Abstract
Objective
To study normative thresholds and latencies for click and tone-burst auditory brainstem response (TB-ABR) for air and bone conduction in normal infants and those discharged from neonatal intensive care units (NICU), who passed newborn hearing screening and follow-up DPOAE. An evoked potential system (Vivosonic Integrity™) that incorporates Bluetooth electrical isolation and Kalman-weighted adaptive processing to improve signal to noise ratios was employed for this study. Results were compared with other published data.
Research Design
One hundred forty-five infants who passed two-stage hearing screening with transient-evoked otoacoustic emission (OAE) or automated ABR were assessed with clicks at 70 dB nHL and threshold TB-ABR. Tone-bursts at frequencies between 500 to 4000 Hz were employed for air and bone conduction ABR testing using a specified staircase threshold search to establish threshold levels and Wave V peak latencies.
Results
Median air conduction hearing thresholds using TB-ABR ranged from 0-20 dB nHL, depending on stimulus frequency. Median bone conduction thresholds were 10 dB nHL across all frequencies, and median air-bone gaps were 0 dB across all frequencies. There was no significant threshold difference between left and right ears and no significant relationship between thresholds and hearing loss risk factors, ethnicity or gender. Older age was related to decreased latency for air conduction. Compared to previous studies, mean air conduction thresholds were found at slightly lower (better) levels, while bone conduction levels were better at 2000 Hz and higher at 500 Hz. Latency values were longer at 500 Hz than previous studies using other instrumentation. Sleep state did not affect air or bone conduction thresholds.
Conclusions
This study demonstrated slightly better Wave V thresholds for air conduction than previous infant studies. The differences found in the current study, while statistically significant, were within the test step size of 10 dB. This suggests that threshold responses obtained using the Kalman weighting software were within the range of other published studies using traditional signal averaging, given step-size limitations. Thresholds were not adversely affected by variable sleep states.
INTRODUCTION
Congenital hearing loss is an important cause of developmental delay in speech, language and social-emotional development. About 1 in 500 babies are born with permanent hearing loss, making it one of the one of the most common birth defects in developed countries (Ross et al., 2008; Watkin and Baldwin, 2012). About 8,000 children are born in the United States each year with congenital hearing loss (White, 2008). In the past decade, universal newborn hearing screening (NHS) has reduced the mean age of diagnosis of all hearing loss from about 30 months prior to universal newborn screening to about 3-4 months in 2009 (Harrison and Roush, 1996; CDC, 2011; Watkin and Baldwin, 2012). In order to achieve the best outcomes for NHS programs, early and accurate diagnosis of congenital hearing loss is essential to allow appropriate and early intervention in order to lessen developmental sequelae (JCIH, 2007). The Joint Committee on Infant Hearing (JCIH), and participating organizations such as the American Academy of Pediatrics, American Academy of Otolaryngology, American Academy of Audiology and the American Speech-Language Hearing Association, recommend the use of frequency-specific physiologic measures to diagnose hearing loss by no later than 3 months of age (JCIH 2007).
Auditory Brainstem Response (ABR) is an electrophysiological measure used to predict hearing sensitivity in infants for whom reliable behavioral thresholds cannot be obtained, and is currently the clinical standard for diagnosing degree and type of hearing loss in young infants unable to respond behaviorally (Stapells and Oates, 1997; Gorga et al., 2006). ABR testing was introduced in the 1970's as a physiologic tool to assess and diagnose disorders affecting the auditory pathways (Galambos & Hecox, 1978; Galambos, Hicks, & Wilson, 1984; Schulman-Galambos & Galambos, 1979). ABR tone-burst (TB) thresholds are highly correlated with behavioral audiometric thresholds (Gorga et al., 2006; Stapells, 2000; Stapells, Gravel, & Martin, 1995; Vander Werff, Prieve, & Georgantas, 2009), and as such may be used to estimate hearing thresholds for the purpose of initiating amplification prior to validation with behavioral audiometry. The use of air conduction (AC) and bone conduction (BC) TB-ABR for hearing diagnosis in newborns has been advocated by both the Joint Committee on Infant Hearing (JCIH, 2007) and clinical guidelines from the American-Speech-Language-Hearing Association (ASHA, 2004). Once the infant is developmentally able to respond reliably to sound, behavioral audiometry is the accepted gold standard for hearing function, but ABR testing is of particular importance in infants below six months of age (Mason, McCormick, & Wood, 1988).
Despite the long history of the use of ABR in NHS and diagnostic programs, several problems in the clinical application of AC and BC TB-ABR have limited effective and timely diagnosis of all newborns referred from screening programs. Some audiologists believe it is not feasible to obtain accurate frequency-specific ABR due to a lack of frequency specificity or neural synchrony (Baldwin & Watkin, 2013; Stapells, 2011). Concerns about obtaining adequate recordings in naturally sleeping or quiet, awake infants, and time required to obtain TB recordings have caused many audiologists to persist with using only click stimuli. There are also concerns about the accuracy of BC recordings. Despite a number of studies documenting the reliability and validity of bone-conduction recordings in infants (Yang, Stuart, Stenstrom & Green, 1993a; Yang, Stuart, Mencher, Mencher & Vincer, 1993b; Small, Hatton & Stapells, 2007), some clinicians believe BC testing is not feasible or accurate. A lack of consensus exists about how to couple the bone transducer to the infant's skull, and whether and how to employ contralateral masking. Additionally, threshold data are lacking for some TB frequencies, notably 1000 and 4000 Hz, which are important for defining the shape of the predicted audiogram (Hatton, Janssen and Stapells, 2012). Normative data have been published for AC and BC TB-ABR testing, but most studies have consisted of retrospective clinical samples, or infants who referred from NHS and were later deemed to have normal hearing (e.g. Vander Werff et al., 2009; Hatton, Janssen and Stapells, 2012). Infants referred for clinical concerns do not represent a true normative population since failed newborn screening tests could be associated with mild hearing loss or middle ear problems, thus the normative sample is affected by the clinical interpretation of what is considered “normal” after the test, rather than based on a set of a priori inclusion criteria.
Difficulties with obtaining adequate recordings in natural sleep in young infants can lead to delays in diagnosis. Loss to follow-up from NHS programs continues to be a challenge, with only 57% of infants who refer receiving a complete diagnostic evaluation by 3 months of age, and 35% classified as lost to follow-up (Centers for Disease Control, 2011). Thus, the ability to obtain a complete air and bone conduction ABR on the first attempt is highly desirable to achieve an early age of accurate diagnosis.
A major problem encountered in obtaining a high quality recording in non-sedated infants is myogenic noise produced as the infant moves, suckles or cries. Variable sleep state may cause increased myogenic noise, thus the small auditory signal must be extracted from a background of larger myogenic and electrical signals. An improvement in signal to noise ratio is traditionally obtained through a combination of signal averaging and rejection of recordings above a certain threshold value, called artifact rejection. However, when recordings are noisy, the time needed to acquire an averaged waveform is increased since many recordings may be rejected. An alternative approach is to retain these noisy recordings, but to weight them less than quiet recordings. This approach is known as “robust weighted averaging” (Leski & IEEE, 2002).
A type of robust weighted averaging that has been applied to auditory evoked responses is known as Kalman-weighted averaging (Marcoux & Kurtz, 2012). Kalman weighting is a recursive filter that estimates the true state of dynamically changing noisy measurements, so is well suited to applications such as auditory evoked potentials which seek to extract low-level electrical signals within high background noise (Georgiadis, 2005). This type of weighting has been developed in the Vivosonic Integrity system (Toronto, ON, Canada), a commercially available ABR instrument designed for use in pediatric diagnostic applications. A primary claim of the manufacturer is that ABR recordings can be obtained more readily in non-sedated infants. The instrument reduces electric and magnetic field-induced interference by using in-situ amplification on the ground electrode. The pre-amplifier, called the Amplitrode, has short, shielded leads to further isolate electrical contamination. Kalman weighting, as implemented in the Vivosonic system with digital filtering, further reduces electromagnetic artifacts and estimates the ABR signal by extracting the signal from each sweep, without rejection of artifacts as is done in traditional ABR systems. Instead, each response is weighted according to the estimated electrical noise, as derived from the pre-stimulus recording. This adaptive processing method provides cleaner waveforms by recalculating the weighting according to the relationship between covariance in sweeps and residual noise.
The electrodes are connected via a Bluetooth wireless interface, which reduces conducted electrical power contamination. An AC isolation transformer is used to reduce power-line noise. Normative ABR threshold data using this system are not currently available, and comparisons to thresholds published for other ABR test systems are needed. It is possible that ABR thresholds may be improved using this measurement system, but the effect of using Kalman weighting on measurements of ABR latency in infants has not been evaluated. Additionally, the impact of sleep state has not been evaluated in this system, which purports to improve threshold estimates under less than optimal conditions.
The primary goal of this study was to provide normative data for AC and BC TB-ABR thresholds for 500 to 4000 Hz. The normative group was defined as infants who passed the NHS exam, and also passed a distortion product otoacoustic emission (DPOAE) test on the follow-up date on which the TB-ABR data were acquired. There is a lack of published normative data for air and bone conduction TB-ABR at 1000 and 4000 Hz, thus these frequencies were prioritized. The specific objectives of this study were to:
Develop normative data for AC and BC TB Wave V thresholds and latencies at four audiometric frequencies (500, 1000, 2000 and 4000 Hz) in unsedated infants.
Determine relationship to age, ear, gender, sleep state and risk factors.
Compare the resulting normative TB-ABR thresholds and latencies to previously published studies.
MATERIALS AND METHODS
Subjects
This study is a part of a more extensive and ongoing longitudinal, prospective study of newborns and infants screened in normal nurseries and neonatal intensive care units, funded by the National Institute of Deafness and other Communication Disorders. The overall goal is to improve accuracy of hearing screening to discriminate conductive hearing loss from sensorineural and neural hearing loss, and to determine risk for recurrent/chronic otitis media with effusion.
Infants were enrolled from the well-baby nursery and Neonatal Intensive Care Unit (NICU) at Good Samaritan Hospital (GSH) and the NICU at Cincinnati Children's Hospital Medical Center (CCHMC), both urban hospitals in Cincinnati, Ohio. Table I summarizes the demographic characteristics of the infants included in the normal ABR analysis. A total of 145 infants (males = 89, females = 56) and 265 ears comprised the study sample, in which 120 infants were tested in both ears. Nineteen infants were recruited from the NICU and 126 from the well-baby nursery. The mean age at testing was 1.8 months (SD=1.3), and the median age was 1.4 months, with a range of 0.8-6.9 months. While a few infants were older due to prematurity and the need to reschedule when they were healthy enough to travel for outpatient visits, 75% of subjects were tested at age 2 months or less. The sample reflects the race and ethnicity of the Cincinnati metropolitan area, with the majority of infants identified as white, non-Hispanic (65%) or African-American (27%), as well as smaller numbers of Hispanic ethnicity and mixed race or other categories. Risk factors for hearing loss were reported in 28% of infants, while 13% had a NICU stay. Some of the babies in the normal newborn nursery had risk factors, such as family history of hearing loss, ear tags or pits, or brief courses of ototoxic drugs. Many of the infants cared for in the NICU had multiple risk factors; thus, the percentages for risk factors in Table 1 add up to more than 100%.
Table 1.
Study subject characteristics (N=145 subjects).
| Characteristic | |
|---|---|
| Male | 89 (61.4%) |
| Race/Ethnicity | |
| Non Hispanic white | 94 (64.8%) |
| African American | 39 (26.9%) |
| Mixed & Other | 11 (7.6%) |
| Hispanic | 1 (0.7)% |
| NICU | 19 (13%) |
| Mean age (SD) | 1.8 (1.3) |
| Range [0.8-6.9] | |
| Risk Factors* | |
| None listed | 105 (72.4%) |
| NICU >5 days | 19 (13.1%) |
| Family history | 12 (8.3%) |
| Resuscitation required | 6 (4.1%) |
| Craniofacial anomaly | 5 (3.5%) |
| Hyperbilirubenemia | 9 (6.2%) |
| Ototoxic drugs | 11 (7.6%) |
| Birth weight <1500g | 4 (2.8%) |
| Ear tag or earpit | 6 (4.1%) |
Percentages add up to more than 100% due to multiple risk factors for individuals.
Test Protocol
The research protocol was approved by the Institutional Review Boards of CCHMC and GSH. The standard hospital newborn screening protocol was performed prior to enrollment so as not to interfere with the normal clinical process. After explanation of the study and agreement by the primary caregiver to participate via signed informed consent, infants were tested using a two-stage hearing screening protocol of transient-evoked (TE) OAE, followed by automated (A) ABR for infants who did not pass TEOAE. Infants in the GSH NICU were tested with both TEOAE and AABR, while infants in the CCHMC NICU were tested with DPOAE and manual click ABR rather than AABR. Infants who passed the two-stage hearing screening hospital protocol and returned for diagnostic ABR were selected for this normative study. Follow-up diagnostic ABR testing was scheduled at age 1 month and preparation instructions for testing were sent to caregivers. Instructions included keeping the infant awake and delaying feeding until after arrival at the outpatient clinic. Testing was scheduled in synchronization with the infant's sleep schedule when possible. Infants were tested after bottle or breast feeding while resting in their caregiver's arms or in an infant carrier.
DPOAE testing using the Vivosonic Integrity system version 5.2 (Toronto, ON, Canada) was performed prior to the diagnostic threshold ABR. Primary tone levels were set at 65 (L1) and 55 (L2) dB SPL, and primary tone frequencies f1 and f2 were set at f2/f1 equal to 1.22. In-situ calibration was performed prior to testing each ear, followed by a DP-gram acquisition. Two trials were run and the overall better of the two tests was chosen to optimize signal to noise ratio (SNR). Pass criteria were SNR of 6 dB or greater at 3 of 5 test (f2) frequencies (2000, 3000, 4000, 5500, 8000 Hz). In addition, DP levels were required to be at or above 0 dB SPL to reduce the possibility of mild hearing loss (Gorga et al., 2005). Wideband absorbance and admittance tympanograms were also measured using a research system with custom software, although these results were not analyzed in the present report with one exception: wideband admittance tympanograms were examined at 1000 Hz to determine whether the compensated admittance was in a normal range. This normal range was a magnitude exceeding 0.6 mmho relative to the positive baseline, which is similar to the criterion used with a single-frequency admittance test at 1000 Hz (Margolis, Bass-Ringdahl, Hanks, Holte, & Zapala, 2003). Some ears (n=39) were excluded due to either not passing DPOAE or 1000-Hz tympanometry.
The ABR diagnostic examination was conducted by licensed pediatric research audiologists within a shielded double-walled sound-attenuated booth, using the Vivosonic Integrity system, Version 5.2 (Toronto, ON, Canada). Stimuli for AC were presented via insert earphones (Etymotic Research ER-3A, Elk Grove Village, IL) using pediatric ear foam tips, trimmed as necessary to accommodate newborn ear canals. BC stimuli were presented via a standard hand-held Radioear B-71 bone vibrator (Radioear Corp, New Eagle, PA), which was placed at the temporal bone at the superior post-auricular area as recommended by Yang, Rupert and Moushegian (1987) and by Stuart, Yang, and Stenstrom (1990). Broadband contralateral masking was applied using ER-3A insert phones with a masker level 10 dB above the level of the test stimulus. Stimuli were presented at 37.1 per second using Blackman-gated tones with ramping of 2-0-2 cycles, thus the rise/fall time was 4 ms at 500 Hz, 2 ms at 1000 Hz, 1 ms at 2000 Hz and 0.5 ms at 4000 Hz. Reference values at 0 dB nHL for the ER-3A insert earphones in peSPL were 25 dB at 500 Hz, 26 dB at 1000 Hz, 31 dB at 2000 Hz, and 35 dB at 4000 Hz. The reference peak-equivalent (pe) SPLs at 0 dB nHL for the B-71 bone vibrator were 67, 54, 49, and 41 dB at 500, 1000, 2000, and 4000 Hz, respectively. The system was calibrated by the manufacturer prior to the study and annually. Independent acoustic calibration measurements (peak dB SPL in a standard 2 cc coupler) done by our laboratory for dB HL pure tone conversion confirmed these values for insert earphones.
Signal averaging using the default Kalman weighting algorithm was employed, with the high pass filter cutoff frequency set at 30 Hz and the low pass filter cutoff frequency set at 3000 Hz. Rarefaction, condensation and summed alternating averages were recorded in a “split alternating” protocol which allowed comparison of polarities and the ability to correlate waveforms for reliability measures. The high forehead to ipsilateral mastoid recording montage was used with disposable pre-gelled electrodes, and inter-electrode impedance was maintained at less than 5 K ohms. Clicks were delivered at 70 dB nHL, referenced to 38 dB peSPL to assess waveform morphology and latencies for Waves I, III and V. Toneburst thresholds were collected for AC and BC at octave frequencies between 500 and 4000 Hz, starting at 4000 Hz, at 30 dB nHL, with initial steps of 20 dB higher or lower, depending on presence of Wave V, decreasing after the first reversal to 10 dB steps to seek threshold. The next frequency tested was 1000 Hz, and then 500 Hz and 2000 Hz as time and infant state allowed. The minimum test protocol was AC for 1000 and 4000 Hz, and BC for at least one corresponding frequency. The rationale for selection of 1000 and 4000 Hz as the primary frequencies tested was that 500 Hz may show air-bone gaps in normal hearing infants (Vander Werff et al., 2009), and tends to be more difficult to distinguish at threshold. It was desirable to test a lower and higher frequency TB, to characterize presence, type and shape of hearing loss for the larger study.
The latency of wave V was recorded at all intensities. Stopping rules are necessary in order to improve confidence that a response is biologically present, and not due to myogenic or electrical artifacts (Don & Elberling, 1996). Multiple decision criteria were constructed based on (1) logical latency-intensity functions, (2) correlation coefficients meeting specified criteria, (3) acceptable residual noise and (4) inter-examiner agreement. In order to determine the presence of Wave V, split-half waveforms were compared using a correlation coefficient, set manually from the onset prior to Wave V to the negative trough after Wave V as shown in Figure 1. The ongoing correlation value was tracked during the assessment with a stopping rule of visual acceptance of the waveform by the testing audiologist and correlation of 0.7 or higher, to indicate high probability of a non-random response (Sokolov, Kurtz, Sokolova, Steinman, & Mahon, 2008). Rarefaction and condensation waveforms were subtracted to determine when the residual noise was low relative to the averaged waveform; in the case of threshold responses, ideally below .05 μV or 50 nV (Don & Elberling, 1996). Normally, 2000-4000 averages were obtained, but a minimum of 1000 averages were accepted if the waveform was clearly present within an expected latency region, correlations were high and residual noise was low. In addition, the waveforms were examined by a second audiologist experienced in ABR measurements to concur with the presence and latency of marked waveforms. If the threshold interpretation was not in agreement, the next higher intensity marked was used for Wave V threshold. It is possible that lower thresholds could have been achieved with longer averaging, but these criteria were designed as a reasonable balance between time and accuracy, and to be clinically realistic for naturally sleeping infants.
Figure 1.
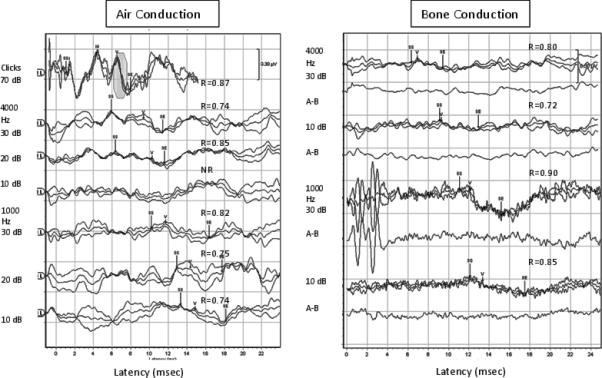
Typical Click and TB-ABR recording from the left ear of an infant aged 5 weeks for clicks, 4000 and 1000 Hz for AC (left panel) and BC (right panel). Correlation coefficients (R values) are shown above each waveform. Wave V is marked for each set of waveforms (rarefaction, condensation and alternating), along with the region used to calculate the correlations from peak to trough of Wave V, indicated by the Statistical Start (SS) and Statistical End (SE) markers before and after the onset of the Wave V component. Residual noise waveforms (A-B) are also shown below each set of BC recordings.
Statistical Analysis
Wave V threshold and latency values were analyzed for mean and standard deviation (SD), medians and percentiles to establish normative ranges. Median levels are helpful for clinical use because the 10 dB step size used for our testing and by many clinicians does not correspond with the mean levels, thus the median provides clinically-useful normative values. Previous studies have reported mean values, regardless of step size, so mean values were also calculated to allow comparison to published studies. Mean differences between the current study and published data were tested using the Student t-test represented as mean ± SD. Differences in median values were tested using a non-parametric Wilcoxon Rank Sum test. When available, raw data from the publications were used in the analysis, otherwise statistical comparisons were done using the published summary statistics. Because the limited number of multiple tests (multiple comparisons between published and study data across frequencies and intensities) introduced a risk of a Type I error, statistical significance for analyses with multiple tests was evaluated using a Bonferroni correction. Mean threshold differences between ears and gender were tested using the Student t-test as well. Since measurements were taken on the same infant/ear across frequencies, linear mixed models were used to assess changes across frequencies (repeated measures – within-subject variation) while testing for ear, gender, and age effects. Random effects were used to account for multiple measurements (multiple frequencies) on the same subject. Different covariance models were considered and compound symmetry was selected based on model fit. Separate models were constructed on air conduction and bone conduction measurements for both threshold and latency values. In the event of a statistically significant finding, an interaction term between frequency and that significant factor (e.g., frequency × gender) was tested in the model. Results were analyzed in the final model for ear, gender, age, and frequency.
RESULTS
Typical recordings of AC and BC TB-ABR for 1000 and 4000 Hz from an infant aged 5 weeks of age are shown in Figure 1. The intensity steps used for recording are shown, at 70 dB nHL for clicks and starting at 30 dB nHL for tonebursts. Wave V cross correlations (R) are shown for each waveform, along with the statistical start (SS) and statistical end (SE) used to calculate the correlations for Wave V. The SS and SE were placed manually prior to the onset of Wave V, to the offset (trough) for consistency. In each set of overlapped waveforms, separate polarity and alternating (combined) waveforms are shown. The stimulus artifact and cochlear microphonic (where present) can be seen within the first 1-2 msec of the recording. The neural components (Waves I, III, V) were marked if the correlation was adequate (R values are shown by each panel of responses). Residual noise recordings are also shown for BC recordings in order to show that responses obtained were not the result of myogenic or other noise.
For all ears included in the study, Figure 2 shows the mean and standard deviation for click ABR components, and Figure 3 shows box and whisker plots for AC and BC wave V thresholds at the octave test frequencies from 500 Hz to 4000 Hz. The box overlaps the whiskers at some frequencies in Figure 3 due to the non-normal distribution. Cumulative percentiles were constructed to determine normative ranges for each frequency, and are shown in Figures 4 and 5 for AC and BC, respectively. Functions were fit to determine optimal cut points for normal ABR threshold based on the 95th percentile. Normative threshold values recommended for clinical application, along with other descriptive statistics are provided in Table 2 for AC, BC and air-bone gap (ABG). The median ABG was 0 at all frequencies, and the 95th percentile was 20 dB at 500 and 1000 Hz, decreasing to 0 dB at 2000 Hz and 10 dB at 4000 Hz, validating the presence of normal hearing in this group of infants. Note that negative numbers in ABG indicate the BC threshold was worse than the AC threshold. The number of test ears at each frequency varied due to the protocol that tested first at 4000 and 1000 Hz, and then at other frequencies, depending on the time remaining and the infant state.
Figure 2.
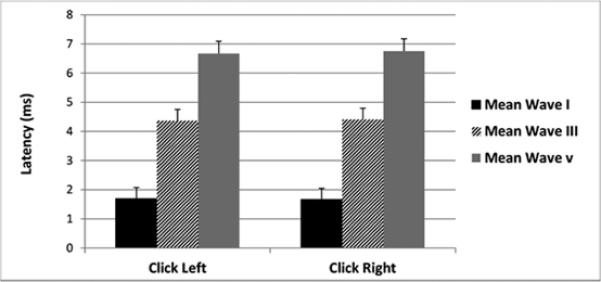
Click ABR at 70 dB nHL. The black bars represent the mean wave I latency, diagonal bars represent the mean wave III latency and the grey bars represent the mean wave III latency with standard deviation indicated above each bar.
Figure 3.
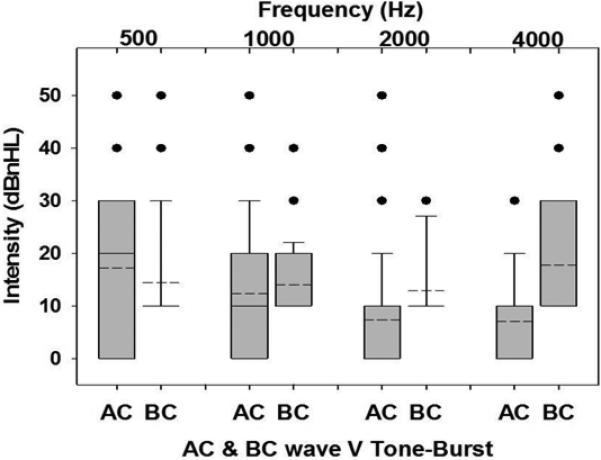
Box and whisker plots of the mean (dotted lines) and median (solid lines) for AC TBABR thresholds and BC TB-ABR thresholds. The gray bars represent the 25th to 75th percentiles. Whiskers of the plots are the 10th to 90th percentiles. The dots represent outliers. Note that at some frequencies, box and whiskers overlap due to non-normal distribution.
Figure 4.
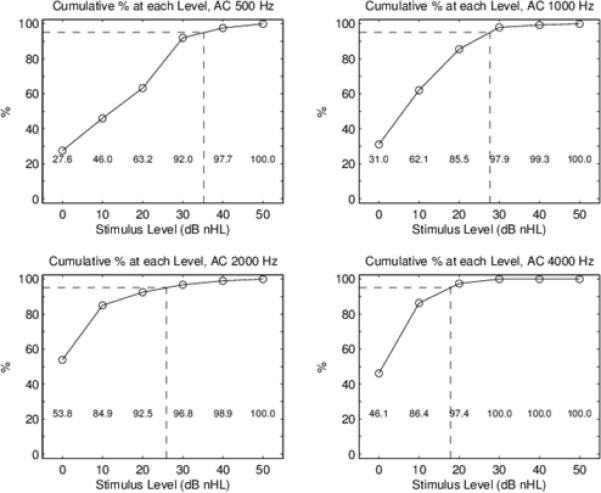
AC thresholds: Cumulative percentage of thresholds up to and including each stimulus level. This cumulative percentage is normalized by the number of test ears, which varies across the four TB frequencies. The numerical cumulative percent is shown in text at the bottom of each panel. The 95th percentiles are marked by the dotted line at each of the four TB frequencies to the nearest 5 dB.
Figure 5.
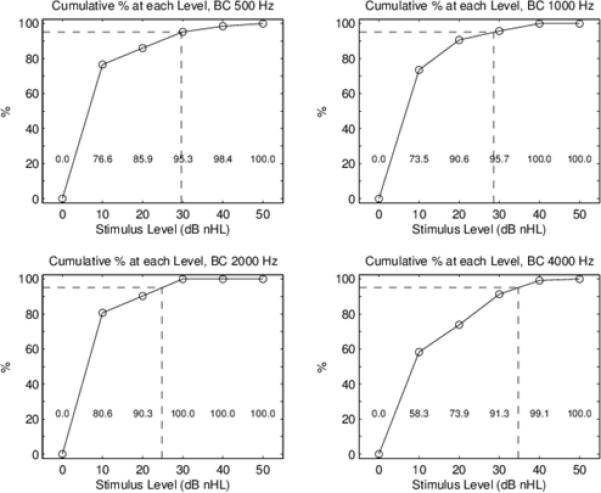
BC thresholds: Cumulative percentage of thresholds up to and including each stimulus level. This cumulative percentage is normalized by the number of test ears, which varies across the four TB frequencies. The numerical cumulative percent is shown in text at the bottom of each panel. The 95th percentiles are marked by the dotted line at each of the four TB frequencies to the nearest 5 dB.
Table 2.
Air conduction (AC) and bone conduction (BC) thresholds (dB nHL), and air bone gap (ABG) in dB for toneburst wave V thresholds. The number of ears tested varied with frequencies. The 95th percentile was extrapolated from cumulative distribution graphs to the nearest 5 dB value between adjacent levels at 10 dB increments.
| Frequency (Hz) | 500 | 1000 | 2000 | 4000 |
|---|---|---|---|---|
| Mean AC | 17.2 | 12.3 | 7.3 | 7.0 |
| Median AC | 20 | 10 | 0 | 10 |
| 95th percentile AC | 35 | 30 | 25 | 20 |
| No of ears | 86 | 144 | 93 | 154 |
| Mean BC | 14.4 | 14.0 | 12.9 | 17.7 |
| Median BC | 10 | 10 | 10 | 10 |
| 95th percentile BC | 30 | 30 | 25 | 35 |
| No of ears | 64 | 117 | 62 | 115 |
| Mean ABG | 2.36 | 1.71 | −1.38 | −6.88 |
| Median ABG | 0 | 0 | 0 | 0 |
| 95th percentile ABG | 20 | 20 | 0 | 10 |
Results of linear mixed models for AC are provided in Table 3. Wave V tone-burst thresholds had an overall significant decrease with increasing frequencies (p < 0.0001), as thresholds were better for higher frequencies. In contrast, bone conduction wave V tone-burst thresholds significantly increased (p = 0.003) with increasing frequencies, particularly at 4000 Hz. There were no significant effects of age, ear or gender on AC thresholds on AC or BC thresholds, thus no apparent need for separate normative data for ear or gender, or within the age range birth to 6 months. The latency of wave V was also analyzed in linear mixed models, as detailed in Table 3B. Wave V latency decreased with increasing stimulus intensity as expected between 10 and 30 dB nHL for both AC and BC tests. There was also a significant decrease in latency as expected for higher frequencies, due to shorter cochlear travel time to the basal regions tuned to higher frequencies. There were no significant age or ear effects on latency, but there was an effect of gender at the lowest intensity (10 dB nHL), with shorter latencies for females.
Table 3.
Statistical results for mixed models. A. Threshold models across frequencies for AC and BC wave V tonebursts.
| Frequency | Age Effects | Ear Effects | Gender | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | DF | F | p-value | DF | F | p-value | DF | F | p-value | DF | F | p-value |
| AC | 1,361 | 104.79 | <.0001 | 1,105 | 3.06 | 0.083 | 1,464 | 0.20 | 0.66 | 1,113 | 0.26 | 0.61 |
| BC | 1,213 | 8.93 | 0.003 | 1,61 | 1.13 | 0.29 | 1,246 | 0.14 | 0.71 | 1,67 | 1.36 | 0.25 |
| B. Latency models by intensity for AC and BC wave V. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Age Effects | Ear Effects | Gender | |||||||||
| Models | DF | F | p-value | DF | F | value | DF | F | value | DF | F | value |
| AC 10 dB nHL* | 1,172 | 632.51 | <.0001 | 1,81 | 1.62 | 0.21 | 1,189 | 1.39 | 0.24 | 1,90 | 8.63 | 0.004 |
| AC 30 dB nHL | 1,359 | 1817.81 | <.0001 | 1,10 | 3.72 | 0.057 | 1,392 | 1.10 | 0.30 | 1,109 | 5.13 | 0.026 |
| BC 10 dB nHL | 1,158 | 290.62 | <.0001 | 1,70 | 0.20 | 0.66 | 1,192 | 1.30 | 0.26 | 1,79 | 0.66 | 0.42 |
| BC 30 dB nHL | 1,243 | 630.75 | <.0001 | 1,80 | 0.10 | 0.76 | 1,226 | 0.01 | 0.92 | 1,91 | 2.81 | 0.10 |
Significant gender × frequency interaction noted. To account for 6 models, the Bonferroni corrected significance level is set at 0.0083.
The mean wave V thresholds for this study were compared with similar published studies and are shown in Figures 6 and 7 for AC and BC thresholds, respectively. Mean AC and BC thresholds were compared between the present study and Vander Werff et al. (2009) as shown in Table 4. This statistical analysis used the raw data from the appendix of Vander Werff et al (2009). Significant differences were found in both mean and median thresholds at 500, 2000 and 4000 Hz, with the present study showing lower (better) thresholds than Vander Werff et al. (2009) at all three frequencies for AC. A comparison at 1000 Hz was not possible since it was not studied in Vander Werff et al. (2009) or other previous studies. The comparison for BC thresholds (Fig 7) showed no difference at 500 Hz, while the current study found significantly better thresholds at 2000 Hz. BC thresholds in the present study at 1000 and 4000 Hz were not compared because of the lack of previously published normative data at these frequencies. It should be noted that the mean and SD calculated from raw data in the appendices from Vander Werff et al. (2009) are slightly different from the summary tabled values at 2000 and 4000 Hz for AC, but we used the raw data in order to calculate statistical significance. The differences in the summary and raw data for Vander Werrf et al. (2009) are less than 1 dB, so should not affect statistical comparisons with the present study.
Figure 6.
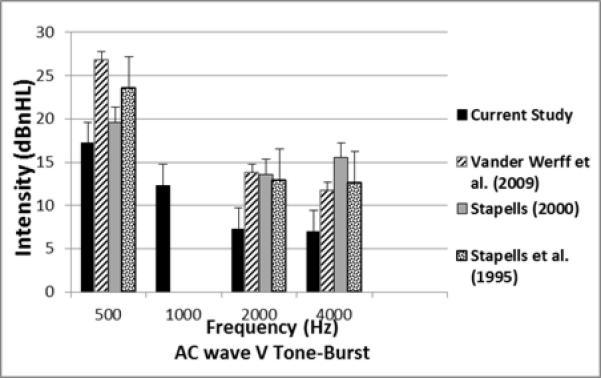
Comparison of the mean and SD for AC TB-ABR thresholds for the current study and previous studies in infants.
Figure 7.
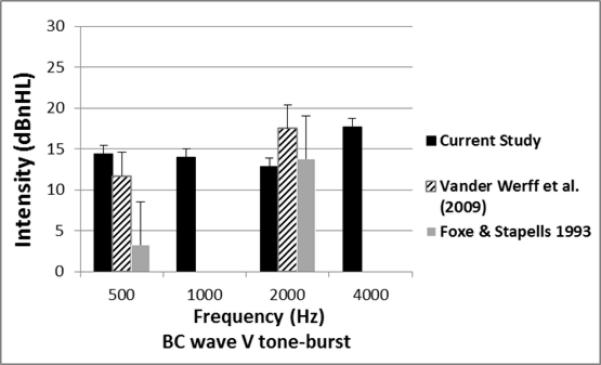
Comparison of the mean and SD for BC TB-ABR thresholds for the current study and previous studies in infants.
Table 4.
Comparison of mean wave V threshold for air conduction (AC) and bone conduction (BC) for the current study and Vander Werff et al. (2009). Standard deviations are in parentheses. All threshold values are expressed in dB nHL.
| AC thresholds (dB nHL) | Current Study | Vander Werff et al. (2009) | t value | p-value |
|---|---|---|---|---|
| 500 Hz | ||||
| Mean (SD) | 17.2 (14.0) | 26.8 (8.3) | −3.98 | <0.0001* |
| 95% CI around mean | 14.2-20.2 | 24.1-29.4 | ||
| Median (5th-95th percentile) | 20 (0-40) | 30 (20-40) | 0.0002* | |
| 2000 Hz | ||||
| Mean (SD) | 7.3 (10.4) | 13.8 (6.3) | −4.38 | <0.0001* |
| 95% CI around mean | 5.2-9.5 | 11.7-15.8 | ||
| Median (5th-95th percentile) | 0 (0-30) | 10 (0-20) | <0.0001* | |
| 4000 Hz | ||||
| Mean (SD) | 7.0 (7.7) | 11.7 (6.5) | −3.11 | 0.002* |
| 95% CI around mean | 5.8-8.2 | 9.2-14.1 | ||
| Median (5th-95th percentile) | 10 (0-20) | 10 (0-20) | 0.0007* |
| BC thresholds (dB nHL) | Current Study | Vander Werff et al. (2009) | t value | p-value |
|---|---|---|---|---|
| 500 Hz | ||||
| Mean (SD) | 14.4 (9.1) | 11.3 (7.9) | 1.79 | 0.08 |
| 95% CI around mean | 12.1-16.6 | 8.7-13.8 | ||
| Median (5th-95th percentile) | 10 (10-30) | 10 (0-30) | 0.15 | |
| 2000 Hz | ||||
| Mean (SD) | 12.9 (6.4) | 17.4 (7.2) | −3.32 | 0.001* |
| 95% CI around mean | 11.3-14.5 | 15.1-19.8 | ||
| Median (5th-95th percentile) | 10 (10-30) | 20 (10-30) | 0.0001* |
Statistically significant
The differences found in the current study, while statistically significant, were within the intensity step size of 10 dB. Differences in calibration values for the Vivosonic system and subject selection for other published studies may account for the differences found. While small differences exist, threshold responses obtained using the Kalman weighting software were within the 10 dB step size of other published studies using traditional signal averaging methods.
Mean AC and BC wave V latencies were also compared with latencies from the Vander Werff et al. (2009) study. Table 5 shows mean latency results from the present study along with the results of the statistical analysis comparing mean latencies in the two studies. For AC latencies, differences were not systematic; there were significant differences for 500 Hz at 20 dB, for 2000 Hz at 30 dB, and for 4000 Hz at 10 dB. Otherwise, mean latencies were similar in these studies, although the limited data at some frequencies and intensities reported by Vander Werff et al. (2009) precluded a direct statistical comparison. For BC latency comparisons (Table 5), significant differences were found at 500 for all intensities, with the present study showing slightly longer latency values. At 2000 Hz, a significantly longer latency was found in the present study only at 10 dB nHL. BC latencies were not compared at 1000 and 4000 Hz due to a lack of any previously published normative data at these frequencies.
Table 5.
Comparison of mean wave V latency for AC and BC (BC in the current study and Vander Werff et al. (2009). Standard deviations are in parentheses.
| AC | BC | |||||||
|---|---|---|---|---|---|---|---|---|
| Frequency (Hz) | 500 | 1000 | 2000 | 4000 | 500 | 1000 | 2000 | 4000 |
| Current Study | ||||||||
| Number of ears | 25 | 58 | 44 | 82 | 50 | 86 | 50 | 67 |
| 10 dB nHL | 15.3 (2.3) | 13.6 (1.7) | 11.0 (1.3) | 9.43 (0.7) | 15.92 (1.9) | 14.1 (1.7) | 12.84 (1.5) | 11.0 (2.1) |
| Number of ears | 15 | 34 | 7 | 19 | 6 | 20 | 6 | 19 |
| 20 dB nHL | 14.91 (0.95) | 12.9 (1.3) | 9.9 (0.5) | 9.3 (1.1) | 15.65 (1. 4) | 13.5 (1.8) | 12.1 (1.1) | 10.5 (1.6) |
| Number of ears | 80 | 137 | 91 | 151 | 57 | 108 | 61 | 103 |
| 30 dB nHL | 14.0 (1.8) | 11.5 (0.9) | 9.32 (0.9) | 8.2 (0.7) | 13.82 (1.8) | 11.96 (1.4) | 10.4 (1.0) | 8.99 (1.4) |
| Vander Werff et al. (2009) | ||||||||
| 10 dB nHL | 10.99 (0.87) | 9.86 (0.68) | 14.6 (0.9) | 12.0 (0.62) | ||||
| 20 dB nHL | 13.95 (1.13) | 10.35 (0.89) | 9.5 (1.07) | 13.2 (1.31) | 11.4 (0.84) | |||
| 30 dB nHL | 14.01 (1.19) | 10.04 (0.61) | 11.9 (1.69) | 10.9 (0.66) | ||||
Statistically Significant
p=0.006
p<0.0001
p=0.002
p=0.001
p=0.0001
All latency values are in msec.
Sleep state may be related to ABR results, and was recorded in this study by the test audiologist as variable sleeping/awake in 58% of infants, sleeping in 27%, awake and moving in 5%, awake and fussy in 4%, and awake and quiet in 2%. Sleep state was not recorded in 4% of infants. Analysis of sleep state on threshold and latency values showed no significant effect on AC latency at 10 or 30 dB nHL (p = 0.21 and p = 0.92, respectively). For BC, a significant effect was found for sleep state latency at 10 dB HL (awake and fussy), with slightly poorer latency values compared to other sleep states (p = 0.0055). However, there was no effect of sleep state on BC at 30 dB nHL (p = 0.25).
DISCUSSION
In order to achieve high reliability and timely detection of congenital hearing loss, clinical diagnostic tools that are optimized for infant hearing screening follow-up are critical. ABR threshold testing can provide the audiologist with valuable information about an infant's sensorineural hearing loss to initiate treatment with amplification if frequency-specific stimuli are employed. The overall goal of this study was to improve the accuracy of hearing screening in infants by developing a clinical standard for diagnostic TB-ABR thresholds in naturally sleeping infants. This goal was achieved by measuring thresholds for air and bone conduction at four octave intervals between 500-4000 Hz. Previous studies have not provided normative data across the frequency range for both AC and BC stimuli using a clinically applicable protocol. Additionally, previous studies have not included a normative sample of infants who passed newborn hearing screening and a follow-up comparison test, which was a DPOAE test in this study. Strengths of this study included a longitudinal, prospective design to acquire data in a large sample of infants enrolled and screened in both normal nurseries and NICUs. ABR testing was performed at both screening hospital and outpatient follow-up visits. The study population was diverse and reflective of Ohio demographic characteristics.
The 95th percentile are recommended as normative cut-off for ABR thresholds at each frequency, and are similar to those recommended . For AC, the normal cut-off value decreases with frequency, from 35 dB nHL at 500 Hz to 20 dB nHL at 4000 Hz. To decrease test time, AC stimulus levels might initially start at 30 dB nHL for stimuli between 1000-4000 Hz, and decrease in a 10 dB step size. If responses were present at both levels within the expected latency range, the ABR would be considered normal. Testing at 1000 and 4000 Hz provides assessment across the most important audiometric range for speech perception. Responses at these frequencies are generally more defined and less prone to interference from myogenic activity than at 500 Hz, and they reveal the presence and configuration of both conductive and/or sensorineural hearing loss. We found that testing at 1000 and 4000 Hz is a time-efficient procedure to identify ears at risk for hearing loss. Moreover, the presence of Wave V is often more difficult to ascertain at 500 Hz due to the broad, shallow morphology characteristics at that frequency. Clinical cut-offs for BC Wave V threshold suggested from this study are 30 dB at 500 Hz, 30 dB at 1000 Hz, 25 dB at 2000 Hz, and 35 dB at 4000 Hz. On comparing mean AC and BC thresholds, BC thresholds were better than AC thresholds at 500 Hz, similar at 1000 and 2000 Hz, and worse at 4000 Hz.
Use of BC was found to be highly feasible in this study, and just as reliable as AC in terms of cross-correlation values and residual noise (shown for a typical case in Fig. 1). Two pilot methods were tested for applying the BC transducer to the infant's head. In the initial phases of the study, we tried using an elastic headband with a Velcro fastener, and experimented with several types, including a bone-anchored hearing aid (BAHA) soft band to hold the B71 transducer. The elastic headband methods proved to be difficult to manage, as they would slip off the infant's head when enough pressure was applied to achieve adequate force, as measured using a force transducer. We switched to hand-holding the transducer, based on previous research that has reported acceptable reliability with hand-held measurements (Small Hatton & Stapells, 2007). We found a distinct advantage in that the examiner could feel if the transducer was slipping at any point in order to pause averaging.
In a sidearm procedural study, thresholds were measured with the hand held method in 12 infants in two conditions, with and without measurement of force using a calibrated force transducer designed by Etymotic Research (Elk Grove Village, IL). Wave V thresholds were first measured without using the force transducer, and then measured using the force transducer. No significant differences in mean thresholds were found at 1000 or 4000 Hz. We found the hand held technique to be just as accurate and more convenient, in which either the audiologist or a trained assistant held the transducer firmly at the temporal bone above the ear with a fingertip.
No effect of age, ear or gender was found on wave V thresholds, suggesting that normative values may be collapsed. While no significant effect of risk factors was found, the small NICU sample size precluded any firm conclusions about normal ranges in high-risk infants. There were no significant effects of ageor gender on latency, except for gender at 10 dB nHL.
Four previous published studies have reported normative TB-ABR thresholds in the infant age group. The age range was 1 week to 8 years in Stapells et al. (1995), 2 weeks to 13 months in Foxe & Stapells (1993), and less than 2 years in Stapells (2000). Similar to our study, Vander Werff et al. (2009) measured the ABR thresholds in normal hearing infants less than three months old. As depicted in Figures 6 and 7, our AC and BC results showed slightly lower (better) mean thresholds at the three frequencies studied compared to Vander Werff et al. (2009). These mean threshold differences may be explained in part by our subject population, who all passed NHS and follow-up DPOAE testing. In addition, the Vivosonic reference calibration values were higher than the comparative studies.
Comparisons for latency across a range of frequency and intensity were similar in our study for AC-TB at most intensities, and were generally consistent with trends from a previous study that measured TB-ABRs with a Bio-Logic system (Vander Werrf et al., 2009). A similar comparison was performed for BC TB-ABR latencies measured at 500 and 2000 Hz. Significantly longer latency differences at 500 Hz were found for intensities from 10-30 dB nHL. Thus, there may be an impact of the weighting algorithm for lower frequency stimuli, but this would require a direct comparison with and without weighting to determine. The fact that latency values were generally similar to those reported by Vander Werff et al. (2009) for most intensity levels is evidence for similarity in auditory and neural function between the populations in the two studies, and demonstrates that calibration values are unlikely to explain the threshold differences we found, since higher intensity stimuli would result in shorter latencies, not longer.
This study used a conservative approach for determining the presence of Wave V, in which a high cross-correlation, low residual noise level, and agreement in data interpretation between two audiologists were required. While it is not clinically feasible to require agreement between two audiologists for every recording, training and quality control could ideally include this approach for a subset of recordings. Use of online correlational analysis and examining the residual noise are highly desirable quality controls for clinical reliability, and might be considered when selecting equipment for clinical use.
In pilot testing, we measured thresholds in the NICU environment infants for click and TB stimuli were compared using the Bio-Logic and Vivosonic systems. Slightly better thresholds were measured for clicks and TB stimuli at 500 and 1000 Hz for the Vivosonic system reported here compared to published studies in other systems. Inter-test agreement in the pilot testing showed click thresholds within 10 dB for 15/20 ears (75% agreement), and thresholds of 10-20 dB better in the Vivosonic system in 5/20 ears (25%). No thresholds were higher with the Vivosonic system.
A primary claim of the manufacturer of the Vivosonic Integrity system is the ability to achieve adequate recordings in awake infants. While it is always ideal to record in sleeping or sedated infants, the results of this study showed the ability to successfully record thresholds in awake quiet infants when an adequate sleep was not achieved. In the fussy state, recordings were paused until the infant stopped crying; thus, the majority of recordings were in variable awake/sleeping infants. Notably, threshold and latency values were not significantly affected by sleep state, except at 10 dB for BC latency.
CONCLUSIONS
This study measured normative values in young infants for AC and BC TB-ABR thresholds, as well as for the ABG for Wave V. Low intensity thresholds were successfully obtained for masked BC with a hand-held bone vibrator over the temporal bone in infants. The large normal sample size tested across frequencies (500 to 4000 Hz) is a strength of the study and estimates the distribution of responses expected in a normal population that passed NHS and were tested under typical clinical conditions in natural sleep. We found that clicks at 70 dB nHL and AC-BC TB-ABR thresholds for both ears at 1000 and 4000 Hz could be completed in nearly all cases regardless of sleep state. The results of this study using a Vivosonic system for latency-intensity functions were similar to previous studies using a Bio-Logic system for 500, 2000 and 4000 Hz. The primary advantage of the Vivosonic system was the demonstrated ability to successfully measure AC and BC thresholds in both ears at multiple frequencies in lightly sleeping or awake infants in less than 1.5 hours, including time for explanations, feeding, OAE, and wideband immittance.
ACKNOWLEDGMENTS
We are grateful to the families and infants who participated in our study. We would like to thank Candice Dixon, Cassandra Guarneros, Jennifer Wright, Samantha Gustafson, and Erin Hegner, who assisted in enrollment and infant assessment. Thanks as well to 3 reviewers who provided many helpful comments.
Research reported in this publication was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the United States Government.
The Vivosonic Integrity system was provided on loan by Vivosonic, Inc., Toronto, ON, Canada.
REFERENCES
- ASHA, American-Speech-Language-Hearing Association Guidelines for the Audiologic Assessment of Children from Birth to 5 Years of Age. 2004 [PubMed] [Google Scholar]
- Baldwin M, Watkin P. Predicting the Degree of Hearing Loss Using Click Auditory Brainstem Response in Babies Referred From Newborn Hearing Screening. Ear Hear. 2013;34:361–69. doi: 10.1097/AUD.0b013e3182728b88. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) National Center on Birth Defects and Developmental Disabilities [Feb 11, 2014];2011 from http://www.cdc.gov/ncbddd/hearingloss/ehdi-history.html.
- Don M, Elberling C. Use of quantitative measures of auditory brain-stem response peak amplitude and residual background noise in the decision to stop averaging. J Acoust Soc Am. 1996;99:491–9. doi: 10.1121/1.414560. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Stapells DR. Normal infant and adult auditory brainstem responses to bone-conducted tones. Audiology. 1993;32:95–109. doi: 10.3109/00206099309071860. [DOI] [PubMed] [Google Scholar]
- Galambos R, Hecox KE. Clinical applications of the auditory brain stem response. Otolaryngol Clin North Am. 1978;11(3):709–722. [PubMed] [Google Scholar]
- Galambos R, Hicks GE, Wilson MJ. The auditory brain stem response reliably predicts hearing loss in graduates of a tertiary intensive care nursery. Ear Hear. 1984;5(4):254–260. doi: 10.1097/00003446-198407000-00011. [DOI] [PubMed] [Google Scholar]
- Georgiadis SD, Ranta-aho PO, Tarvainen MP, Karjalainen PA, IEEE Trans Biomed Eng. Single-trial dynamical estimation of event-related potentials: a Kalman filter-based approach. Aug. 2005;52(8):1397–406. doi: 10.1109/TBME.2005.851506. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinial application of multivariate analysis of distortion product otoacoustioc emission data. Ear and Hearing. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Johnson TA, Kaminski JR, Beauchaine KL, Garner CA, Neely ST. Using a combination of click- and tone burst-evoked auditory brain stem response measurements to estimate pure-tone thresholds. Ear Hear. 2006;27(1):60–74. doi: 10.1097/01.aud.0000194511.14740.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton JL, Janssen RM, Stapells DR. Auditory brainstem responses to bone-conducted brief tones in young children with conductive or sensorineural hearing loss. Intl J or Otolaryngol. 2012 doi: 10.1155/2012/284864. [Electronically published, Sept. 4, 2012]. DOI: 10.1155/2012/284864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M, Roush J. Age of suspicion, identification, and intervention for infants and young children with hearing loss: a national study. Ear Hear. 1996;17(1):55–62. doi: 10.1097/00003446-199602000-00007. [DOI] [PubMed] [Google Scholar]
- JCIH, Joint Committee on Infant Hearing Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- Lee CY, Hsieh TH, Pan SL, Hsu CJ. Thresholds of tone burst auditory brainstem for infants and young children with normal hearing in Taiwan. Journal of the Formosan Medical Association. 2007;106:847–53. doi: 10.1016/S0929-6646(08)60050-9. [DOI] [PubMed] [Google Scholar]
- Lee CY, Jaw FS, Pan SL, Hsieh TH, Hsu CJ. Effects of age and degree of hearing loss on the agreement and correlation between sound field audiometric thresholds and tone-burst auditory brainstem response thresholds in infants and young children. Journal of the Formosan Medical Association. 2008;107:869–75. doi: 10.1016/S0929-6646(08)60203-X. [DOI] [PubMed] [Google Scholar]
- Leski JM, Member IEEE. Robust weighted averaging. Trans Biomed Eng. 2002;49:796–804. doi: 10.1109/TBME.2002.800757. [DOI] [PubMed] [Google Scholar]
- Marcoux A, Kurtz I. Noise reduction to achieve quality ABR measurement. Canadian Hearing Report. 2012;8(3):19–23. [Google Scholar]
- Margolis RH, Bass-Ringdahl S, Hanks WD, Holte L, Zapala DA. Tympanometry in newborn infants: 1 kHz norms. J Am Acad Audiol. 2003;14:383–92. [PubMed] [Google Scholar]
- Mason S, McCormick B, Wood S. Auditory brainstem response in paediatric audiology. Arch Dis Child. 1988;63(5):465–467. doi: 10.1136/adc.63.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Tomlin D, Rickards F. Comparison of auditory steady-state responses and tone-burst auditory brainstem responses in normal babies. Ear and Hearing. 2006;27:751–762. doi: 10.1097/01.aud.0000240491.68218.ed. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Carvallo RM. Tone-evoked ABR in full-term and preterm neonats with normal hearing. International Journal of Audiology. 2008;47:21–29. doi: 10.1080/14992020701643800. [DOI] [PubMed] [Google Scholar]
- Ross DS, Holstrum WJ, Gaffney M, Green D, Oyler RF, Gravel JS. Hearing screening and diagnostic evaluation of children with unilateral and mild bilateral hearing loss. Trends Amplif. 2008;12(1):27–34. doi: 10.1177/1084713807306241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman-Galambos C, Galambos R. Brain stem evoked response audiometry in newborn hearing screening. Arch Otolaryngol. 1979;105(2):86–90. doi: 10.1001/archotol.1979.00790140032006. [DOI] [PubMed] [Google Scholar]
- Sokolov Y, Kurtz I, Sokolova O, Steinman A, Mahon C. Non-sedated ABR evaluation.. Paper presented at the EHDI Conference; New Orleans, LA. 2008. Available from: http://www.infanthearing.org/meeting/ehdi2008/topical%205/sokolov1%20session%205.pdf. [Google Scholar]
- Small S, Hatton J, Stapells D. Effects of bone oscillator coupling, placement location, and occlusion on bone-conduction auditory steady-state responses in infants. Ear Hear. 2007;28(1):83–98. doi: 10.1097/01.aud.0000249787.97957.5b. [DOI] [PubMed] [Google Scholar]
- Stapells DR. Threshold estimation by the tone-evoked auditory brainstem response: a literature meta-analysis. Journal of Speech Language Pathology and Audiology. 2000;24:74–83. [Google Scholar]
- Stapells DR, Oates P. Estimation of the pure-tone audiogram by the auditory brainstem response: A review. Audiology and Neurotology. 1997;2:257–280. doi: 10.1159/000259252. [DOI] [PubMed] [Google Scholar]
- Stapells D. Frequency-specific threshold assessment in young infants usig the transient ABR and the brainstem ASSR. In: Seewald R, Tharpe AM, editors. Comprehensive Handbook of Pediatric Audiology. Plural Publishing; San Diego: 2011. pp. 409–448. [Google Scholar]
- Stapells DR, Gravel JS, Martin BA. Thresholds for Auditory Brain Stem Responses to Tones in Notched Noise from Infants and Young-Children with Normal-Hearing or Sensorineural Hearing-Loss. Ear and Hearing. 1995;16(4):361–371. doi: 10.1097/00003446-199508000-00003. [DOI] [PubMed] [Google Scholar]
- Stuart A, Yang EY, Stenstrom R. Effect of temporal area bone vibrator placement on auditory brain stem response in newborn infants. Ear Hear. 1990;11:363–369. doi: 10.1097/00003446-199010000-00007. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Prieve BA, Georgantas LM. Infant air and bone conduction tone burst auditory brain stem responses for classification of hearing loss and the relationship to behavioral thresholds. Ear Hear. 2009;30(3):350–368. doi: 10.1097/AUD.0b013e31819f3145. [DOI] [PubMed] [Google Scholar]
- Watkin P, Baldwin M. The longitudinal follow up of a universal neonatal hearing screen: the implications for confirming deafness in childhood. Int J Audiol. 2012;51:519–28. doi: 10.3109/14992027.2012.673237. [DOI] [PubMed] [Google Scholar]
- White KR. The current status of EHDI programs in the United States. Ment Retard Dev Disabil Res Rev. 2008;9(2):70–88. doi: 10.1002/mrdd.10063. [DOI] [PubMed] [Google Scholar]
- Yang EY, Rupert AL, Moushegian G. A developmental study of bone conduction auditory brain stem response in infants. Ear Hear. 1987;12:55–60. doi: 10.1097/00003446-198708000-00009. [DOI] [PubMed] [Google Scholar]
- Yang EY, Stuart A, Stenstrom R, Green WB. Test-retest variability of the auditory brainstem response to bone-conducted clicks in newborn infants. Audiology. 1993a;32:89–94. doi: 10.3109/00206099309071859. [DOI] [PubMed] [Google Scholar]
- Yang EY, Stuart A, Mencher GT, Mencher LS, Vincer MJ. Auditory brain stem responses to air- and bone-conducted clicks in the audiological assessment of at-risk infants. Ear Hear. 1993b;14:175–82. doi: 10.1097/00003446-199306000-00004. [DOI] [PubMed] [Google Scholar]


