2. TIP60 is required for RNAPII activation and histone acetylation at the ANKRD37 locus.
Quantitative ChIP analysis of (A) HIF1A, (B) TIP60, (C) Reptin, (D) TRRAP, (E) total RNAPII, (F) serine-5-phosphorylated RNAPII CTD (S5P) and (G–H) histone acetylation (H3K9ac and pan-H4ac) at the ANKRD37 locus in control and shTIP60 HCT116 cells in normoxia or hypoxia (1% O2 24 hr). To represent profiles across the locus, values are plotted as percentage of maximum signal for each epitope. Data are represented as mean ± SEM from three independent replicates. Gray area indicates the transcribed region.
We next asked whether the TIP60 complex localizes to this gene by assessing occupancy of TIP60, Reptin and TRRAP (Figure 2B–D). All three subunits were found to associate with the ANKRD37 promoter, with their occupancy peaking in hypoxia. Interestingly, Reptin and TRRAP exhibit relatively strong enrichment even in normoxic conditions. This could be explained by their reported roles within other chromatin-associated complexes distinct from the TIP60 complex, or by basal but undetectable recruitment of TIP60 itself in normoxia (see next section). Note that TIP60 depletion modestly affects occupancy of both Reptin and TRRAP (Figure 2C, D), and TIP60 enrichment is expectedly low in TIP60-depleted cells (Figure 2B).
TIP60 depletion compromises HIF1A-dependent RNAPII activation and histone acetylation
Many HIF1A target genes, including ANKRD37, exhibit promoter-proximal paused RNAPII during normoxia and their activation under hypoxia requires conversion of paused RNAPII into an elongation-competent form (Galbraith et al., 2013). This process typically involves phosphorylation of the C-terminal domain (CTD) of RNAPII. Therefore, we tested the influence of TIP60 on total and phosphorylated RNAPII. Expectedly, hypoxia provokes an increase in total RNAPII levels throughout the body of the ANKRD37 gene with a peak near the transcription start site (Figure 2E), and stimulates RNAPII activation, as indicated by CTD serine 5 phosphorylation (S5P) (Figure 2F). While TIP60 depletion has a small effect on normoxic levels, it causes a substantial reduction in induced enrichment of both total and S5P-RNAPII across the ANKRD37 locus. This suggests that TIP60 is a direct positive regulator of RNAPII activity at HIF1A-responsive genes.
Since histone acetylation is associated with HIF1A-dependent gene activation (Johnson et al., 2008) and the TIP60 complex has well established lysine acetyl-transferase activity, we next asked if this complex promotes HIF1A-dependent histone acetylation. We found that TIP60 depletion diminishes acetylation of histone H3 lysine 9 (H3K9) and histone H4 (Figure 2G, H), two acetylation events previously shown to depend on HIF1A (Galbraith et al, 2013). Although TIP60 recruitment is maximal during hypoxia, its depletion also impairs histone acetylation during normoxia. This might reflect a low basal recruitment of the TIP60 complex, which is consistent with levels of HIF1A, Reptin and TRRAP observed during normoxia at the ANKRD37 locus (Figure 2A, C, D). Notably, ANKRD37 is one of a minority of HIF1A-dependent genes whose normoxic expression is affected by depletion of TIP60. Overall, these results demonstrate that TIP60 is required for maximal HIF1A-dependent RNAPII activation and histone hyper-acetylation at the ANKRD37 locus.
HIF1A directs the TIP60 complex to chromatin
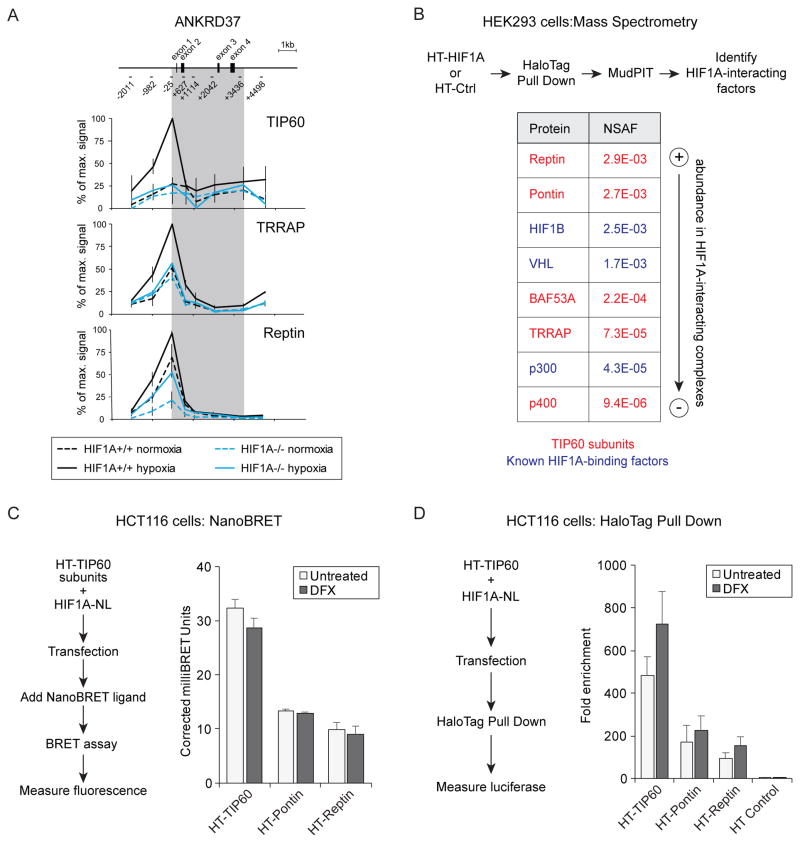
We next asked if HIF1A is required for recruitment of the TIP60 complex to chromatin. Using ChIP in isogenic wild-type and HIF1A−/− HCT116 cells, we found that maximal occupancy of the ANKRD37 locus by the TIP60, Reptin and TRRAP subunits of the TIP60 complex under hypoxia requires HIF1A (Figure 3A).
Figure 3. HIF1A directs the TIP60 complex to chromatin.
(A) Quantitative ChIP analysis of TIP60, TRRAP and Reptin at the ANKRD37 locus in wild-type and HIF1A−/− HCT116 cells in normoxia or hypoxia. To represent profiles across the locus, values are plotted as percentage of maximum signal for each epitope. Data are represented as mean ± SEM from three independent replicates. Gray area indicates the transcribed region. (B) Identification of HaloTag (HT)-HIF1A interactors by pulldown and LC-MS/MS analysis. Average normalized spectral abundance factors (NSAF) from two biological replicates are shown for selected proteins isolated in complex with HT-HIF1A from desferrioxamine (DFX)-treated HEK293T cells. Known HIF1A interactors are shown in blue and subunits of the TIP60 complex are shown in red. (C) NanoBRET assays measuring proximity of HIF1A to TIP60, Pontin, and Reptin. HCT116 cells were co-transfected with HIF1A-NL (donor) and either HT-TIP60, HT-Pontin, or HT-Reptin (acceptors). Graph shows corrected milliBRET units for each acceptor fusion protein when combined with the HIF1A-NL donor. Higher values indicate closer proximity. Data are represented as mean + SEM from three independent replicates. None of the comparisons between untreated and DFX values gave p-values <0.05 by T test. (D) HaloTag pull down from HCT116 cells co-expressing HT-TIP60, HT-Pontin, HT-Reptin, or HT control with HIF1A-NL. Pulldown of HIF1A-NL by physical association with HT-TIP60, or HT control, was measured by detection of luciferase activity. Data are represented as mean + SEM from at least three independent replicates. None of the comparisons between untreated and DFX values gave p-values <0.05 by T test. See also Figure S2.
To test whether HIF1A associates physically with the TIP60 complex we performed HaloTag pull down and mass spectrometry analysis of HIF1A-interacting proteins from HEK293T cells expressing Halo-tagged(HT)-HIF1A, or control HaloTag (HT) alone, and treated with desferrioxamine (DFX) to induce HIF1A stabilization. Using this approach, in addition to known HIF1A interactors such as HIF1B (ARNT) and VHL, we detected Reptin and Pontin (Figure 3B, Figure S2A, Table S2). Detection of an interaction with VHL, a subunit of the E3 ligase complex that targets HIF1A for degradation when hydroxylated at key residues during normoxia, suggests that in this context some HT-HIF1A remains hydroxylated, despite treatment with DFX. Also present, albeit at lower abundance, were three other potential TIP60 complex subunits TRRAP, BAF53A (ACTL6a) and p400 (EP400), as well as p300 (EP300).
We next tested for physical proximity between HIF1A and TIP60 complex subunits in live cells using a Nano-luciferase Bioluminescent Resonance Energy Transfer (NanoBRET) assay (Machleidt et al., 2015) in HCT116 and HEK293 cells (Figure 3C, S2B). In agreement with our mass spectrometry analysis we detected energy transfer from HIF1A-NanoLuc (HIF1A-NL) to HT-Pontin and HT-Reptin. These interaction signals were not significantly enhanced by DFX treatment which could be explained by the fact that ectopic expression of HIF1A-NL bypasses the need for hypoxia-induced stabilization. Notably, we detected a stronger signal for HT-TIP60 than for HT-Pontin and HT-Reptin. This suggests that despite not being detected by mass spectrometry, TIP60 can also interact with HIF1A in cells. This could be explained by poor performance or low relative abundance of TIP60-derived peptides in the mass spectrometry assay. Of note, nanoBRET ratios are influenced by many factors including close proximity, relative affinity, occupancy, and expression level and therefore higher values do not necessarily indicate a higher affinity interaction. Importantly, all signals were greater than for the HaloTag (HT) control (Figure S2C). To confirm the HIF1A-TIP60 interaction we performed HaloTag pull down from HCT116 co-expressing HT-TIP60, HT-Pontin, HT-Reptin, or HT control with HIF1A-NL (Figure 3D, S2D). After isolation, complexes were assayed for luciferase activity as a measure of interaction with HIF1A-NL. We observed an enrichment in luciferase signal, over the HT control, for all three pulldowns with HT-TIP60 again displaying the strongest signal (Figure 3D). Treatment with DFX induced a small, albeit not statistically significant, increase in signal. The interaction between HT-TIP60 and HIF1A-NL was also observed in HEK293 cells (Figure S3D). Taken together, our data suggest that HIF1A can physically interact with components of the TIP60 complex and direct their recruitment to chromatin during hypoxia.
HIF1A employs TIP60 and CDK8-Mediator as coactivators across much of its transcriptional program
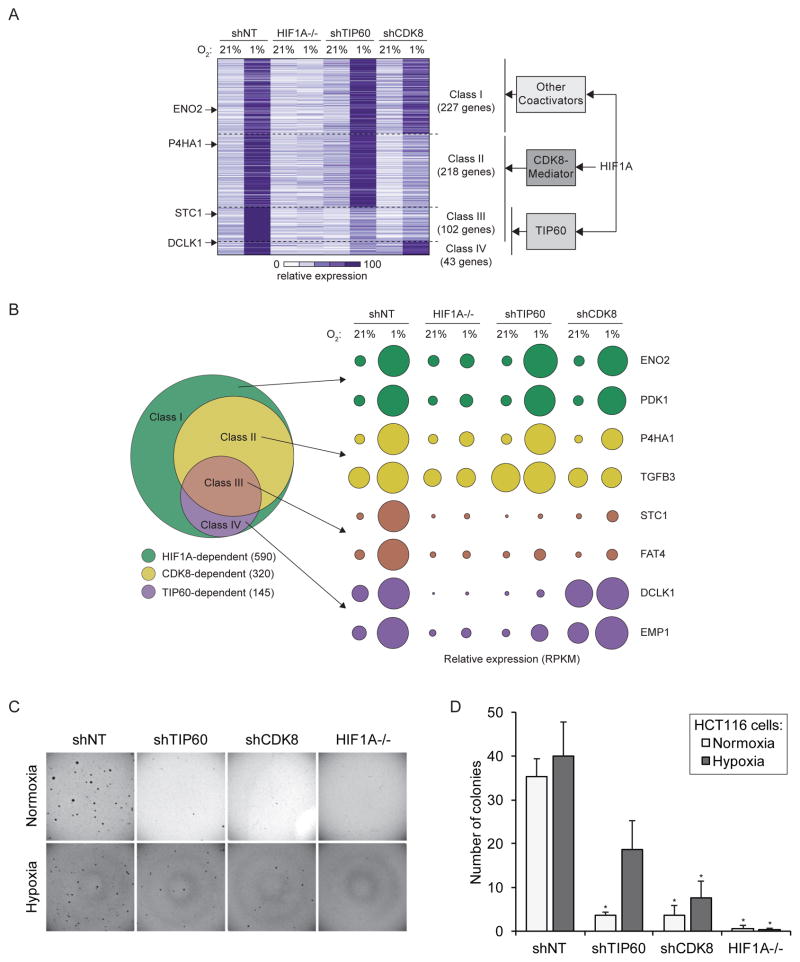
We recently discovered that HIF1A employs the CDK8-Mediator complex as a coactivator at many of its target genes (Galbraith et al., 2013). To define the relative contributions of both TIP60 and CDK8 to the transcriptional response during hypoxia we compared RNA-seq data for HCT116 cells depleted of CDK8 (shCDK8) to our previous data for HIF1A−/− and shTIP60 HCT116 cells (Figure 4A, Table S1). This analysis revealed that TIP60 and CDK8 affect distinct but overlapping sets of genes and, collectively, contribute to the induced expression of >60% (363 out of 590) of genes across the HIF1A network. We defined four classes of HIF1A-dependent genes. Class I genes require HIF1A but not TIP60 or CDK8 for full expression. Class II genes require HIF1A and CDK8, but not TIP60. Class III genes require HIF1A, CDK8 and TIP60. Class IV genes require HIF1A and TIP60, but not CDK8. Each class contains known HIF1A target genes displaying differential requirement for CDK8 and TIP60 (Figure 4B). The differential requirement for TIP60 across select genes in Classes I, III, and IV (PDK1, FAT4 and EMP1) was confirmed by qRT-PCR (Figure S3A). Next, we next examined markers of transcriptional activity at these loci upon TIP60 knockdown (Figure S3B). Depletion of TIP60 largely abrogated enrichment of total and phosphorylated RNAPII at FAT4 (Class III) and EMP1 (Class IV) but not PDK1 (Class I). Interestingly, TIP60 depletion also affected histone H4 acetylation at PDK1, a TIP60-independent gene, suggesting that TIP60 may be recruited and active even at loci where it is not required for RNAPII activity or full mRNA induction. We also confirmed that these genes are likely direct HIF1A target genes, as HIF1A depletion led to reduced levels of total and phosphorylated RNAPII, TIP60 recruitment and histone acetylation (Figure S3C). We also analyzed a hypoxia-inducible gene that does not require HIF1A, TIP60 or CDK8 (CYR61, Figure S3A–C). TIP60 and HIF1A depletion did not affect total RNAPII at this locus (Figure S3B, C). However, their depletion did impact Ser5 phosphorylation and, as observed for the Class I gene PDK1, TIP60 knockdown reduced histone acetylation at CYR61 despite not being necessary for CYR61 mRNA expression. HIF1A depletion had minor effects on TIP60 and histone acetylation at this locus (Figure S3C). Altogether, these results indicate that TIP60 is recruited to many classes of hypoxia-inducible genes where it contributes to histone acetylation, yet it is only required for RNAPII activity and mRNA production at Classes III and IV. Note that the requirement for TIP60 is not simply defined by its chromatin occupancy, and that its ultimate effects on RNAPII at a given locus are likely defined by other, unknown variables (see below). Furthermore, because our ChIP analyses may not have captured the site of maximum TIP60 occupancy at each locus, a quantitative relationship between TIP60 association and its impact on RNAPII activity can not be established. Since histone H4 acetylation is affected by TIP60 knockdown at all genes analyzed, TIP60 effects could be driven by other acetylation targets in the transcriptional apparatus. In fact, several non-histone TIP60 targets, including transcription factors, have been identified (Patel et al., 2004; Sun et al., 2005; Tang et al., 2006; Tang et al., 2008; Xiao et al., 2014).
Figure 4. TIP60 and CDK8 are required for expression of specific subsets of HIF1A target genes.
Differential coactivator requirement as determined by RNA-seq analysis (includes datasets used in Figure 1). (A) Comparison of mRNA levels for hypoxia-inducible genes in shNT control, HIF1A−/−, shTIP60, and shCDK8 HCT116 cells in normoxia and hypoxia (1% O2 24 hr). HIF1A-dependent hypoxia-inducible genes were divided into four non-overlapping classes based on their coactivator dependency: CDK8- and TIP60-independent (Class I), CDK8-dependent (Class II), TIP60-dependent (Class IV) and dependent on both TIP60 and CDK8 (Class III). Hypoxia-inducible genes were defined as ratio (shNT normoxia/shNT hypoxia) ≥2, FDR adjusted p-value <10%; cofactor requirement was defined as ratio ≤0.9, FDR adjusted p-value <10% for HIF1A−/−, shTIP60, or shCDK8 over shNT cells in hypoxia. Heat map shows mRNA levels as RPKM (reads per kilobase per million) for all HIF1A-dependent hypoxia-inducible genes, normalized to the maximum signal for that gene. (B) Left, Venn diagram showing the relative size of each class. Right, bubble plots showing relative mRNA levels for example known direct HIF1A target genes within each class. Surface area corresponds to RPKM values relative to shNT control cells in hypoxia. (C, D) Anchorage-independent growth assay showing the ability of shNT control versus shTIP60, shCDK8, or HIF1A−/− HCT116 cells to form colonies in soft agar. Data are represented as mean + SEM. Asterisks indicate p-values ≤0.05 by T-test against shNT in normoxia or hypoxia. See also Figures S3 and S4.
Of note, the differential requirements for TIP60 and CDK8 across the HIF1A-driven gene expression program are not simply a consequence of a general effect on transcription, as their overall contributions to global mRNA levels in normoxic cells are much smaller, with TIP60 affecting many more genes than CDK8 (Figure S4A). These results suggest that alternative coactivator usage by HIF1A could confer regulatory flexibility to the transcriptional response to hypoxia. Moreover, the differential coactivator requirements of subsets of genes might depend in part on the ability of HIF1A to cooperate with other transcription factors (Dang et al., 2008; Gray et al., 2005; Xia and Kung, 2009). To search for factors with potential to differentially regulate subsets of HIF1A target genes we identified known upstream transcriptional regulators of each gene class in our RNA-seq dataset using Ingenuity Pathway Analysis (IPA) (Figure S4B). Expectedly HIF1A was the top prediction for all HIF1-dependent hypoxia-inducible genes, as well as for the four classes within this group, but not for HIF1A-independent genes. HIF2A is the second inferred regulator for HIF1A-dependent genes as well as a top prediction for gene Classes I and II, which likely reflects its many shared target genes with HIF1A. When comparing the top ten enriched regulators across each of the four gene classes we identified transcription factors that are predicted to regulate genes within specific classes (Figure S4C). For example, while SP3 may regulate only genes in class I, STAT6 could regulate genes in classes II and III, both of which require CDK8 for full expression. Thus, we propose that the combinatorial action of HIF1A with other transcription factors may enable the fine-tuning of the transcriptional response to hypoxia to favor diverse biological responses in a context-dependent fashion. Indeed, analysis of the different gene classes identified here also revealed distinct functional pathways controlled by this putative mechanism (Figure S4D).
Finally, to examine the roles of TIP60 and CDK8 in oncogenic properties of colorectal cancer cells, we measured the ability of shNT control, shTIP60, shCDK8, and HIF1A−/− HCT116 cells to initiate and sustain growth of three-dimensional colonies in soft agar, which quickly reach a size large enough to create hypoxic conditions at their core (Indovina et al., 2007; Lin et al., 2012; Meng et al., 2012; Sutherland et al., 1986). These assays are considered a good surrogate of the ‘tumor initiating ability’ of cancer cells (i.e. ‘stemness’) and their ability to adapt to hypoxic conditions. Importantly, this assay is exquisitely sensitive to HIF1A activity in multiple cell types (Dang et al., 2006; Leek et al., 2005; Onnis et al., 2013; Rohwer et al., 2008), including when incubated at 21% oxygen (Dang et al., 2006). Whereas HCT116 HIF1A−/− cells do not show significant proliferation defects in monolayers, they completely fail to form colonies in soft agar (Figure 4C–D). Therefore, this assay provides a great opportunity to gauge the contribution of TIP60 and CDK8 to a cellular process that is fully dependent on HIF1A. Hypoxia is known to stimulate ‘stemness’ and clonogenicity, along with induction of HIF1A target genes (Lee and Simon, 2012; Yeung et al., 2011). We therefore performed the assay under conditions of normoxia and hypoxia. In wild-type HCT116 cells, introducing hypoxia from the time of seeding stimulates colony formation efficiency, as seen by increased colony numbers, although without reaching statistical significance (Figure 4C–D). The stimulatory effect of hypoxia is completely lost in HIF1A−/− cells, consistent with the ability of HIF1A to promote stemness in colon cancer cells (Yeung et al., 2011). However, the stimulatory effect of hypoxia can be observed, to varying degrees, in TIP60- and CDK8-depleted cells, which can be explained by the fact that these cells retain the ability to induce a fraction of the HIF1A-dependent transcriptional program (Figure 4A). In fact, the amount of colonies formed under hypoxia inversely correlates with the contributions of HIF1A, TIP60, and CDK8 to the hypoxia-inducible program. Altogether these data suggest that HIF1A, TIP60 and CDK8 may promote tumorigenesis through their contributions to a common transcriptional program.
DISCUSSION
The cellular response to hypoxia is important in normal physiology and disease (Semenza, 2012a). In particular, hypoxia favors tumor progression by promoting angiogenesis, epithelial to mesenchymal transition, and metabolic reprogramming (Mucaj et al., 2012; Semenza, 2012b). It is therefore imperative to understand the molecular basis by which cells respond to this stress. The transcription factors HIF1A and HIF2A are the key factors governing the transcriptional response to hypoxia, yet the mechanisms by which they regulate RNAPII have yet to be fully elucidated (Dengler et al., 2014). Here we identified a conserved role for the TIP60 complex in HIF1A-dependent transactivation, and investigated differential requirements for TIP60 and CDK8 as coactivators in the regulation of HIF1A target genes in human cancer cells.
Repeatedly, the related KATs p300 and CBP have been reported as key HIF1A coactivators (Arany et al., 1996; Cho et al., 2007; Ebert and Bunn, 1998; Ema et al., 1999; Kallio et al., 1998; Ruas et al., 2010). However, mutation of their CH1 domains to disrupt HIF interaction was shown to affect expression of only a small number of HIF target genes (Kasper et al., 2005). Our data show that a different KAT, TIP60, contributes to the induction of specific HIF1A targets and suggest that it may be a common driver of HIF1A-driven histone acetylation. We found that TIP60 is required for HIF1-dependent gene expression in Drosophila and two different human cancer cell lines; HIF1A interacts with and recruits TIP60 to chromatin; TIP60 is dispensable for HIF1A association with its target genes but is required for HIF1A-dependent chromatin modification and RNA polymerase II activation in hypoxia.
It has been suggested that Pontin and Reptin play opposite roles in HIF1-dependent transcription (Lee et al., 2011; Lee et al., 2010). However, we found here that both Pontin and Reptin are required for HIF1 transcriptional activity in Drosophila cultured cells and embryos (Figure 1A, B). This contrasting requirement could be explained by locus-specific functions and the critical role of Pontin and Reptin in assembly of the TIP60 complex and its acetyl-transferase activity (Jha et al., 2013). In agreement with this notion, we found that depletion of the Tip60 or domino (p400) subunits reproduced the gene-specific effects of Pontin and Reptin depletion in S2 cells (Figure 1B). Thus, we focused our investigation on the defining subunit of the complex, TIP60 (KAT5).
Recently, we demonstrated that a specific variant of the Mediator complex plays a widespread role in HIF1A coactivation (Galbraith et al., 2013). Our transcriptome analysis shows that HIF1A employs TIP60, CDK8-Mediator, or both as coactivators across much of its transcriptional program. Together these two coactivators contribute to induced expression of >60% of HIF1A-driven genes. It would be interesting to determine in our system what fraction of HIF1A targets require other known coactivators such as PKM2 (Luo et al., 2011).
Why do different HIF1A target genes display differential requirement for TIP60, CDK8 or other coactivators? Using publicly available ENCODE data for the HCT116 cell line, we looked for differences in regulatory chromatin modifications but did not find any striking differences between our gene classes that might explain differential cofactor requirement. Another possibility is that coactivator requirement is defined by the combinatorial action of HIF1A and its partner transcription factors (Dang et al., 2008; Gray et al., 2005; Xia and Kung, 2009). Our bioinformatics analysis supports this notion by predicting multiple transcription factors in addition to HIF1A as upstream regulators of our various gene classes (Figure S4B, C). Mechanistically, the coordinated binding of HIF1A and a specific partner transcription factor may allow recruitment of different coactivators that are required at specific genes. In turn, this layered action of partner transcription factors and coactivators may provide flexibility to the HIF1A-driven transcriptional program veering it toward different cellular outcomes in a context-dependent manner. Furthermore, we observed enrichment of different cellular pathways between our gene classes (Figure S4D) and it would therefore be interesting to examine the role of HIF1A coactivators and putative partner TFs in regulation of these pathways and phenotypic outcomes during hypoxia.
Many cancer cells experience hypoxia due to uncontrolled proliferation and aberrant blood supply within tumors. In hypoxic cancer cells HIFs regulate genes involved in metabolic reprograming, angiogenesis, stemness, epithelial-to-mesenchymal transition, invasion, metastasis, apoptosis, and resistance to radiation and chemotherapy (Dengler et al., 2014; Semenza, 2012b). Accordingly, hypoxic tumors expressing high levels of HIFs are known to be more aggressive and resistant to various therapies. Our data here suggest that TIP60 and CDK8, by regulating a fraction of the HIF1A transcription program, may contribute to survival and proliferation of cancer cells during hypoxia. Since both TIP60 and CDK8 are potentially amenable to pharmacological inhibition (Cee et al., 2009; Ghizzoni et al., 2012), it is possible to envision strategies for therapeutic remodeling of the hypoxic response by selectively blocking the activity of these HIF1A coactivators.
Altogether, the results presented here demonstrate a conserved role for the TIP60 complex in the transcriptional response to hypoxia, alone or in combination with CDK8-Mediator, at HIF1A target genes. This paves the road for future studies aimed at defining the mechanistic basis and biological implications of this functional relationship between three well recognized players in gene expression control.
EXPERIMENTAL PROCEDURES
Fly stocks and β-galactosidase assay
Flies were yw; yw, LDH-LacZ (Lavista-Llanos et al., 2002). Pont5.1/TM3 (pontin-) and rept35/TM3 (reptin-) flies were generously provided by Dr. Peter Gallant (Julius Maximilians University of Würzburg). To measure β-galactosidase activity of the hypoxia-responsive reporter, embryos were exposed to 21% or 5% O2 for 4 hr. Embryos were then bleach-dechorionated, fixed with 0.5% glutaraldehyde, washed with PBS + 1% Triton, and incubated with a solution of X-gal.
Drosophila S2 cell culture, dsRNA synthesis and RNAi
Drosophila S2 cells were maintained at 25°C in Schneider medi um (Sigma), supplemented with 10% fetal bovine serum (Gibco), 50 μg/mL streptomycin and 50 units/mL penicillin. dsRNAs were synthesized from cDNA with the T7 Megascript kit (Ambion). See Table S3 for dsRNA sequences. Transfection of S2 cells with dsRNAs was performed in 24-well plates (Greiner) using the “bathing” method as previously described (Clemens et al., 2000). After 4 days, cells were exposed to 1% O2 or kept in normoxia for 20 hr. RNA was extracted using Trizol (Life Technologies), cDNA synthesized using the SuperScript III First-Strand Synthesis System (Life Technologies) and gene expression analyzed by real-time PCR in a Stratagene Mx3005P system (Agilent Technologies). Gene expression was normalized to RPL29 levels.
Mammalian cell culture and stable shRNA knockdown cell lines
Cells were cultured in McCoy’s 5A (Gibco/Life Technologies) or DMEM medium (Sigma) supplemented with 10% fetal bovine serum and antibiotic-antimycotic mixture (Gibco/Life Technologies) under 5% CO2 at 37°C. HCT116 HIF1A −/− cells were created by disrupting exons 3 and 4 of the HIF1A locus using adeno-associated virus-mediated homologous recombination, resulting in a 226 bp deletion with translation stop codons in all three reading frames (Dang et al., 2006). Cells were plated 24 hr prior to experimental treatments and harvested in RIPA lysis buffer for protein or Trizol for total RNA. Hypoxia treatments were carried out in incubation chambers (Billups-Rothenberg) by flushing twice with 120 L of a mixture of 1% O2/5% CO2/94% N2 (Airgas) and incubated for 24 hr at 37° C. Individ ual knockdown cell lines were generated using Sigma Mission shRNA lentiviral plasmids (pLKO.1-puro), as described previously (Galbraith et al., 2013). See Table S3 for shRNA sequences.
RNA-seq analysis, chromatin immunoprecipitation, HaloTag pull down, and NanoBRET assay
See Extended Experimental Procedures.
Supplementary Material
Highlights.
TIP60 is required for expression of HIF1-dependent genes in Drosophila
The role of TIP60 at HIF1-dependent genes is conserved in human colorectal cancer cells
HIF1A interacts with and recruits TIP60 to chromatin
Many HIF1A targets require TIP60, CDK8, or both for full expression in hypoxia
Acknowledgments
We are grateful to all members of the Espinosa lab for invaluable advice and reagents, and Bruce Dickinson for helpful discussions. We thank Dr. Dylan Taatjes for the TRRAP antibody and Dr. Bruno Amati for TIP60 antibodies. We thank Long Dang for the HCT116 HIF1A−/− cells. This work was supported primarily by a grant from the National Science Foundation (NSF-MCB MCB-1243522) and by NIH grant 5R01CA117907, NIH Training Grant T32 GM08759 to VLD, and NIH grant P30-CA046934-27. Promega Corporation is the commercial owner by assignment of patents of the HaloTag technology and its applications.
Footnotes
SUPPLEMENTAL INFORMATION. Supplemental information includes Extended Experimental Procedures, four figures, and three tables. RNA-seq data are available under GEO accession number GSE68297.
AUTHOR CONTRIBUTIONS
JIP-P and VLD conceived and designed experiments, acquired, analyzed and interpreted data, and drafted the article. KAA, MU, JM, and DLD acquired and analyzed data. AP analyzed data. PW conceived the study and revised the article. MDG conceived and designed experiments, acquired, analyzed and interpreted data, drafted and revised the article. JME conceived and designed experiments, interpreted data, drafted and revised the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cee VJ, Chen DY, Lee MR, Nicolaou KC. Cortistatin A is a high-affinity ligand of protein kinases ROCK, CDK8, and CDK11. Angew Chem Int Ed Engl. 2009;48:8952–8957. doi: 10.1002/anie.200904778. [DOI] [PubMed] [Google Scholar]
- Cho H, Ahn DR, Park H, Yang EG. Modulation of p300 binding by posttranslational modifications of the C-terminal activation domain of hypoxia-inducible factor-1alpha. FEBS Lett. 2007;581:1542–1548. doi: 10.1016/j.febslet.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci USA. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- Dang DT, Chen F, Gardner LB, Cummins JM, Rago C, Bunz F, Kantsevoy SV, Dang LH. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 2006;66:1684–1936. doi: 10.1158/0008-5472.CAN-05-2887. [DOI] [PubMed] [Google Scholar]
- Dekanty A, Romero NM, Bertolin AP, Thomas MG, Leishman CC, Perez-Perri JI, Boccaccio GL, Wappner P. Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia. PLoS Genet. 2010;6:e1000994. doi: 10.1371/journal.pgen.1000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler VL, Galbraith MD, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Bunn HF. Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol. 1998;18:4089–4096. doi: 10.1128/mcb.18.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, et al. HIF1A Employs CDK8-Mediator to Stimulate RNAPII Elongation in Response to Hypoxia. Cell. 2013;153:1327–1339. doi: 10.1016/j.cell.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P. Control of transcription by Pontin and Reptin. Trends Cell Biol. 2007;17:187–192. doi: 10.1016/j.tcb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Ghizzoni M, Wu J, Gao T, Haisma HJ, Dekker FJ, George Zheng Y. 6-alkylsalicylates are selective Tip60 inhibitors and target the acetyl-CoA binding site. Eur J Med Chem. 2012;47:337–344. doi: 10.1016/j.ejmech.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- Indovina P, Collini M, Chirico G, Santini MT. Three-dimensional cell organization leads to almost immediate HRE activity as demonstrated by molecular imaging of MG-63 spheroids using two-photon excitation microscopy. FEBS Lett. 2007;581:719–726. doi: 10.1016/j.febslet.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Jha S, Gupta A, Dar A, Dutta A. RVBs are required for assembling a functional TIP60 complex. Mol Cell Biol. 2013;33:1164–1174. doi: 10.1128/MCB.01567-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res. 2008;640:174–179. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson ZO, Jha S, Wohlschlegel JA, Dutta A. Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol Cell. 2004;16:465–477. doi: 10.1016/j.molcel.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Okamoto K, O’Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 2005;24:3846–3858. doi: 10.1038/sj.emboj.7600846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavista-Llanos S, Centanin L, Irisarri M, Russo DM, Gleadle JM, Bocca SN, Muzzopappa M, Ratcliffe PJ, Wappner P. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol Cell Biol. 2002;22:6842–6853. doi: 10.1128/MCB.22.19.6842-6853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim Y, Bhin J, Shin HJ, Nam HJ, Lee SH, Yoon JB, Binda O, Gozani O, Hwang D, et al. Hypoxia-induced methylation of a pontin chromatin remodeling factor. Proc Natl Acad Sci USA. 2011;108:13510–13515. doi: 10.1073/pnas.1106106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim Y, Kim IS, Kim B, Choi HJ, Lee JM, Shin HJ, Kim JH, Kim JY, Seo SB, et al. Negative regulation of hypoxic responses via induced Reptin methylation. Mol Cell. 2010;39:71–85. doi: 10.1016/j.molcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr Opin Cell Biol. 2012;24:232–235. doi: 10.1016/j.ceb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Leek RD, Stratford I, Harris AL. The role of hypoxia-inducible factor-1 in three-dimensional tumor growth, apoptosis, and regulation by the insulin-signaling pathway. Cancer Res. 2005;65:4147–4152. doi: 10.1158/0008-5472.CAN-04-2184. [DOI] [PubMed] [Google Scholar]
- Lin HH, Li X, Chen JL, Sun X, Cooper FN, Chen YR, Zhang W, Chung Y, Li A, Cheng CT, et al. Identification of an AAA ATPase VPS4B-dependent pathway that modulates epidermal growth factor receptor abundance and signaling during hypoxia. Mol Cell Biol. 2012;32:1124–1138. doi: 10.1128/MCB.06053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt T, Woodroofe CC, Schwinn MK, Mendez J, Robers MB, Zimmerman K, Otto P, Daniels DL, Kirkland TA, Wood KV. NanoBRET-A Novel BRET Platform for the Analysis of Protein-Protein Interactions. ACS Chem Biol. 2015;10:1797–1804. doi: 10.1021/acschembio.5b00143. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. Journal of cellular and molecular medicine. 2009;13:4319–4328. doi: 10.1111/j.1582-4934.2009.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- Mucaj V, Shay JE, Simon MC. Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol. 2012 doi: 10.1007/s12185-012-1070-5. [DOI] [PubMed] [Google Scholar]
- Onnis B, Fer N, Rapisarda A, Perez VS, Melillo G. Autocrine production of IL-11 mediates tumorigenicity in hypoxic cancer cells. J Clin Invest. 2013;123:1615–1629. doi: 10.1172/JCI59623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JH, Du Y, Ard PG, Phillips C, Carella B, Chen CJ, Rakowski C, Chatterjee C, Lieberman PM, Lane WS, et al. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N, Welzel M, Daskalow K, Pfander D, Wiedenmann B, Detjen K, Cramer T. Hypoxia-inducible factor 1alpha mediates anoikis resistance via suppression of alpha5 integrin. Cancer Res. 2008;68:10113–10120. doi: 10.1158/0008-5472.CAN-08-1839. [DOI] [PubMed] [Google Scholar]
- Ruas JL, Berchner-Pfannschmidt U, Malik S, Gradin K, Fandrey J, Roeder RG, Pereira T, Poellinger L. Complex regulation of the transactivation function of hypoxia-inducible factor-1 alpha by direct interaction with two distinct domains of the CREB-binding protein/p300. J Biol Chem. 2010;285:2601–2609. doi: 10.1074/jbc.M109.021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. J Biol Chem. 2002;277:38723–38730. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- Ruas JL, Poellinger L, Pereira T. Role of CBP in regulating HIF-1-mediated activation of transcription. J Cell Sci. 2005;118:301–311. doi: 10.1242/jcs.01617. [DOI] [PubMed] [Google Scholar]
- Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int J Biochem Cell Biol. 2006;38:1496–1509. doi: 10.1016/j.biocel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012a;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012b;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B, Mueller-Klieser W. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46:5320–5329. [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Kung AL. Preferential binding of HIF-1 to transcriptionally active loci determines cell-type specific response to hypoxia. Genome Biol. 2009;10:R113. doi: 10.1186/gb-2009-10-10-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Nagai Y, Deng G, Ohtani T, Zhu Z, Zhou Z, Zhang H, Ji MQ, Lough JW, Samanta A, et al. Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep. 2014;7:1471–1480. doi: 10.1016/j.celrep.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung TM, Gandhi SC, Bodmer WF. Hypoxia and lineage specification of cell line-derived colorectal cancer stem cells. Proc Natl Acad Sci USA. 2011;108:4382–4387. doi: 10.1073/pnas.1014519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.