Abstract
BACKGROUND: In northwest Nigeria in 2013 and 2014, two sequential, localized outbreaks of meningitis were caused by a new strain of Neisseria meningitidis serogroup C (NmC). In 2015, an outbreak caused by the same novel NmC strain occurred over a wider geographical area, displaying different characteristics to the previous outbreaks. We describe cases treated by Médecins Sans Frontières (MSF) in the 2015 outbreak.
METHODS: From February 10 to June 8, 2015, data on cerebrospinal meningitis (CSM) cases and deaths were recorded on standardized line-lists from case management sites supported by MSF. Cerebrospinal fluid (CSF) samples from suspected cases at the beginning of the outbreak and throughout from suspected cases from new geographical areas were tested using rapid Pastorex® latex agglutination to determine causative serogroup. A subset of CSF samples was also inoculated into Trans-Isolate medium for testing by the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo. Reactive vaccination campaigns with meningococcal ACWY polysaccharide vaccine targeted affected administrative wards.
RESULTS: A total of 6394 (65 confirmed and 6329 probable) cases of CSM including 321 deaths (case fatality rate: 5.0%) were recorded. The cumulative attack rate was 282 cases per 100,000 population in the wards affected. The outbreak lasted 17 weeks, affecting 1039 villages in 21 local government areas in three states (Kebbi, Sokoto, Niger). Pastorex® tests were NmC positive for 65 (58%) of 113 CSF samples. Of 31 Trans-Isolate medium samples, 26 (84%) tested positive for NmC (14 through culture and 12 through PCR); all had the same rare PorA type P1.21-15,16 as isolates from the 2013 and 2014 outbreaks. All 14 culture-positive samples yielded isolates of the same genotype (ST-10217 PorA type P1.21-15,16 and FetA type F1-7). More than 222,000 targeted individuals were vaccinated relatively early in the outbreak (administrative coverage estimates 98% and 89% in Kebbi and Sokoto, respectively).
CONCLUSIONS: The outbreak was the largest caused by NmC documented in Nigeria. Reactive vaccination in both states may have helped curtail the epidemic. A vaccination campaign against NmC with a long-lasting conjugate vaccine should be considered in the region.
Keywords: disease outbreak, Epidemiology, meningococcal disease, Nigeria
Background
Large-scale epidemics of invasive meningococcal meningitis in the African meningitis belt, a region of sub-Saharan Africa comprising 22 countries including Nigeria, have been attributed commonly to Neisseria meningitidis serogroup A (NmA), and less frequently to serogroups W and X.1 , 2 , 3 Epidemics in the meningitis belt occur in the dry season, which brings low humidity and dusty conditions and ends with the onset of rain.4 , 5 , 6 , 7 , 8 Since the introduction of the meningococcal A conjugate vaccine (MenAfriVac®), NmA cases have declined and NmA epidemics have been eliminated.9 However, in parallel, the proportion of cases and epidemics caused by other Nm serogroups such as W, X, and C has increased.
In 2013 and 2014, northwest Nigeria experienced two sequential outbreaks of meningitis in the adjacent Sokoto and Kebbi states caused by a new strain of N. meningitidis serogroup C (NmC).10 By contrast with NmA outbreaks, the NmC outbreaks were relatively localized and confined to small areas.11 , 12 In 2015, a meningitis outbreak occurred in the same region caused by the same novel NmC strain, but with different characteristics to the previous two outbreaks and wider geographic spread including a concurrent large outbreak in the neighbouring country of Niger where 8500 cases including 573 deaths were reported.13 , 14 We describe this unprecedented NmC outbreak in northwest Nigeria in the 2015 meningitis season.
Methods
Case Definition
The following case definition for cerebrospinal meningitis (CSM) was used throughout the outbreak.15
Suspected case of acute meningitis: sudden onset of fever with neck stiffness or petechial rash for adults and children over 1 year of age; sudden onset of fever with bulging fontanelle or petechial rash for children less than 1 year of age.
Probable case of acute meningitis: a suspected case (as defined above) within an ongoing CSM outbreak or with cloudy cerebrospinal fluid (CSF) with or without a Gram stain.
Confirmed case of acute meningitis: a suspected or probable case (as defined above) with positive CSF antigen detection via positive latex agglutination test or positive culture.
Data Collection
From February 10 to June 8, 2015, morbidity, mortality, and demographic data for CSM cases treated by MSF were recorded daily on a standardized line-list from each MSF supported case management site in Kebbi and Sokoto states. Only patients presenting for medical care at a case management site during this time period and who met the case definition were included on the line-list.
Within Kebbi and Sokoto states there are 21 and 23 local government areas (LGAs) respectively, and within each LGA there are approximately 10-15 wards, with variable population (estimated range 2,000-130,000). Population estimates for some affected wards in Kebbi state were provided by the Kebbi State Ministry of Health. All other population estimates were calculated using annual projections based on the most recent national census (2006). Weekly and overall incidence rates per 100,000 population were calculated using the combined population of all wards with cases from the start of the outbreak until that week.
The aggregated data analysed in this paper were collected as part of the routine activities that MSF has approval to conduct from the Ministries of Health. This work met the standards set by the independent MSF Ethics Review Board for retrospective analyses of routinely collected programmatic data.
Laboratory Methods
CSF samples were collected from suspected CSM cases at the beginning of the outbreak, as well as from suspected cases from new geographical areas during the outbreak. All samples were tested using the rapid Pastorex® (Bio-rad Laboratories USA) latex agglutination kit to determine the causative agent. Test kits were stored and transported at 2-8°C. Prior to usage, quality control tests on the kits were conducted.
A subset of CSF samples, being approximately 10 at the beginning of the outbreak and at least 3 from new areas during the course of the outbreak if practical, were also inoculated into Trans-Isolate medium16 within 1 hour of collection and sent to the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo for confirmation testing. Briefly, Gram staining and standard biochemical reactions were done to identify bacteria, followed by serogrouping of N. meningitidis strains using Remel, GA, USA commercial antisera. Antimicrobial susceptibility was determined using minimal inhibitory concentrations (MIC) and classified using the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Multilocus sequence typing (MLST) was done on each strain identified and compared to the MLST website. PCR analysis was done on culture-negative samples using QiAmp DNA mini kit (Qiagen) and analysed by real-time PCR for speciation followed by genogrouping. The PorA variant was determined by DNA sequencing of the porA gene using a nested porA-PCR.17 As part of routine practice, all cases who presented at an MSF-supported treatment site were tested for malaria infection using the rapid Paracheck-Pf® diagnostic test.
Data Analysis
Data from line-lists were entered weekly into an MSF standardized database in Microsoft Excel; quality checks and data validation were conducted weekly. Descriptive analysis including frequencies, summaries, and epidemic curves were produced using Microsoft Excel 2010.
Reactive Vaccination
In Kebbi state, MSF supported two rounds of reactive vaccinations with meningococcal ACWY polysaccharide vaccine targeting individuals aged 2-30 years in wards that were affected at the time of making the vaccine request to the International Coordinating Group (ICG) on Vaccine Provision for Epidemic Meningitis Control. A subsequent round was conducted solely by the Kebbi State Ministry of Health targeting 1-29 year olds in wards newly affected as the outbreak spread. In Sokoto, MSF supported one round of reactive vaccination with meningococcal ACWY polysaccharide vaccine targeting individuals aged 2-30 years in wards affected at the time of making the vaccine request to the ICG. The second round was conducted solely by the Sokoto State Ministry of Health targeting 1-29 year olds in newly affected wards. In this paper, vaccination results are only reported from MSF-supported vaccinations.
Results
Case Counts and Rates
Between February 10 and June 8, 2015, 6394 (65 confirmed and 6329 probable) cases of CSM were treated at MSF-supported treatment sites in Kebbi and Sokoto states (Table 1). The cumulative attack rate was 282 cases per 100,000 population in the affected wards. Treatment sites recorded 321 deaths, giving a case fatality rate (CFR) of 5.0%. Kebbi state treated the most patients, with 5714 cases (52 confirmed and 5662 probable) including 292 deaths (CFR: 5.0%), compared with Sokoto’s 680 cases (13 confirmed and 667 probable) including 29 deaths (CFR: 4.2%). However, the burden of illness was higher in the affected wards of Sokoto compared to Kebbi (attack rates: 317 vs. 279 cases per 100,000 population, respectively).
Table 1. Number, deaths, and rates of confirmed and probable CSM cases treated by MSF in Kebbi and Sokoto States, February 10 – June 8, 2015.
*Population figures are based on the most recent census of the affected wards that had at least one case.
| State | Population in Affected Wards* | Number of Confirmed Cases | Number of Probable Cases | Total Number of Cases | Cumulative Attack Rate (per 100,000 population) | Number of Deaths | Case Fatality Rate (%) |
|---|---|---|---|---|---|---|---|
| Kebbi | 2,049,883 | 52 | 5662 | 5714 | 279 | 292 | 5.1 |
| Sokoto | 214,782 | 13 | 667 | 680 | 317 | 29 | 4.3 |
| Total | 2,264,665 | 65 | 6329 | 6394 | 282 | 321 | 5.0 |
Case Demographics
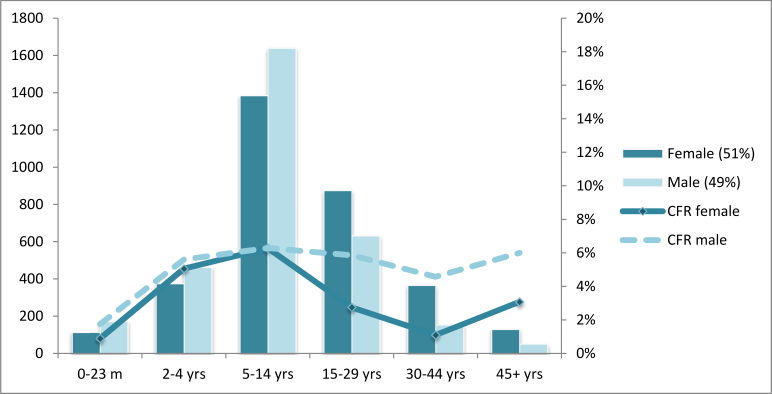
Of the 6394 cases treated, 3270 (51%) were female, 48% (3023/6354 [age missing for 40 cases]) were aged 5-14 years and 24% (1507) 15-29 years (Figure 1). Malaria co-infection was detected in 1358 (21%) cases. Of 4771 cases where immunization status for meningococcal meningitis C was recorded, 104 (2%) reported having received the meningococcal ACWY vaccine during the reactive vaccination campaign, of whom 88 (85%) presented their vaccination cards.
Total number of cases and case fatality rate (CFR) by age group and sex of CSM cases treated by MSF in Kebbi and Sokoto states, February 10 – June 8, 2015.
Total of 6354 cases with both age and sex recorded.
Outbreak Description and Spread
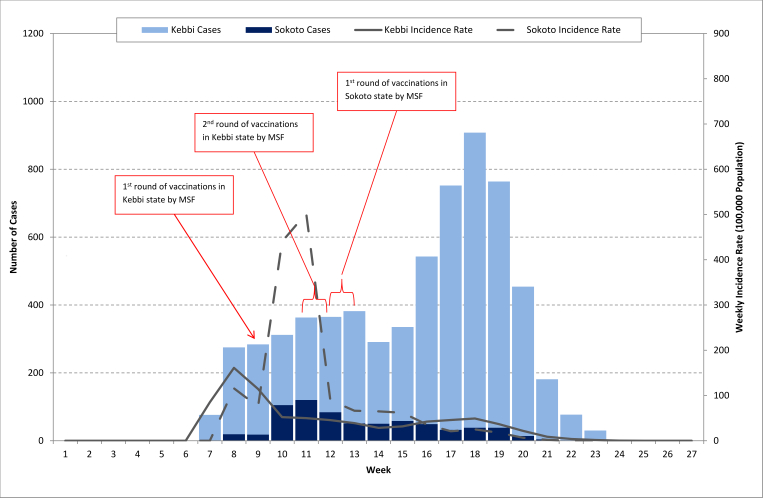
The outbreak started in epidemiological week 7 and lasted 17 weeks, with the highest number of cases (870; Figure 2) presenting for treatment by MSF in Kebbi state in week 18. This peak correlated with the opening of three more MSF-supported treatment sites in Kebbi. The epidemic threshold of 5 cases in one week in a localized area of population <30,00015 was first reached in a ward in Kebbi state in week 7 and a ward in Sokoto state in week 8. The incidence rate in the early-affected wards of Kebbi state peaked in week 9 and then remained relatively stable for the remainder of the outbreak. In Sokoto, the number of cases treated by MSF peaked in week 11 which also saw a large spike in incidence rate. However, this followed a rapid decline thereafter, and steadily decreased until the end of the outbreak. Both states saw a rapid decline in cases, particularly in Kebbi, from week 19, which closely followed the onset of rain in weeks 19 and 20.
Number and weekly incidence rate of CSM cases treated by MSF in Kebbi and Sokoto states, February 10 – June 8, 2015.
*Population estimates used to calculate incidence rates changed over the course of the outbreak as the population affected increased as the outbreak spread.
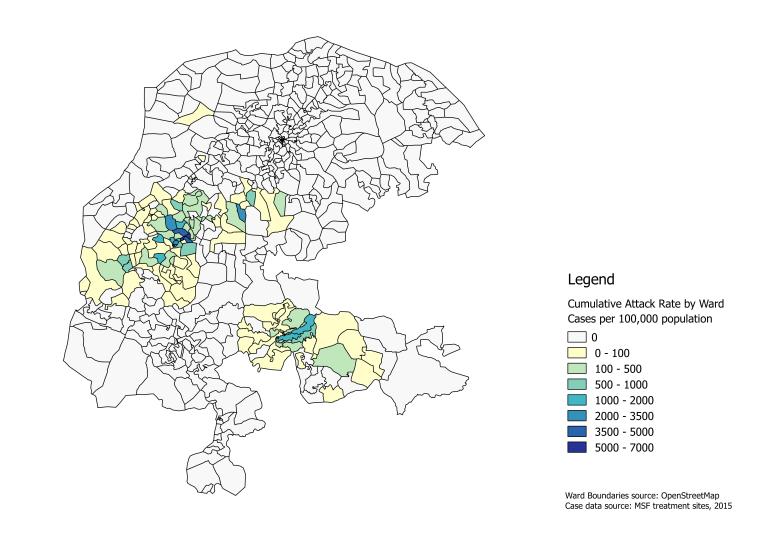
The outbreak spread over approximately 19,000km2, affecting 1039 villages within 113 wards in 21 LGAs in three states (Kebbi, Sokoto, and Niger) (Figure 3). The single case treated from Niger state was from a ward sharing a boundary with Kebbi.
Cumulative attack rate by ward for CSM cases treated by MSF in Kebbi and Sokoto states, February 10 – June 8, 2015, Nigeria.
Reactive Vaccination
In Kebbi state, MSF-supported reactive vaccinations were held in weeks 9, 11, and 12 in 16 wards within four LGAs. More than 140,000 targeted individuals were vaccinated, providing an administrative coverage estimate of 98%. In Sokoto, MSF-supported vaccinations were conducted in weeks 12 and 13 in 10 wards within four LGAs. More than 82,000 targeted individuals were vaccinated, providing an administrative coverage estimate of 89%.
Laboratory Results
Of 113 CSF samples tested using Pastorex®, 65 (58%) were positive for NmC (52 [80%] from Kebbi; 13 [20%] Sokoto) and 48 (42%) were negative for any causative bacteria. Of 31 Trans-Isolate medium samples, 26 (84%) tested positive for NmC (14 through culture and 12 through PCR) and none tested positive for any other causative bacteria. All 26 NmC positive samples exhibited meningococcal DNA with the same genetic characteristics as isolates collected from the 2013 and 2014 outbreaks. The 14 culture-positive isolates were ST-10217 PorA type P1.21-15,16 and FetA type F1-7, while the 12 PCR-positive samples harbored the porA gene coding for type P1.21-15,16. By state, 14/18 (78%) samples from Kebbi and 12/13 (92%) from Sokoto were culture and/or PCR positive for NmC (Table 2).
Table 2. Trans-Isolate test results conducted on CSF samples collected from patients treated by MSF in Kebbi and Sokoto states, by location, February 16 to March 26, 2015.
*All 26 samples had PorA type P1.21-15,16. The 14 culture-positive isolates had genetic sequencing of ST-10217 PorA type P1.21-15,16 and FetA type F1-7. **PCR stands for Real-time polymerase chain reaction.
| Kebbi State | Sokoto State | Total | |
|---|---|---|---|
| All Trans-Isolate Tests (Culture and PCR**) | |||
| Total number of CSF samples tested | 18 | 13 | 31 |
| Total number of positive NmC samples (%) | 14 (78%) | 12 (92%) | 26* (84%) |
| Culture | |||
| Number of CSF samples tested | 8 | 6 | 14 |
| Number of positive NmC samples (%) | 8 (100%) | 6 (100%) | 14 (100%) |
| PCR | |||
| Number of CSF samples tested | 10 | 7 | 17 |
| Number of positive NmC samples (%) | 6 (60%) | 6 (86%) | 21 (71%) |
Discussion
We report on the largest outbreak of NmC ever recorded in Nigeria with 6394 confirmed and probable cases treated by MSF. This is almost 4000 cases more than the 2670 in Nigeria during 2015 included in the WHO meningitis bulletin, which also showed 8500 meningitis cases in neighbouring Republic of Niger.18 Our data show the continued expansion of NmC in the meningitis belt, increasing justification for a regional mass vaccination campaign with a long-lasting conjugate vaccine in order to help curtail further spread of NmC. The 2015 outbreak in northwest Nigeria was caused by the same NmC strain as the 2013 and 2014 outbreaks but caused more cases over a wider geographical area in Nigeria. NmC of the same strain (personal communication, DAC) was also identified from cases in the large concurrent meningitis outbreak in the Republic of Niger. The globally unique strain of NmC was first identified in Nigeria in 2013, and a WHO expert group meeting recently concluded that there is a high risk of continuing expansion of meningococcal meningitis in the meningitis belt, partly because of the emergence of this strain.19
Case numbers in 2015 were five times greater than the previous two outbreaks combined, and almost ten times more villages were affected. The spread of this outbreak was both rapid and geographically extensive, mimicking characteristics historically seen with NmA outbreaks.11 , 12 The 2015 outbreak followed typical meningitis seasonal patterns in the African meningitis belt, in that it began in the dry season following the Harmattan winds and stopped at the onset of rain.4 , 5 , 6 , 7 , 20 Age and sex distribution of cases were also consistent with those typically seen with meningitis outbreaks, with the 5-14 followed by 15-29 year age groups being the most affected and with equal proportions of males and females.10
The proportion (46%) of negative results for the Pastorex® latex agglutination tests was quite similar to previous outbreaks (64% [2013] and 48% [2014], respectively). This may be due to patients not volunteering a history of home management with antibiotics prior to presentation or showing signs of meningitis from non-bacterial causes. Similar to the 2013 and 2014 outbreaks, none of the CSF samples tested positive for NmA, which may show the effectiveness of the MenAfriVac® mass vaccination against NmA in this region in 2012 and 2013.
It is unclear why this outbreak was far greater in magnitude and spread than the two previous outbreaks. One explanation could be that it started in an urban area, facilitating transmission to more people than in the previous outbreaks, which were confined to small rural settings. Environmental factors also influence the pattern of meningitis,4 , 5 , 6 , 7 , 8 , 20 , 21 with epidemic cessation in Africa related to the onset of rain.4 , 5 , 6 , 7 In 2015 the onset of rain occurred later (week 19) than in previous years; in 2013 and 2014, the onset of rain occurred in weeks 18 and 15, respectively. Social-cultural factors are also believed to influence transmission patterns of meningitis; hypothetically, such behaviours could have differed in 2015 compared with the previous two outbreak years,22 but this was not assessed. Finally, vaccination uptake in Muslim Hausa communities may have been affected by vaccinee-vaccinator gender differences and the cultural requirement for male head-of-household consent to women being vaccinated. All these factors could have influenced rapid and widespread transmission of this outbreak.
The 2015 outbreak season marked the first reactive vaccination campaign against NmC in northwest Nigeria. In 2014, requests for vaccines against NmC were made by the Kebbi State Ministry of Health but were not approved by the ICG. In 2015, over 222,000 at-risk individuals were vaccinated in certain affected wards relatively early in the outbreak, which likely changed its course. However, a vaccine effectiveness study is needed in order to properly determine its impact. Due to the magnitude and rapid spread of the outbreak, reactive vaccination was not available for all affected wards, nor were there enough vaccines to reach herd immunity in some locations. As such, during future meningitis epidemic seasons it will be important that these areas are monitored closely, along with other locations that did not receive vaccination.
Limitations
The data used to describe and analyze the description of these cases and the outbreak was based solely on individuals who presented to an MSF-supported case management facility and were treated by MSF. Complete data related to NmC cases treated at non-MSF supported facilities were not available, and thus, were excluded from analysis. Furthermore, additional suspected NmC cases are likely to have been undetected and unreported. However, the proportion of cases not included in our report is believed to be low as MSF provided treatment to a large proportion of the affected areas that were reporting cases. Nevertheless, incomplete case ascertainment may mean that the characteristics of the cases and the outbreak described in this report are not perfectly representative of the entire cohort of NmC cases. However, the characteristics of the cases in this outbreak were consistent with those in the two previous NmC outbreaks in the region. It is possible that some suspected or probable cases were due to a different causative organism, as was the case in the concurrent outbreak in the country of Niger where NmW was also isolated,14 , 18 however NmC was the only organism identified in CSF samples tested using Pastorex®, PCR or culture.
Conclusion and Recommendations
This outbreak was the largest caused by N. meningitidis serogroup C ever documented in this part of the meningitis belt in northwest Nigeria. Since meningococcal meningitis ACWY polysaccharide vaccine should provide protection for at least 2 to 3 years,23 , 24 it can be anticipated that the geographic spread and case numbers should be reduced in the next few meningitis seasons. Nonetheless, to help further curtail outbreaks of NmC, a vaccination campaign with a long-lasting conjugate vaccine similar to MenAfriVac® should be considered in the region.
Competing Interests
The authors have declared that no competing interests exist.
Data Availability Statement
The underlying data is provided as a supplementary table in the manuscript.
Supplementary Material
Table S1. Number, deaths and weekly incidence rate of CSM cases treated by MSF in Kebbi and Sokoto states, February 10 – June 8, 2015.
| Kebbi | Sokoto | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Epidemiological week | Cases (n) | Deaths (n) | Incidence | Cases (n) | Deaths (n) | Incidence | Cases (n) | Deaths (n) | Incidence |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 76 | 9 | 85 | 0 | 0 | 0 | 76 | 9 | 85 |
| 8 | 256 | 17 | 161 | 19 | 3 | 116 | 275 | 20 | 157 |
| 9 | 266 | 4 | 114 | 18 | 5 | 75 | 284 | 9 | 111 |
| 10 | 207 | 8 | 52 | 105 | 4 | 440 | 312 | 12 | 74 |
| 11 | 243 | 6 | 50 | 120 | 7 | 503 | 363 | 13 | 71 |
| 12 | 281 | 14 | 46 | 84 | 2 | 90 | 365 | 16 | 51 |
| 13 | 331 | 32 | 39 | 51 | 0 | 66 | 382 | 32 | 41 |
| 14 | 241 | 17 | 28 | 50 | 0 | 65 | 291 | 17 | 31 |
| 15 | 277 | 13 | 32 | 58 | 1 | 62 | 335 | 14 | 35 |
| 16 | 494 | 16 | 42 | 49 | 0 | 36 | 543 | 16 | 42 |
| 17 | 720 | 38 | 46 | 32 | 0 | 21 | 752 | 38 | 43 |
| 18 | 870 | 61 | 49 | 38 | 3 | 25 | 908 | 64 | 47 |
| 19 | 726 | 43 | 37 | 38 | 4 | 18 | 764 | 47 | 35 |
| 20 | 440 | 7 | 22 | 14 | 0 | 7 | 454 | 7 | 20 |
| 21 | 177 | 5 | 9 | 4 | 0 | 2 | 181 | 5 | 8 |
| 22 | 77 | 1 | 4 | 0 | 0 | 0 | 77 | 1 | 4 |
| 23 | 30 | 1 | 1 | 0 | 0 | 0 | 30 | 1 | 1 |
| 24 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 5714 | 292 | 279 | 680 | 29 | 317 | 6394 | 321 | 282 |
Table S2. Number, deaths and rates of CSM cases treated by MSF, by patient residence, February 10 – June 8, 2015
Acknowledgments
Many thanks to the members of the Nigeria Emergency Response Unit (NERU) of MSF, especially Titilope Osamika, Gillian Onions, Niall Holland, Peter Ndunga, Fredoh Macharia Mathenge, Dorothy Wuyep, Na Dickson, Nicole Desi, Davies Mpundu Mumba, Zubairu Ibrahim, Rik Vaassen, Dieudonne Kongolo Sango, Abubakar Umar, George Gonet, Chiabua S. Michael, all NERU drivers, and the many daily staff workers for their contributions to case management, surveillance, and reactive vaccination during this outbreak. Our appreciation also extends to Dr. Femi Odunsi, the assistant medical coordinator and Michelle Chouinard, the head of mission for their support during this 2015 outbreak. We would also like to acknowledge the Sokoto and Kebbi state Ministry of Health teams for their support, especially the public health directors Shehu Tureta (Sokoto) and Alhassan Kabiru (Kebbi) for their review of this paper. We thank Sarah Venis (MSF UK) for editing assistance and Holly Baker (MSF UK) for formatting assistance.
Biographies
I read medicine at the University of Benin, Benin City, Edo state, Nigeria and currently works as the Medical Team Leader of the Nigeria Emergency Response Unit (NERU) Project of Médecins sans Frontières (Doctors without Borders) - OCA Nigeria mission.
BSc.
Chief scientist at the Norwegian Institute of Public Health Head of the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo, Norway Professor in International Health
I graduated from the college of medical sciences University of Maiduguri Borno State Nigeria. Am the Director Disease control and Immunization with the state primary Health Care Development Agency Sokoto, Sokoto State Nigeria.
Funding Statement
This study was funded as part of MSF routine operations. The WHO Collaborating Centre for Reference and Research on Meningococci, Oslo funded sample transport media and laboratory analyses of the strains.
Contributor Information
Jaime Chow, Médecins sans Frontières, Sokoto, Nigeria.
Kennedy Uadiale, Nigeria Emergency Response Unit (NERU), Médecins sans Frontières, Sokoto, Nigeria.
Agatha Bestman, Médecins sans Frontières, Sokoto, Nigeria.
Charity Kamau, Médecins sans Frontières, Amsterdam, Netherlands.
Dominique A. Caugant, WHO Collaborating Centre for Reference and Research on Meningococci, Norwegian Institute of Public Health, Oslo, Norway
Aminu Shehu, State Primary Health Care Development Agency, State Ministry of Health, Sokoto, Nigeria.
Jane Greig, Manson Unit, Médecins Sans Frontières, London, United Kingdom.
References
- 1.Koutangni T, Boubacar Maïnassara H, Mueller JE. Incidence, carriage and case-carrier ratios for meningococcal meningitis in the African meningitis belt: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0116725. PubMed PMID:25658307. [DOI] [PMC free article] [PubMed]
- 2.Abio A, Neal KR, Beck CR. An epidemiological review of changes in meningococcal biology during the last 100 years. Pathog Glob Health. 2013 Oct;107(7):373-80. PubMed PMID:24392681. [DOI] [PMC free article] [PubMed]
- 3.Meningococcal disease in countries of the African meningitis belt, 2012 - emerging needs and future perspectives. Wkly Epidemiol Rec. 2013 Mar 22;88(12):129-36. PubMed PMID:23544241. [PubMed]
- 4.Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis. 2003 Oct;9(10):1287-93. PubMed PMID:14609465. [DOI] [PMC free article] [PubMed]
- 5.Pérez García-Pando C, Stanton MC, Diggle PJ, Trzaska S, Miller RL, Perlwitz JP, Baldasano JM, Cuevas E, Ceccato P, Yaka P, Thomson MC. Soil dust aerosols and wind as predictors of seasonal meningitis incidence in Niger. Environ Health Perspect. 2014 Jul;122(7):679-86. PubMed PMID:24633049. [DOI] [PMC free article] [PubMed]
- 6.Thomson MC, Molesworth AM, Djingarey MH, Yameogo KR, Belanger F, Cuevas LE. Potential of environmental models to predict meningitis epidemics in Africa. Trop Med Int Health. 2006 Jun;11(6):781-8. PubMed PMID:16771998. [DOI] [PubMed]
- 7.Sultan B, Labadi K, Guégan JF, Janicot S. Climate drives the meningitis epidemics onset in west Africa. PLoS Med. 2005 Jan;2(1):e6. PubMed PMID:15696216. [DOI] [PMC free article] [PubMed]
- 8.Yaka P, Sultan B, Broutin H, Janicot S, Philippon S, Fourquet N. Relationships between climate and year-to-year variability in meningitis outbreaks: a case study in Burkina Faso and Niger. Int J Health Geogr. 2008 Jul 2;7:34. PubMed PMID:18597686. [DOI] [PMC free article] [PubMed]
- 9.Meningococcal disease control in countries of the African meningitis belt, 2014. Wkly Epidemiol Rec. 2015 Mar 27;90(13):123-31. PubMed PMID:25816448. [PubMed]
- 10.Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013-14. PLoS Curr. 2014 Dec 29;6. PubMed PMID:25685621. [DOI] [PMC free article] [PubMed]
- 11.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009 Jun 24;27 Suppl 2:B51-63. PubMed PMID:19477562. [DOI] [PubMed]
- 12.Control of Epidemic Meningococcal Disease. WHO Practical Guidelines. Second edition. 1998.
- 13.Burki T. Meningitis outbreak in Niger is an urgent warning. Lancet Infect Dis. 2015 Sep;15(9):1011. PubMed PMID:26333332. [DOI] [PubMed]
- 14.World Health Organization. Meningococcal Disease – Niger (Update). Disease Outbreak News. 2015 Jul 23. Retrieved on September 24, 2015
- 15.Médecins Sans Frontières. (2008). Management of epidemic meningococcal meningitis – fourth edition.
- 16.Ajello GW, Feeley JC, Hayes PS, Reingold AL, Bolan G, Broome CV, Phillips CJ. Trans-isolate medium: a new medium for primary culturing and transport of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. J Clin Microbiol. 1984 Jul;20(1):55-8. PubMed PMID:6430956. [DOI] [PMC free article] [PubMed]
- 17.Caugant DA, Høiby EA, Frøholm LO, Brandtzaeg P. Polymerase chain reaction for case ascertainment of meningococcal meningitis: application to the cerebrospinal fluids collected in the course of the Norwegian meningococcal serogroup B protection trial. Scand J Infect Dis. 1996;28(2):149-53. PubMed PMID:8792481. [DOI] [PubMed]
- 18.World Health Organization Inter country support team – West Africa. Meningitis weekly bulletin December 2015. Retrieved on May 05, 2016
- 19.World Health Organization. Serogroup C in the Meningitis Belt: Facing the Challenge. Report of meeting held in Geneva, October 2015. Retrieved on June 14, 2016
- 20.Agier L, Deroubaix A, Martiny N, Yaka P, Djibo A, Broutin H. Seasonality of meningitis in Africa and climate forcing: aerosols stand out. J R Soc Interface. 2013 Feb;10(79):20120814. PubMed PMID:23221989. [DOI] [PMC free article] [PubMed]
- 21.Palmgren H. Meningococcal disease and climate. Glob Health Action. 2009 Nov 11;2. PubMed PMID:20052424. [DOI] [PMC free article] [PubMed]
- 22.Tobías A, Caylà JA, Pey J, Alastuey A, Querol X. Are Saharan dust intrusions increasing the risk of meningococcal meningitis? Int J Infect Dis. 2011 Jul;15(7):e503. PubMed PMID:21511508. [DOI] [PubMed]
- 23.U.S. Food and Drug Administration. Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined Menomune® – A/C/Y/W-135. Retrieved on September 28, 2015
- 24.World Health Organization. Meningococcal disease – vaccine. Retrieved on September 28, 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The underlying data is provided as a supplementary table in the manuscript.