Abstract
Background
The role of single-agent nab-paclitaxel in relapsed or platinum-refractory advanced non-small cell lung cancer (NSCLC) has not been well reported in Western populations. We reviewed our own institution's experience using nab-paclitaxel in these settings.
Patients and Methods
We analyzed the records of stage IV NSCLC patients with relapsed or platinum-refractory disease treated with single-agent nab-paclitaxel at Weill Cornell Medical College between October 2008 and December 2013. The primary endpoint of the study was treatment failure-free survival (TFFS), defined as the time from the start of nab-paclitaxel therapy to discontinuation of the drug for any reason. The best overall response was recorded for each patient and overall response and disease control rates were calculated.
Results
Thirty-one stage IV NSCLC patients received a median of 4 cycles (range 1-40) of nab-paclitaxel. Dose reduction or drug discontinuation due to toxicity occurred in 10 patients, mainly because of grade 2/3 fatigue or peripheral neuropathy. The overall response rate was 16.1% and the disease control rate was 64.5%. Median TFFS was 3.5 months (95% CI = 1.3-5.3 months). No statistically significant difference in TFFS based on line of therapy or prior taxane exposure was identified. There was a statistically significant decrease in TFFS for patients with non-adenocarcinoma histology, although there were only 5 patients in this group. There was a trend toward reduction in the risk of treatment failure with increasing age. One patient remained on nab-paclitaxel therapy for over 3 years.
Conclusions
Single-agent nab-paclitaxel was well tolerated and demonstrated efficacy in advanced NSCLC patients with relapsed or platinum-refractory disease. Further prospective clinical trials with nab-paclitaxel in these settings are warranted.
Keywords: Carcinoma, Non-Small-Cell Lung, Taxane, 130-nm albumin-bound paclitaxel, Neoplasm Recurrence, Treatment Failure
1. Background
Lung Cancer is the leading cause of cancer mortality worldwide, accounting for approximately 1.4 million deaths per year[1]. About 85% of cases are non-small cell lung cancer (NSCLC) and 40% of these patients will present with metastatic disease[2, 3]. In the front-line setting, the standard of care treatment is platinum-based doublet chemotherapy[4]. Standard 2nd/3rd line treatment typically involves the use of single agents given sequentially. Current options in this setting include pemetrexed, erlotinib, anti-PD-1 antibodies, and the taxane drug docetaxel with or without the VEGFR2 antibody ramucirumab[5].
Paclitaxel is a mitotic inhibitor that has poor solubility and is typically dissolved in Cremophor El as a delivery vehicle. However, Cremophor is associated with several toxicities such as hypersensitivity reactions, severe anaphylaxis, hyperlipidemia, and peripheral neuropathy[6]. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel, Abraxane® [Celgene Corporation, Summit, NJ, USA]) is a formulation of paclitaxel which is not dissolved in Cremophor EL, reducing many of the toxicities associated with this solvent. This formulation also potentially increases the delivery of paclitaxel into tumor cells via the endogenous albumin pathways[7, 8].
Nab-paclitaxel was first approved in the United States for the treatment of metastatic breast cancer after failure of combination chemotherapy, based on the results of the phase III CA012 trial[9]. Efficacy has also been reported in the front-line treatment of advanced NSCLC, both as a single agent[8, 10] and in combination with other agents[11-13]. CA031 was a randomized phase III trial that compared carboplatin plus nab-paclitaxel with carboplatin plus solvent-based paclitaxel as frontline chemotherapy in patients with advanced stage NSCLC. The carboplatin plus nab-paclitaxel arm had a significantly higher overall response rate vs the carboplatin plus solvent-based paclitaxel arm (33% vs 25% respectively, p = 0.005), which was the primary endpoint of the trial. A trend towards improvement in both progression-free and overall survival was also identified in the nab-paclitaxel containing arm[13]. This trial lead to approval by the FDA of nab-paclitaxel for the first-line treatment of advanced NSCLC in combination with carboplatin.
While several studies have examined the efficacy of nab-paclitaxel for the initial management of advanced NSCLC, studies on the use of this drug as a single agent in the second-line setting and beyond have predominantly been done in East Asia[14-16]. Limited data therefore exists for nab-paclitaxel when used as a single agent in later lines of therapy in Western populations. Here we report our institution's experience using single-agent nab-paclitaxel in patients with relapsed or platinum-refractory advanced NSCLC.
2. Patients and Methods
2.1 Study Population
This study was approved by the Institutional Review Board of Weill Cornell Medical College (protocol number: 1112012070). After obtaining approval we analyzed the records of patients with Stage IV pathologically-confirmed NSCLC treated with single-agent nab-paclitaxel (Abraxane®) in the outpatient Thoracic Oncology Clinic at Weill Cornell Medical College between October 2008 and December 2013. Lung cancer staging was determined based on the American Joint Committee on Cancer Manual for Staging of Cancer[17]. Patients included in the analysis had received at least one line of systemic therapy (either platinum-based chemotherapy or erlotinib) for Stage IV NSCLC prior to receiving nab-paclitaxel and received at least one dose of nab-paclitaxel prior to censoring of the data. Patients were considered platinum-refractory if they demonstrated radiographic evidence of disease progression at the time of their first imaging scan while on platinum-based chemotherapy, which occurred after receiving 2-3 cycles and could be a CT or PET-CT scan. Patient data included age, sex, Karnofsky Performance Status (KPS), histology (as determined by pathological review at our institution), line of systemic therapy for stage IV disease, prior taxane exposure, number of cycles of nab-paclitaxel administered, dose adjustments, toxicity leading to dose adjustments, treatment response, and treatment failure-free survival (TFFS) estimated by Kaplan-Meier analysis.
2.2 Treatment and Follow-up Evaluations
Standard dosing of nab-paclitaxel was defined as 260 mg/m2 IV every 21 days. However, some patients initiated therapy at reduced doses of 230mg/m2 IV or 200mg/m2 IV every 21 days, based on the clinical judgment of the treating oncologist. No pre-medications were given prior to administration of nab-paclitaxel and each dose was infused over 30 minutes. Dose and schedule reductions could be made at any point following the first cycle of therapy due to the development of toxicities. These adjustments involved either an increase to 28 days between doses or a 20-25% dose reduction of nab-paclitaxel in conjunction with an increase to a 28-day cycle.
Patients underwent imaging every 6-9 weeks (CT scan of the measurable disease or PET-CT). When the dosing interval was increased or the patient remained on nab-paclitaxel therapy for more than 6 months, scans were performed every 12 weeks. Toxicities were graded according to the NCI's Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0[18].
Data were censored on December 31st, 2013, after 26 of the 31 patients discontinued treatment with nab-paclitaxel. TFFS was defined as the time between the start of nab-paclitaxel therapy and permanent discontinuation of the drug for any reason, including disease progression, symptom progression, reduced performance status, patient preference, treatment toxicity, or death resulting from any cause. The determination of overall response rate (ORR) and best overall response - complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) - were based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1[19], except for one patient with symptomatic progression of disease at the time of first imaging scan who was classified as PD despite stable findings radiographically.
2.3 Statistical Analysis
Descriptive statistics (including mean, standard deviation, median, range, frequency, and percent) were calculated to characterize the study cohort. The primary endpoint of the study was TFFS as defined above. TFFS was expressed in months by dividing the value in days by 30.41. Univariate associations between treatment/prognostic variables and TFFS were assessed by the log-rank test. Univariate hazards ratios for the association between continuous prognostic variables (i.e., age) and TFFS were estimated by univariable Cox proportional hazards regression analysis. Ninety-five percent confidence intervals (95% CI) for hazard ratios and median treatment failure-free survival time estimates were calculated to assess the precision of the obtained estimates. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in Stata Version 13.0 (StataCorp, College Station, TX).
3. Results
3.1 Patient Characteristics
Thirty-one patients with Stage IV NSCLC received single-agent nab-paclitaxel between October 2008 and October 2013. All patients had previously received platinum-based doublet chemotherapy for Stage IV disease. Patient characteristics are summarized in Table 1. Median patient age was 69 years, range 48 to 83. The vast majority had a KPS of 70-80 and had adenocarcinoma histology. Five patients were platinum-refractory and for each of these patients nab-paclitaxel was the next line of therapy. Five of the 31 patients had EGFR activating mutation positive disease and 5 were treated with erlotinib as first-line therapy. Forty-two percent of the patients had received only one prior line of systemic therapy before starting nab-paclitaxel, while 35% had received 2 prior lines and 23% had received 3. Twenty-three percent of patients had received taxane therapy previously, and 77% were taxane-naïve.
Table 1.
Characteristics of Patients Receiving Single-Agent nab-Paclitaxel
| Characteristic | Number of Patients (%) |
|---|---|
| N = 31 | |
| Age (Median Age = 69; Range = 48-83) | |
| <70 | 18 (58) |
| >/= 70 | 13 (42) |
| Sex | |
| Female | 15 (48) |
| Male | 16 (52) |
| Karnofsky Performance Status | |
| 50 | 1 (3) |
| 60 | 2 (6) |
| 70 | 16 (52) |
| 80 | 11 (35) |
| 90 | 1 (3) |
| Histology | |
| Adenocarcinoma | 26 (84) |
| Squamous Cell Carcinoma | 3 (10) |
| Large Cell Carcinoma | 1 (3) |
| Mixed Squamous Cell and Adenocarcinoma | 1 (3) |
| EGFR Mutation | |
| Tested | 13 (42) |
| Mutation Positive | 5 (38) |
| Received Erlotinib First-Line | 5 (16) |
| Refractory to Platinum-Based Chemotherapy | |
| Yes | 5 (16) |
| No | 26 (84) |
| Line of Systemic Therapy for Stage IV Disease | |
| 2nd | 13 (42) |
| 3rd | 11 (35) |
| 4th | 7 (23) |
| Received Prior Taxane | |
| Yes | 7 (23) |
| No | 24 (77) |
| Received Chemotherapy for Earlier Stage Disease | |
| Yes | 6 (19) |
| No | 25 (81) |
3.2 Nab-Paclitaxel Treatment
Fifteen of the 31 patients initiated nab-paclitaxel therapy at a dose of 260mg/m2 on day 1 of a 21-day cycle. Six started therapy with initial dosing of 230mg/m2 and 10 started at a dose of 200mg/m2 every 21 days. A total of 198 cycles of nab-paclitaxel were administered, with a median of 4 cycles (range 1-40) per patient (Table 2). No hypersensitivity reactions were seen amongst our patients.
Table 2.
Individual Data on Patients Receiving Single-Agent nab-Paclitaxel
| Patient # | Age | Sex | Histology | KPS | Chemotherapy For Earlier Stage Disease | Platinum-Refractory | Prior Taxane | Line of Therapy | Started Standard Dose | Dose Reduced | # of Cycles Given | Best Overall Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Male | AdenoCA | 80 | No | No | Yes | 4th | No | No | 3 | PD |
| 2 | 72 | Male | AdenoCA | 60 | No | No | No | 2nd | No | Yes | 40 | PR |
| 3 | 75 | Female | AdenoCA | 70 | No | No | No | 3rd | No | No | 19 | SD |
| 4 | 60 | Female | AdenoCA | 70 | Yes | No | No | 3rd | Yes | Yes | 11 | PR |
| 5 | 71 | Male | Mixeda | 70 | No | No | No | 2nd | Yes | No | 2 | PD |
| 6 | 67 | Female | AdenoCA | 80 | No | No | No | 3rd | Yes | No | 18 | SD |
| 7 | 71 | Female | AdenoCA | 70 | No | No | Yes | 4th | No | No | 9 | SD |
| 8 | 55 | Female | AdenoCA | 80 | Yes | No | Yes | 2nd | Yes | No | 3 | SD |
| 9 | 78 | Male | SCC | 70 | No | Yes | No | 2nd | No | No | 4 | SD |
| 10 | 82 | Female | SCC | 80 | No | No | No | 3rd | Yes | N/A | 1 | SD |
| 11 | 59 | Female | AdenoCA | 70 | Yes | No | Yes | 4th | Yes | N/A | 1 | PD |
| 12 | 70 | Male | AdenoCA | 70 | No | No | No | 2nd | Yes | No | 2 | PD |
| 13 | 48 | Male | AdenoCA | 70 | Yes | No | Yes | 2nd | Yes | Yes | 2 | PD |
| 14 | 61 | Male | AdenoCA | 80 | Yes | No | Yes | 4th | Yes | No | 5 | SD |
| 15 | 58 | Female | AdenoCA | 70 | No | No | No | 4th | Yes | No | 2 | PD |
| 16 | 79 | Female | Large Cell | 70 | Yes | Yes | Yes | 2nd | No | Yes | 6 | PR |
| 17 | 69 | Male | AdenoCA | 70 | No | No | No | 3rd | No | No | 6 | SD |
| 18 | 78 | Male | AdenoCA | 70 | No | Yes | No | 2nd | Yes | No | 2 | PD |
| 19 | 60 | Female | AdenoCA | 80 | No | No | No | 4th | No | No | 4 | SD |
| 20 | 57 | Female | AdenoCA | 70 | No | No | No | 3rd | Yes | No | 2 | PD |
| 21 | 66 | Female | AdenoCA | 80 | No | No | No | 2nd | No | Yes | 9 | SD |
| 22 | 65 | Female | AdenoCA | 90 | No | No | No | 3rd | Yes | No | 5 | SD |
| 23 | 56 | Female | AdenoCA | 70 | No | No | No | 2nd | No | No | 3 | PD |
| 24 | 81 | Male | AdenoCA | 80 | No | No | No | 2nd | No | No | 8 | SD |
| 25 | 69 | Male | SCC | 70 | No | No | No | 3rd | No | No | 2 | PD |
| 26 | 78 | Male | AdenoCA | 50 | No | No | No | 4th | No | Yes | 6 | SD |
| 27 | 80 | Male | AdenoCA | 80 | No | No | No | 2nd | No | No | 2 | PD |
| 28 | 61 | Female | AdenoCA | 80 | No | No | No | 2nd | Yes | No | 8 | PR |
| 29 | 83 | Male | AdenoCA | 80 | No | Yes | No | 3rd | No | No | 4 | SD |
| 30 | 69 | Male | AdenoCA | 60 | No | No | No | 3rd | No | Yes | 3 | SD |
| 31 | 60 | Male | AdenoCA | 70 | No | Yes | No | 3rd | Yes | Yes | 6 | PR |
Mixed = Mixed Adenocarcinoma and Squamous Cell Carcinoma
Abbreviations: AdenoCA = Adenocarcinoma; SCC = Squamous Cell Carcinoma; KPS = Karnofsky Performance Status; PR = Partial Response; SD = Stable Disease; PD = Progressive Disease; N/A = Not Applicable
Twenty-one patients discontinued nab-paclitaxel therapy because of disease progression identified on imaging. Two patients discontinued the drug after a single dose, one due to clinical deterioration and one due to grade 3 myalgias and arthralgias. One patient discontinued therapy after developing grade 3 peripheral sensory neuropathy after 4 cycles, and one discontinued therapy after being diagnosed with active tuberculosis. One patient was hospitalized with steroid-induced myopathy and died in the hospital. At the time of data censoring, 5 patients were alive and receiving nab-paclitaxel therapy, including one who had received 40 cycles.
3.3 Analysis of Outcomes
There were no complete responses observed. A partial response was identified in 5 patients (16.1%). Two of these 5 patients were platinum-refractory and one had known EGFR activating mutation positive disease. Fifteen patients (48.4%) demonstrated disease stability after at least one cycle of nab-paclitaxel and the remaining 11 patients (35.5%) had disease progression (Table 2). This translated into a disease control rate (PR + SD) of 64.5%. The seven patients with prior taxane exposure demonstrated an overall response rate (ORR) of 14.3% and a disease control rate (DCR) of 57.1%. Twenty-six patients with adenocarcinoma had an ORR of 15.4% and DCR of 65.4%. The ORR and DCR for the 3 patients with squamous cell carcinoma were 0% and 66.7%, respectively. Among the 5 patients with known EGFR activating mutations the DCR was 100%.
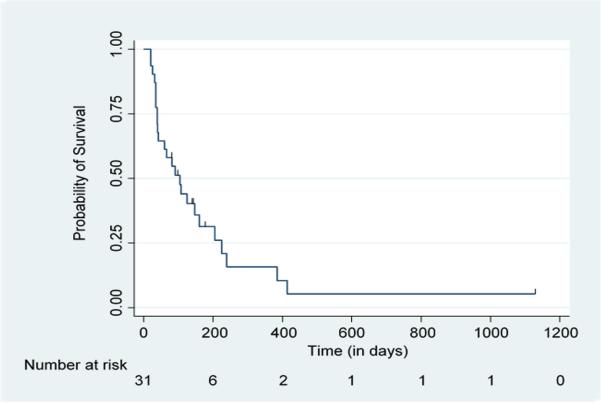
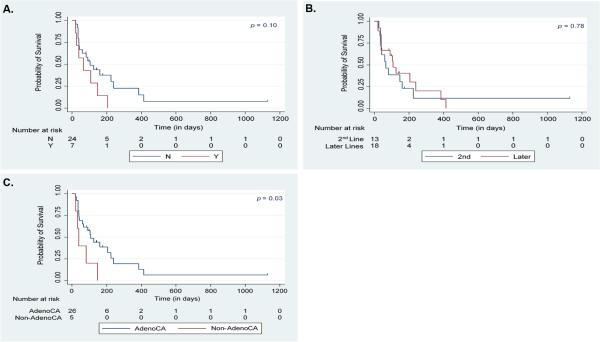
Table 3 shows the median TFFS for all patients in the study as well as for different subsets of patients. Median TFFS for all patients was 3.5 months [95% CI = 1.3-5.3 months] (Figure 1). There was a trend toward shorter TFFS with prior taxane exposure [median TFFS of 3.5 months without prior taxane vs. 2.2 months with prior taxane; p=0.10] (Figure 2A). No statistically significant difference in TFFS was identified for patients who received nab-paclitaxel as 2nd line therapy compared to those who received the drug beyond the 2nd line [median TFFS of 2.2 months vs. 3.6 months; p=0.78] (Figure 2B). Patients with adenocarcinoma had a longer TFFS compared to those who had non-adenocarcinoma histologies [median TFFS of 3.6 months vs. 1.3 months; p=0.03] (Figure 2C). There was a trend toward reduction in the risk of treatment failure with increasing age [hazard ratio=0.96 for each 1-year increase in age (95% CI = 0.91-1.01); p=0.09].
Table 3.
Median Treatment Failure-Free Survival for Patients Treated with nab-Paclitaxel
| Patient Characteristic | Number of Patients | Median Time to Treatment Failure (in Months) | p Value (by log-rank test) |
|---|---|---|---|
| All Patient | 31 | 3.5 (95% CI = 1.3 – 5.3) | |
| Prior Taxane | 7 | 2.2 (95% CI = 0.7 – 4.8) | 0.10 |
| No Prior Taxane | 24 | 3.5 (95% CI = 1.3 – 7.9) | |
| Nab-Paclitaxel as 2nd Line | 13 | 2.2 (95% CI = 1.3 – 5.3) | 0.78 |
| Nab-Paclitaxel beyond 2nd Line | 18 | 3.6 (95% CI = 1.2 – 7.9) | |
| Adenocarcinoma | 26 | 3.6 (95% CI = 1.4 – 7.4) | 0.03 |
| Non-Adenocarcinoma (Including Mixed Adenocarcinoma and Squamous Cell Carcinoma) | 5 | 1.3 (95% CI = 0.7 – not estimated) |
Figure 1. TFFS for All Patients.
Kaplan-Meier Analysis of Treatment Failure-Free Survival (TFFS) for All 31 Patients Treated with Nab-Paclitaxel for Recurrent or Platinum-Refractory Stage IV Non-Small Cell Lung Cancer (NSCLC)
Figure 2. TFFS for Patient Subsets.
Kaplan-Meier Analysis of Treatment Failure-Free Survival (TFFS) for Patients Treated with Nab-Paclitaxel for Recurrent or Platinum-Refractory Stage IV Non-Small Cell Lung Cancer (NSCLC) Based on A. Prior Taxane Exposure (N = No prior Taxane, Y = Prior Taxane); B. Line of Therapy; and C. Histology (AdenoCA = Pure Adenocarcinoma Histology)
Dose adjustments due to toxicity occurred in 8 patients (Table 4). The most common toxicities requiring dose adjustments were grade 2 or 3 fatigue and grade 2 peripheral sensory neuropathy. Other toxicities leading to dose adjustments included anemia and arthralgias/myalgias.
Table 4.
Toxicities for which Dose Adjustment or Drug Discontinuation Occurred
| Patient # | Starting Dose | Toxicities (Grade) | Dose Reduced To |
|---|---|---|---|
| 2 | 200mg/m2 every 21 days | Fatigue (Grade 2) | 200mg/m2 every 28 days |
| 4 | 260mg/m2 every 21 days | Facial Edema (Grade 2) | 260mg/m2 every 28 days |
| 9 | 200mg/m2 every 21 days | Peripheral Sensory Neuropathy (Grade 3) | Drug Discontinued |
| 10 | 260mg/m2 every 21 days | Arthralgias/Myalgias (Grade 3) | Drug Discontinued |
| 13 | 260mg/m2 every 21 days | Fatigue (Grade 3) | 200mg/m2 every 28 days |
| 16 | 200mg/m2 every 21 days | Fatigue (Grade
3) Arthralgias/Myalgias (Grade 2) Peripheral Sensory Neuropathy (Grade 2) |
160mg/m2 every 28 days |
| 21 | 200mg/m2 every 21 days | Peripheral Sensory Neuropathy (Grade 2) | 200mg/m2 every 28 days |
| 26 | 200mg/m2 every 21 days | Fatigue (Grade 2) | 150mg/m2 every 28 days |
| 30 | 230mg/m2 every 21 days | Anemia (Grade 2) | 230mg/m2 every 28 days |
| 31 | 260mg/m2 every 21 days | Fatigue (Grade 2) Peripheral Sensory Neuropathy (Grade 2) |
200mg/m2 every 28 days |
4. Discussion
Nab-paclitaxel is well tolerated and efficacious in the front-line treatment of advanced NSCLC, both as a single agent and in combination with carboplatin[8, 10, 13]. However, patients with this disease inevitably progress after initial therapy and subsequent treatment options are needed, especially as pemetrexed is more commonly used in the front-line setting. Nab-paclitaxel can add to the now growing armamentarium of agents effective in later lines of therapy.
In studies of single-agent nab-paclitaxel as initial therapy for metastatic NSCLC, a DCR of 50% was reported, with a median time to progression of 5-6 months[8, 10]. The DCR in our study was 64.5% for patients who received nab-paclitaxel in second-line therapy or later, with a median TFFS of 3.5 months. Although a direct comparison cannot be made, the TFFS of 3.5 months seen in our study is similar to the median progression free survival (PFS) of 2.9 months reported with use of pemetrexed or docetaxel as second-line treatment[20]. The median PFS for erlotinib as a single agent in the second or third line setting is 2.2 months[21] and the anti-PD-1 antibodies nivolumab and pembrolizumab have median progression-free survivals ranging from 2.3–3.7 months when used after font-line therapy in populations that include both PD-L1 positive and negative tumors[22-24]. The ORR of nab-paclitaxel in our study was 16%, comparable to the 7-9% response rates reported for docetaxel, pemetrexed, and erlotinib when used as monotherapy after front-line, platinum-based chemotherapy[20, 21]. Our data therefore suggest that nab-paclitaxel may have comparable activity to other single-agents used following progression on first-line therapy for NSCLC.
Our data is also consistent with that of similar single-institution studies from China which have also examined nab-paclitaxel as monotherapy for metastatic NSCLC following disease progression. A retrospective study by Xing et al of 21 patients treated with single-agent nab-paclitaxel for recurrent, advanced NSCLC found an ORR of 28.6% and a DCR of 76.2%. The PFS was 4 months, similar to the 3.5 month TFFS found in our group of US patients[14]. The results of a single-arm, prospective phase II trial of 56 patients in China with stage IIIB/IV NSCLC treated with single agent nab-paclitaxel as second-line therapy at 100mg/m2 on days 1, 8, and 15 of a 28-day cycle was reported by Hu et al[15]. This trial showed an ORR of 16.1% and a median PFS of 3.5 months, again comparable to the results of our study. Finally, a phase II trial by Liu et al randomized 111 advanced NSCLC patients treated at the General Hospital of Chinese PLA to second-line therapy with either nab-paclitaxel at 150mg/m2 on days 1 and 8 of a 21-day cycle or standard pemetrexed at 500mg/m2 on day 1 of a 21-day cycle. The ORR in the nab-paclitaxel arm was 14.5% with a disease control rate of 65.5%. The median PFS was 5.1 months for nab-paclitaxel and 4.6 months for pemetrexed, with the difference not being statistically significant[16]. Together these data as well as those presented in our study provide evidence for the efficacy of nab-paclitaxel beyond front-line therapy in advanced NSCLC and suggest that Eastern and Western populations may respond similarly to the drug in this setting.
Patients in our study tolerated nab-paclitaxel well as salvage therapy, consistent with other reports showing a favorable toxicity profile for the drug as a single agent[8, 14-16, 25]. A phase I/II trial by Rizvi et al of nab-paclitaxel given weekly at 125mg/m2 for the front-line treatment of advanced NSCLC reported neutropenia, leukopenia, sensory neuropathy, and fatigue as the most common grade 3/4 toxicities[10]. A study by Green et al using 260mg/m2 of nab-paclitaxel every 21 days also reported similar grade 3 toxicities, as well as a small percentage of grade 3 arthralgias[8]. The main grade 3 or 4 toxicities reported in the retrospective study by Xing et al also included neutropenia, peripheral neuropathy, myalgias/arthralgias, and fatigue[14], while the prospective trials done in China reported dizziness, fatigue, pulmonary embolism, nausea, fever, anemia, and thrombocytopenia as the most commonly seen grade 3 or 4 adverse events[15, 16]. Twenty-six percent of the patients in our study required dose adjustment because of toxicity, mostly grade 2/3 fatigue and grade 2 peripheral sensory neuropathy. Interestingly, dose adjustments in our study were not more frequent in patients who initiated therapy with the standard dose and schedule of 260mg/m2 every 21 days. Four patients in our study (13%) had nab-paclitaxel either dose adjusted or discontinued because of peripheral neuropathy, a common toxicity in many studies with nab-paclitaxel[26]. There is evidence from other studies, however, that nab-paclitaxel is associated with less peripheral neuropathy than solvent-based paclitaxel. In an analysis of patient- and physician-reported neuropathy symptoms in the CA031 phase III trial of first-line therapy for NSCLC, solvent-based paclitaxel plus carboplatin appeared to be more neurotoxic compared to nab-paclitaxel plus carboplatin[27].
In a phase I study of single-agent nab-paclitaxel for the treatment of several different advanced solid tumors in a population of Japanese patients who were refractory to or ineligible for standard treatment, 50% of patients with NSCLC had a partial response to the drug and two-thirds of those that responded had received prior taxane therapy[25]. In our study there was a trend toward shorter TFFS with prior taxane therapy that did not meet statistical significance. We also found no statistically significant difference in TFFS when nab-paclitaxel was used as second-line treatment vs third or fourth-line therapy. Together, these data suggest that nab-paclitaxel may be an appropriate single-agent therapy in advanced recurrent or platinum-refractory NSCLC regardless of prior taxane exposure or number of prior lines of therapy received.
Our study also showed a trend toward reduction in the risk of treatment failure with increasing age. An exploratory analysis of patients in the CA031 trial who were above age 70 demonstrated a near doubling of median overall survival with carboplatin plus nab-paclitaxel versus carboplatin plus solvent-based paclitaxel. There was no difference in overall survival between these two arms in the subset of patients less than 70 years old and there was no statistically significant difference in PFS between the two arms in either age group[28]. Both retrospective and prospective studies done in East Asia which specifically involved patients 65 years old or greater who were treated with single-agent nab-paclitaxel beyond the front-line setting have shown this drug to be safe and efficacious in this elderly patient population[29, 30].
Patients with squamous cell histology in the CA031 trial demonstrated a statistically significantly higher response rate in the arm containing nab-paclitaxel vs solvent-based paclitaxel. For patients with other histologies, the difference in response rate was not statistically significant. The authors concluded that first-line carboplatin plus nab-paclitaxel has a favorable risk-benefit profile in NSCLC patients regardless of tumor histology[31]. In our study, there were only 5 patients who did not have pure adenocarcinoma histology. We therefore cannot draw meaningful conclusions from our study regarding differential efficacy of nab-paclitaxel based on tumor histology.
5. Conclusion
Further treatment options for patients with advanced NSCLC who progress after or are refractory to standard platinum-doublet chemotherapy would be beneficial. Our data suggest that single-agent nab-paclitaxel can be a good therapeutic option for these patients. This drug can be efficacious in these settings and is well tolerated. Our data also indicate that nab-paclitaxel may be an appropriate treatment option even in patients who have previously been exposed to taxanes or received multiple lines of therapy. The results of our study are in line with similar single-institution trials done in East Asian countries and suggest that nab-paclitaxel may be equally beneficial for Western populations. Further formal prospective phase II and phase III trials to evaluate this drug in the recurrent/refractory setting and compare it to other standard therapies are warranted. These studies may also better delineate whether specific subgroups, such as elderly patients or those with specific histologies or mutations, could derive particular benefit from nab-paclitaxel, as well as what the optimal dosing of the drug in this setting would be.
Acknowledgements
Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06).
Footnotes
Declaration of Competing Interests
Dr. Ashish Saxena declares that he has no competing interests.
Dr. Bryan J. Schneider receives research funding from Celgene Corporation. This funding was not used for the conduction of this study, for the analysis or interpretation of the data presented, or for the writing of this manuscript.
Dr. Paul J. Christos declares that he has no competing interests.
Lauren Audibert declares that she has no competing interests.
Jennifer M. Cagney declares that she has no competing interests.
Dr. Ronald J. Scheff is on the Speaker's Bureau for Celgene Corporation. Celgene Corporation had no involvement in the study design, in the collection, analysis, or interpretation of data, in writing of this manuscript, or in the decision to submit this article for publication.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic proceedingsMayo Clinic. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 4.Cufer T, Ovcaricek T, O'Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. European journal of cancer (Oxford, England : 1990) 2013;49:1216–1225. doi: 10.1016/j.ejca.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 5. [November 29, 2015];Network NCC NCCN Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer (Vesion 2.2016) Available from http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 6.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. European journal of cancer (Oxford, England : 1990) 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 7.Edelman MJ. Novel cytotoxic agents for non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1:752–755. [PubMed] [Google Scholar]
- 8.Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 9.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi NA, Riely GJ, Azzoli CG, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:639–643. doi: 10.1200/JCO.2007.10.8605. [DOI] [PubMed] [Google Scholar]
- 11.Stinchcombe TE, Socinski MA, Lee CB, et al. Phase I trial of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with thoracic malignancies. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:521–526. doi: 10.1097/JTO.0b013e31816de2a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds C, Barrera D, Jotte R, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2009;4:1537–1543. doi: 10.1097/JTO.0b013e3181c0a2f4. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 14.Xing PY, Li JL, Wang Y, et al. Efficacy and safety of albumin-bound paclitaxel in treating recurrent advanced non-small-cell lung cancer. Chin J Cancer Res. 2013;25:200–205. doi: 10.3978/j.issn.1000-9604.2013.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu W, Zhang Z. A phase II clinical study of using nab-paclitaxel as second-line chemotherapy for Chinese patients with advanced non-small cell lung cancer. Med Oncol. 2015;32:498. doi: 10.1007/s12032-015-0498-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Wei Z, Hu Y, et al. A phase II open-label clinical study of comparing nab-paclitaxel with pemetrexed as second-line chemotherapy for patients with stage IIIB/IV non-small-cell lung cancer. Med Oncol. 2015;32:216. doi: 10.1007/s12032-015-0660-5. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. Springer; New York: 2010. [Google Scholar]
- 18. [March 20, 2014];Institute NC Common terminology criteria for adverse events (CTCAE) (Version 4.0) Available from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 22.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Yamamoto N, Yamada Y, Mukohara T, Minami H, Tamura T. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Japanese journal of clinical oncology. 2010;40:404–411. doi: 10.1093/jjco/hyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik PK, James LP, Riely GJ, et al. A phase 2 study of weekly albumin-bound paclitaxel (Abraxane(R)) given as a two-hour infusion. Cancer chemotherapy and pharmacology. 2011;68:1331–1337. doi: 10.1007/s00280-011-1621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsh V, Okamoto I, Hon JK, et al. Patient-reported neuropathy and taxane-associated symptoms in a phase 3 trial of nab-paclitaxel plus carboplatin versus solvent-based paclitaxel plus carboplatin for advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:83–90. doi: 10.1097/JTO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 28.Socinski MA, Langer CJ, Okamoto I, et al. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2013;24:314–321. doi: 10.1093/annonc/mds461. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Q, Yao Y, Nan K. Weekly intravenous nanoparticle albumin-bound paclitaxel for elderly patients with stage IV non-small-cell lung cancer: a series of 20 cases. J Biomed Res. 2012;26:159–164. doi: 10.7555/JBR.26.20110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Huang X, Wang S, et al. Nab-paclitaxel (abraxane)-based chemotherapy to treat elderly patients with advanced non-small-cell lung cancer: a single center, randomized and open-label clinical trial. Chin J Cancer Res. 2015;27:190–196. doi: 10.3978/j.issn.1000-9604.2014.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socinski MA, Okamoto I, Hon JK, et al. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2013 doi: 10.1093/annonc/mdt235. [DOI] [PubMed] [Google Scholar]