Abstract
Endogenous opioids have complex social effects that may depend on specific receptor actions and vary depending on the “stage” of social behavior (e.g., seeking vs. responding to social stimuli). We tested the effects of a nonspecific opioid antagonist, naltrexone (NTX), on social processing in humans. NTX is used to treat alcohol and opiate dependence, and may affect both mu and kappa-opioid systems. We assessed attention (“seeking”), and subjective and psycho-physiological responses (“responding”) to positive and negative social stimuli. Based on literature suggesting mu-opioid blockade impairs positive social responses, we hypothesized that NTX would decrease responses to positive social stimuli. We also tested responses to negative stimuli, which might be either increased by NTX’s mu-opioid effects or decreased by its kappa-opioid effects. Thirty-four healthy volunteers received placebo, 25 mg, or 50 mg NTX across three sessions under double-blind conditions. At each session, participants completed measures of attention, identification, and emotional responses for emotional faces and scenes. NTX increased attention to emotional expressions, slowed identification of sadness and fear, and decreased ratings of arousal for social and nonsocial emotional scenes. These findings are more consistent with anxiolytic kappa-antagonist than mu-blocking effects, suggesting effects on kappa receptors may contribute to the clinical effects of NTX.
Keywords: Naltrexone, opiate system, social processes, psychophysiology, emotion perception
Endogenous opioids are thought to play an important but complex role in social behavior in mammals, yet there has been little research on the effect of opioid manipulations in humans. This is problematic, because drugs that act on the opioid system are routinely used in the treatment of pain, and alcohol and opioid abuse, all conditions that are strongly influenced by social factors (Havassy, Hall, & Wasserman, 1991; Lewis & O’Neill, 2000; López-Martínez, Esteve-Zarazaga, & Ramírez-Maestre, 2008; Rhoads, 1983; Warren, Stein, & Grella, 2007). Thus, it is critical to understand the effect of opioid antagonism on social processes in humans. Here, we examined the effect of naltrexone (NTX), a nonspecific opioid antagonist with actions at both mu and kappa-opioid sites (Ko, Butelman, Traynor, & Woods, 1998; Takemori & Portoghese, 1992), on social processes in humans. We tested the effect of NTX on both positive and negative social stimuli at two stages of social processing, seeking and responding to social stimuli. Secondarily, we assessed the effects of NTX on responses to emotional stimuli that contained either social or nonsocial content, to determine whether the effects of NTX were specific to social stimuli, or generalized to all emotional stimuli.
NTX is thought to most strongly antagonize mu-opioid receptors (Ko et al., 1998), a system that is involved in pleasure and pain in general (Berridge, Robinson, & Aldridge, 2009), but has also been thought to specifically underpin social pleasure and distress (Panksepp, Herman, Vilberg, Bishop, & DeEskinazi, 1981). Preclinical studies suggest mu-opioid antagonism affects both positive and aversive social experiences in complex ways. For example, mu-opioid antagonists increase solicitations for positive social contact, such as grooming in primates and the time animals spend in proximity (Fabre-Nys, Meller, & Keverne, 1982; Kalin, Shelton, & Barksdale, 1988; Martel, Nevison, Simpson, & Keverne, 1995; Schino & Troisi, 1992), but paradoxically impair the ability of social contact to reduce distress vocalizations when animals are reunited after separations and decrease spontaneous play behaviors in rodents (Panksepp et al., 1981; Trezza, Baarendse, & Vanderschuren, 2010; Trezza, Damsteegt, Achterberg, & Vanderschuren, 2011). There are several theories to explain these findings. One posits that mu antagonism not only creates a “social deficit” that increases seeking of positive social contact, but also impairs pleasure responses to that social contact when it is obtained (Panksepp et al., 1981). Another model, the state-dependent μ-opioid modulation of social motivation (SOMSOM), argues that an animal’s initial motivational state may moderate opioid agonist effects (Loseth, Ellingsen, & Leknes, 2014). According to the SOMSOM model, opioid antagonists will produce indifference to social stimuli when animals are in a “state of comfort” (e.g., a non-socially deprived state), but will increase social seeking when animals are in a “state of distress” (e.g., after having been socially isolated, particularly in more social species such as primates). Regardless, it appears to be clear that the mu antagonism produced by NTX may alter social responses in humans in important ways that might depend on the stage of social behavior (seeking vs. responding to social stimuli), or initial motivational state (comfortable vs. distressed).
In addition to mu receptors, NTX also antagonizes kappa-opioid receptors, and studies in laboratory animals suggest a distinct and partially opposing role for kappa-opioid receptors in social behavior. Kappa antagonists are believed to have antidepressant or anxiolytic effects (Bruijnzeel, 2009; Carr et al., 2010; Carr & Lucki, 2010; Van’t Veer & Carlezon, 2013), and in line with this, kappa antagonism appears to dampen responses to distressing social stimuli. For example, in prairie voles, kappa antagonism blocks selective aggression toward unfamiliar animals, or “aversive social motivation” (Resendez & Aragona, 2013; Resendez, Kuhnmuench, Krzywosinski, & Aragona, 2012), and in mice reduces time spent in submissive postures during “social defeat” (McLaughlin, Li, Valdez, Chavkin, & Chavkin, 2005), suggesting blunting of responses to stressful social stimuli. Thus, the kappa effects of NTX might also be active in humans, potentially particularly in response to negative social stimuli.
The limited research that has been done using NTX in humans has largely demonstrated effects consistent with those of mu-opioid antagonism in animals. Several studies have examined the effect of NTX on nonsocial rewards, such as food (Arbisi, Billington, & Levine, 1999; Fantino, Hosotte, & Apfelbaum, 1986; Green et al., 2013; Langleben, Busch, O’Brien, & Elman, 2012; Murray et al., 2014; Yeomans & Gray, 1996, 1997) and gambling wins (Petrovic et al., 2008). Consistent with the proposed broader role of the mu-opiate system in pleasure (Berridge et al., 2009), these studies suggest that NTX dampens positive responses to nonsocial rewards. Only three studies have addressed the effects of NTX on positive social stimuli, specifically feelings after positive autobiographical memories combined with film clips (Schweiger, Stemmler, Burgdorf, & Wacker, 2013), feelings of social connection engendered by holding a warm object (Inagaki, Irwin, & Eisenberger, 2015), and “liking” and “wanting” responses to attractive faces (Chelnokova et al., 2014). In these three cases, NTX reduced feelings of social “warmth” and “liking”, again consistent with expected effects of mu-opioid antagonism on responses to positive social stimuli.
However, there are some key gaps in the literature on NTX in humans that this study seeks to address. First, to our knowledge, no studies have examined the impact of NTX on both positive and negative social stimuli. This is important because NTX might affect responses to negative social stimuli also and, depending on whether mu or kappa effects predominate, might influence them in very different directions. Second, no studies have examined the effects of NTX effects across stages of social behavior (e.g., seeking of social stimuli vs. responding to them). This is critical in light of the “social deficit” theory of opiate antagonism, which predicts different NTX effects based on the stage of social behavior. Third, to our knowledge, no studies have directly compared the effects of NTX on social vs. nonsocial stimuli. It is important to determine whether there is a socially specific function of the opiate system, above and beyond its role in pleasure and pain in general (Panksepp et al., 1981).
In this study, we examined the effect of the opiate antagonist NTX on processing of emotional facial expressions, which are key social stimuli in humans. We studied two components of social processing: (1) seeking of social stimuli, indexed as attention to positive and negative expressions, measured using electroocu-lographic (EOG) tracking of eye movements and (2) responses to social stimuli, indexed as identification of emotional expressions and electromyographic (EMG) activity in facial muscles sensitive to emotional state during viewing of emotional expressions. Based on evidence that NTX reduces positive social responses (Chelnokova et al., 2014; Inagaki et al., 2015; Schweiger et al., 2013), we expected that NTX would impair responses to positive social stimuli, as indicated by slower identification of positive facial expressions and reduced positive psychophysiological responses to positive facial expressions. Because of the theoretically opposing effects of mu and kappa activation on anxiety and stress, we maintained an open hypothesis about the effects of NTX on responses to negative facial expressions. Further, given the complex and potentially contrasting predictions of the “social deficit” model and SOMSOM model, we also maintained an open hypothesis regarding whether NTX would increase or decrease attention (“seeking”) for positive social stimuli.
In addition to these primary outcomes, we also compared the effects of NTX on responses to emotional scenes with or without social content, using subjective ratings and EMG activity in facial muscles. Responses to pictures containing social versus nonsocial content are preferentially affected by other drugs that specifically alter social processing, such as oxytocin and ±3,4-methy-lenedioxymethamphetamine (MDMA) (Bershad, Seiden, & de Wit, 2016; Norman et al., 2011; Wardle, Kirkpatrick, & de Wit, 2014). Previous studies have also used subjective measures of responses to pictures with other opioidergic drugs (Bershad et al., 2016; Chelnokova et al., 2014; Gospic et al., 2008), so the inclusion of this secondary analysis also enhanced comparability to previous research. We hypothesized that NTX would reduce both subjective and psychophysiological responses to all positive pictures, but that this effect would be particularly pronounced for positive social pictures, indicating a specific effect of NTX on processing of positive social stimuli. We also examined the effect of NTX on responses to negative social and nonsocial pictures, with an open hypothesis.
Methods
Study design
The study used a three-session within-subjects design in which healthy volunteers received placebo, 25 mg NTX, or 50 mg NTX in a counterbalanced order under double-blind conditions. Sessions were separated by at least 1 week (M = 14.5 days, SD = 10.5 days). At each session, participants completed subjective measures of drug effects, mood, and physical effects at regular intervals before and after capsule ingestion. Ninety minutes after drug administration, during the expected peak effect, they completed subjective, behavioral, and psychophysiological measures of responses to emotional faces and scenes.
Participants
Healthy adult participants (18 men, 16 women) aged 18–35 were recruited via flyers and online advertisements. They completed an in-person screening consisting of a physical examination, electrocardiogram, modified structured clinical interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (First, Spitzer, Gibbon, & Williams, 1996), and self-reported drug and health history. Inclusion criteria were high school education with English fluency body mass index of 19–30; no regular use of prescription medications (except oral contraceptives); no medical contraindication to NTX (determined by the study physician); no prior negative reactions to NTX; no regular use of opioid drugs; no past-year DSM-IV Axis I diagnosis other than nontreatment seeking abuse of non-opioid drugs; no women who were pregnant or planning a pregnancy. See Table 1 for participant demographics.
Table 1.
Scores on the DEQ Feel Drug and Dislike Drug scales, and the POMS Fatigue scale for placebo, 25 mg, and 50 mg of NTX.
| Time | Measure
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Feel Drug (DEQ)
| ||||||||
| Placebo
|
25 mg
|
50 mg
|
||||||
| M | (SD) | M | (SD) | t-test vs. placebo | M | (SD) | t-test vs. placebo | |
| Baseline | Not rated before capsule | Not rated before capsule | Not rated before capsule | |||||
| 30 min | 15.85 | (22.77) | 14.03 | (18.61) | t(33) = 0.47, p = .64 | 13.18 | (17.84) | t(33) = 0.75, p = .46 |
| 75 min | 13.18 | (16.80) | 15.82 | (20.24) | t(33) = 0.70, p = .49 | 21.82 | (24.71) | t(33) = 2.23, p = .03 |
| 180 min | 5.94 | (8.12) | 18.67 | (22.56) | t(32) = 3.00, p = .005 | 13.74 | (18.22) | t(32) = 2.47, p = 0.02 |
| 210 min | 1.06 | (2.76) | 8.15 | (13.19) | t(32) = 2.78, p = .009 | 9.56 | (18.27) | t(32) = 2.71, p = 0.01 |
|
Dislike Drug (DEQ)
|
||||||||
|
Placebo
|
25 mg
|
50 mg
|
||||||
| M | (SD) | M | (SD) | t-test vs. placebo | M | (SD) | t-test vs. placebo | |
|
|
|
|
||||||
| Baseline | Not rated before capsule | Not rated before capsule | Not rated before capsule | |||||
| 30 min | 11.44 | (20.41) | 12.24 | (21.55) | t(33) = 0.17, p = .86 | 14.06 | (22.69) | t(33) = 0.67, p = .51 |
| 75 min | 13.76 | (24.43) | 13.94 | (22.51) | t(33) = 0.03, p = .98 | 13.65 | (21.99) | t(33) = 0.02, p = .98 |
| 180 min | 7.33 | (15.25) | 25.03 | (33.20) | t(32) = 2.79, p = .009 | 28.85 | (34.22) | t(32) = 3.25, p = .003 |
| 210 min | 2.55 | (9.34) | 16.18 | (27.34) | t(32) = 2.96, p = .006 | 17.21 | (31.76) | t(32) = 2.66, p = .01 |
|
Fatigue (POMS)
|
||||||||
|
Placebo
|
25 mg
|
50 mg
|
||||||
| M | (SD) | M | (SD) | t-test vs. placebo | M | (SD) | t-test vs. placebo | |
|
|
|
|
||||||
| Baseline | 1.91 | (3.02) | 2.44 | (3.15) | t(33) = 1.12, p = .27 | 2.85 | (4.13) | t(33) = 1.54, p = .13 |
| 30 min | 2.79 | (3.89) | 2.71 | (3.26) | t(33) = 0.13, p = .90 | 3.18 | (3.30) | t(33) = 0.73, p = .47 |
| 75 min | 3.09 | (4.07) | 2.74 | (3.14) | t(33) = 0.51, p = .61 | 4.68 | (4.34) | t(33) = 2.03, p = .05 |
| 180 min | 3.58 | (3.70) | 5.59 | (4.86) | t(32) = 2.61, p = .01 | 6.38 | (5.46) | t(32) = 3.81, p = .001 |
| 210 min | 3.06 | (3.34) | 4.79 | (3.83) | t(32) = 3.25, p = .003 | 4.85 | (5.47) | t(32) = 2.41, p = .02 |
Participants were asked to abstain from alcohol and medications (except birth control) for 24 h before each session, from recreational drugs for 48 h before each session, to consume their regular amounts of caffeine and nicotine before each session, and to fast for 2 h before each session. Compliance with presession requirements was verified using self-report, along with breath (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA) for alcohol and drugs. Women not on hormonal contraceptives were scheduled during the follicular phase of their menstrual cycle (Roche & King, 2015). To reduce the influence of expectancies, participants were told they might receive a stimulant, a sedative, a cannabinoid, an opioid antagonist, or a placebo. All participants provided written informed consent, and all procedures were carried out in accordance with the Declaration of Helsinki and approved by the University of Chicago Institutional Review Board.
Procedure
Sessions were conducted from 9:00 am to 1:30 pm in private laboratory rooms designed to resemble comfortable “living room” environments, that were equipped with couches and DVD players. Whenever they were not engaged in study tasks, participants were allowed to read quietly, complete non-stressful work, or watch DVDs from a selection provided. Participants were first given breath and urine tests for alcohol, drugs, and pregnancy, and received a standard snack (granola bar). They then completed baseline cardiovascular measures and subjective measures of drug effects, mood, and physical side effects. At 9:30 am, participants were given a capsule containing NTX (25 or 50 mg) or placebo. At 10:00 am, they completed the same cardiovascular and subjective measures. At 10:15 am, psychophysiological sensors were attached. At 10:45 am, participants again completed the cardiovascular and subjective measures, then began the computerized tasks measuring responses to emotional faces and scenes (see below), in a counterbalanced order. At 12:20 pm, psychophysiology sensors were removed. At 12:30 pm and 1:00 pm, participants again completed the cardiovascular and subjective measures. At 1:30 pm, they completed an end-of-session questionnaire and were discharged.
Drug and doses
We administered placebo, 25 and 50 mg NTX in size 00 opaque capsules with lactose filler, in a counterbalanced order under double-blind conditions. Placebo capsules consisted of lactose filler. NTX is a long-lasting opioid antagonist, with a half-life of 3.9–10.3 h (Crabtree, 1983). A dose of 50 mg is considered an effective dose for the treatment of drug dependence (Volpicelli, Alterman, Hayashida, & O’Brien, 1992), and is the dose most commonly examined in other studies of the drug’s effects on pleasure from food and other rewards in healthy adults (e.g., Arbisi et al., 1999; Chelnokova et al., 2014; Schweiger et al., 2013; Yeomans & Gray, 1996, 1997). The 25 mg dose was included to establish a dose–response curve while remaining within the range of doses appropriate for use in healthy volunteers.
Measures
Cardiovascular measures
Blood pressure and heart rate were measured using portable monitors (Life Source, A&D Company, Tokyo, Japan) to ensure participant safety. NTX had no significant effects on systolic blood pressure, diastolic blood pressure, or heart rate. Thus, these variables are not reported on further.
Subjective measures
Subjective drug effects were assessed using the drug effects questionnaire (DEQ; Fischman & Foltin, 1991), with visual analog scales to rate the extent to which subjects feel a drug effect, like the drug effect, dislike the drug effect, and would want more of the drug if given a choice. Mood was assessed using the profile of mood states (POMS; McNair, Lorr, & Droppleman, 1971), a validated measure consisting of 72 adjectives commonly used to describe momentary mood states. Physical side effects were assessed using the naltrexone side effects questionnaire (NESQ), which has been previously validated in clinical and preclinical trials with NTX (King, Volpicelli, Frazer, & O’Brien, 1997; King, Volpicelli, Gunduz, O’Brien, & Kreek, 1997).
Attention to emotional faces
Attention to emotional faces was measured using a visual probe task (VPT) adapted from Garner and colleagues (Garner, Mogg, & Bradley, 2006; Wardle, Garner, Munafò, & de Wit, 2012). This and similar tasks have previously been shown to be sensitive to drugs that alter social and emotional processing (Harmer, 2008; Stevens, Rist, & Gerlach, 2009). Anger, fear, happiness, and sadness were each posed by eight female and eight male actors in color pictures from the Karolinska Directed Emotional Faces Set (Goeleven, De Raedt, Leyman, & Verschuere, 2008). The task consisted of 64 trials in random order. In each trial, a 1000 ms fixation cross was followed by presentation of two images, one on either side of the screen, for 2000 ms. These images always consisted of a neutral and an emotional face posed by the same actor. Images measured 520 mm × 760 mm, with the inner edges 173 mm apart and were viewed at a distance of approximately 65 cm. When the two faces disappeared, they were replaced by gray rectangles of the same size, one of which contained a white shape that the participants were asked to classify as a circle or square by pressing a key. This response requirement helped disguise the main purpose of the task. Each trial was followed by a variable intertrial interval of 750–1250 ms. Probe shape, probe location, and emotional face location were counterbalanced. Outcomes were initial attentional bias, measured as direction of the first gaze when the faces appeared, and sustained or total attentional bias, measured as total gaze time at the emotional picture minus total gaze time at the neutral picture. Reaction time (RT) is not a valid indicator of attentional bias at this stimulus length, which is optimized for eye-gaze collection (Stevens, Rist, & Gerlach, 2011). Therefore, RT data were not analyzed. Gazes were measured using EOG, collected from two 4 mm Ag/AgCl electrodes filled with electrolyte gel attached 1.5 cm from the outer canthus of each eye, and an 8 mm gel-filled Ag/AgCl common ground sensor on the forehead. Electrode placement sites were cleaned with alcohol and exfoliant, and any with impedance above 20 kΩ (Model 1089 MK III Checktrode; UFI, Morro Bay, CA, USA) were reapplied. EOG was amplified 1000×, digitized, and sampled at 1000 Hz using an EOG100C amplifier, Biopac MP150 system, and AcqKnowledge software (Biopac Systems, Inc., Goleta, CA, USA).
Identification of and response to emotional faces
To measure identification of and response to emotional facial expressions, we used the Dynamic Emotional Identification Task (DEIT), as adapted in our laboratory (Benton et al., 2007; Wardle & de Wit, 2014; Wardle et al., 2012). This task has previously been shown to be sensitive to effects of drugs that alter social and emotional processing (Wardle & de Wit, 2014; Wardle et al., 2012). The task consists of 40 sequences showing five female and five male actors performing angry, fearful, sad and happy expressions. The sequences are presented in random order. Each sequence consists of 50 “frames” progressing from 0% to 100% emotional intensity in 2% increments, presented for an average of 250 ms (in a random range of 100–400 ms), producing a color video of an emotional expression developing. Participants were instructed to press the space bar “as soon as” they recognized the emotion being expressed. They were then prompted to identify the emotion from the four options. Outcomes of this task were identification of and psychophysiological responses to the facial expressions. Identification of expressions was quantified as the intensity (0%–100%) of the face when the participant pressed the space bar for correctly identified sequences (accuracy in this task is generally high and not sufficiently variable for analysis).
Psychophysiological responses to the emotional faces were measured using EMG of the corrugator and zygomatic muscles. Negative stimuli (including negative faces) increase activity in the corrugator (“frown”) muscle, whereas positive stimuli (including positive faces) decrease corrugator activity and increase zygomatic (“smile”) muscle activity (Dimberg & Karlsson, 1997; Dimberg, Thunberg, & Elmehed, 2000; Larsen, Norris, & Cacioppo, 2003; Moody, McIntosh, Mann, & Weisser, 2007). Activity was quantified as mean EMG during the final 1 s of face presentation for correctly identified sequences minus mean EMG in a 1 s pre-sequence baseline. The final 1 s was selected rather than the entire face presentation time to standardize the interval of measurement, as total face presentation times differed from participant to participant, depending upon how quickly they identified the face. The final 1 s was selected rather than the initial 1 s, as it represented a psychologically consistent moment for all participants — i.e., the period of time in which the participant identified the face —while the initial 1 s might contain identification for some participants but not others. However, we recognize that the intensity of the facial expression that the participant was viewing during the final 1 s could differ from participant to participant. Therefore, as described below, EMG analyses included intensity at display termination as a covariate. Of note, this is consistent with our procedure in our two prior studies using this same measure (Wardle & de Wit, 2014; Wardle et al., 2012). EMG was measured using two pairs of 4 mm Ag/AgCl electrodes filled with electrolyte gel attached over the left corrugator and zygomatic muscles and the common ground sensor. Electrode placement sites were cleaned with alcohol and exfoliant, and any with impedance above 20 kΩ (Model 1089 MK III Checktrode; UFI, Morro Bay, CA, USA) were reapplied. EMG signals were amplified 5000×, submitted to a 10—500 Hz band pass filter, digitized at 1000 Hz, submitted to a 60 Hz comb band stop filter, rectified, and integrated over 20 ms using two EMG100C amplifiers, an MP150 Data Acquisition System, and Acqknowledge software from Biopac Systems, Inc.
Responses to emotional scenes
Participants also viewed complete emotional scenes drawn from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1999). IAPS pictures are normatively rated on valence (positivity vs. negativity) and arousal, and our pictures were divided into positive, negative, and neutral groups based on these ratings. Further, based on previous research (Cacioppo, Norris, Decety, Monteleone, & Nusbaum, 2009; Gros, Hawk, & Moscovitch, 2009; Norman et al., 2011), we divided the pictures into “social” pictures, depicting at least two people or parts of people (e.g., two people talking, a hand pointing a gun at another person), and “nonsocial” pictures, depicting no people or parts of people (e.g., a slice of pizza, a car accident with no bodies visible). Thus, there were six subtypes, represented by nine pictures each: social/negative, non-social/negative, social/neutral, nonsocial/neutral, social/ positive, and nonsocial/positive. To avoid adaptation, at each session, the participant saw a unique picture set. We attempted to match valence and arousal across sets and social vs. nonsocial pictures, using normative ratings (Lang et al., 1999), and counterbalanced picture set with drug dose. Pictures were presented in fixed random order, with no more than two of the same valence in a row. Picture trials consisted of a 3 s pre-picture fixation, a 6 s picture period, and a 3 s post-picture fixation, immediately followed by subjective ratings using the evaluative space grid (Larsen, Norris, McGraw, Hawkley, & Cacioppo, 2009), which measures picture valence and arousal. Psychophysiological responses were quantified as mean EMG of the corrugator and zygomatic muscles during the 6 s presentation of each picture, compared to mean activity during the 1 s baseline period preceding each image, using the same procedures and equipment reported above.
Statistical analysis
We used linear mixed effect modeling (LME) and generalized linear mixed effect modeling (GLME) in the lme4 package (v 1.1–6; Bates, Meachler, & Bolker, 2011) of the R statistical computing environment (v 3.1.0; R Development Core Team, 2014) as our primary statistical approach. P-values used Satterthwaite approximations of degrees of freedom as implemented in the lmerTest package in R (v 2.0-6; Kusnetsova, Brockhoff, & Christensen, 2014). As (G)LME is relatively robust to missing data, participants with some missing data were included—extent of missing data is described for each analysis.
We first examined the effect of our doses on the subjective measures expected to be most sensitive to NTX, specifically “Feel Drug” and “Dislike Drug” from the DEQ, and “Fatigue” from the POMS. We entered each of these into LME models with dose and time of measurement as independent (fixed) factors, and subject as a random effect. Significant omnibus dose × time interactions were followed with post hoc paired t-tests comparing each drug dose to placebo at each time point. From the NSEQ, we selected “nausea” as the most common physical side effect of NTX. Although the NSEQ was administered at multiple time points, most people responded “0 – —not at all” at most times. Thus, we collapsed this into a dichotomous indicator, with zero indicating no increase in nausea relative to baseline at any point during the session and one indicating an increase in nausea. We entered this dichotomous nausea variable into a GLME model with dose as an independent (fixed) factor, and subject as a random effect.
Next, we examined the effect of NTX on attention to, identification of and responses to emotional faces and scenes. Initial attentional bias in the VPT (a dichotomous measure) was entered into a GLME model using dose and emotion as fixed factors, and subject as a random factor. Total attentional bias was entered into an LME model with the same fixed and random factors. Intensity at identification, corrugator EMG, and zygomatic EMG from the DEIT were each entered into LME models with dose and emotion as independent (fixed) factors and subject and actor as random effects. EMG analyses also included grand-mean-centered intensity at display termination as a covariate, because, as noted above, display intensity might influence EMG, and might in turn be systematically affected by drug. For analyses of the facial emotion stimuli, significant omnibus dose or dose × emotion effects were followed with post hoc paired t-tests comparing each dose to placebo, for main effects of dose, or each dose to placebo within each emotion, for dose × emotion interactions. Valence ratings, arousal ratings, corrugator EMG, and zygomatic EMG in the IAPS task were each entered into individual LME models using dose, picture type (negative, neutral, positive), and social content as independent (fixed) factors and subject as a random effect. For analyses of the IAPS data, significant omnibus dose, dose × picture valence, or dose × picture valence × social content effects were followed with post hoc paired t-tests comparing each dose to placebo, for main effects of dose, each dose to placebo within each picture valence, for dose × emotion interactions, or each dose to placebo within each picture subtype (i.e., social/positive, nonsocial/positive, social/ neutral, etc.), for dose × emotion × social content interactions.
Results
Subjective drug effects, mood, and physical side effects
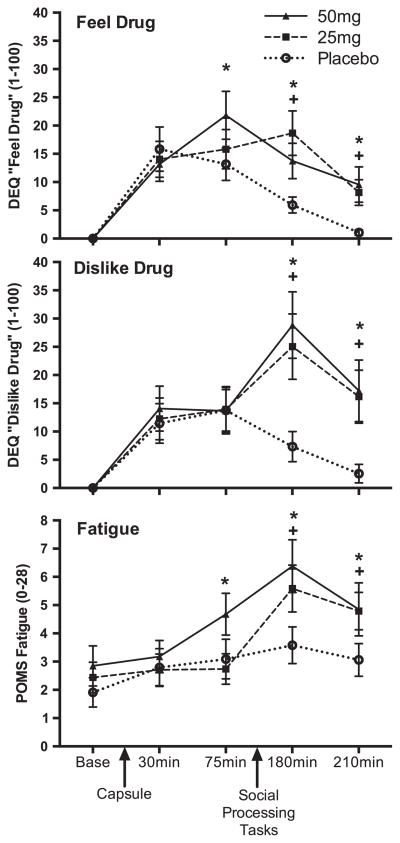
One participant was missing all subjective data from the second half of one session. On the DEQ “Feel Drug”, there was a significant interaction between drug and time (F[8, 459] = 1.95, p = .05. Post hoc paired t-tests showed that NTX (25 and 50 mg) produced small but significant increases in “Feel Drug” from 75 through 210 min after capsule administration, as shown in the top panel of Figure 1 and in Table 1. On the DEQ “Dislike Drug”, there was also a significant interaction between drug and time (F[8, 459] = 2.24, p = .02). Post hoc paired t-tests showed that NTX (25 and 50 mg) significantly increased “Dislike Drug” at the 180 and 210 min time points, as shown in the middle panel of Figure 1 and in Table 1. On the POMS, there was a marginal interaction between drug and time (F[8, 460] = 1.76, p = .08). Post hoc paired t-tests indicated that NTX (25 and 50 mg) slightly increased fatigue, with small effects evident from 75 through 210 min, shown in the bottom panel of Figure 1 and in Table 1. Last, NTX (25 and 50 mg) significantly increased reported nausea from near 0% in the placebo condition, to approximately 24% and 35% of participants reporting nausea under 25 and 50 mg, respectively (linear drug effect on Nausea: B = 3.44, SE = 1.25, z = 2.76, p = .006).
Figure 1.
NTX increased self-reports of feeling a drug effect (“Feel Drug”) and disliking the drug effect (“Dislike Drug”) on the DEQ (top panel and middle panel), and self-reports of Fatigue on the POMS (bottom panel). Of note, some subjective effects of NTX were significantly evident both before and after the social processing tasks, although subjective effects generally appear to have peaked after the social processing tasks. *p < .05 difference between 50 mg NTX and placebo, +p < .05 difference between 25 mg NTX and placebo.
Attention to emotional expressions
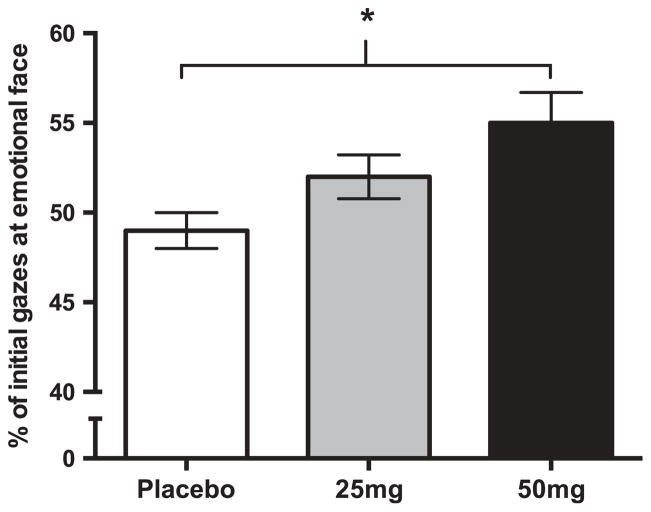
Five participants were missing one session of gaze data due to technical problems, and these sessions were thus excluded from all eye gaze analyses. A further three participants did not have a sufficient number of gazes to be included in the initial bias analysis (two participants for all sessions, and one participant for one session only). This occurred because these participants tended not to look at either picture during the gaze collection period (note, these participants were included in the total bias analysis as having total biases of 0). There was a significant main effect of drug on initial direction of gaze (B = 0.21, SE = 0.08, z = 2.73, p = .006). Post hoc paired t-tests showed that 50 mg NTX significantly increased initial bias for emotional faces, i.e., tendency to look at the emotional face first, with 55% of initial gazes directed toward the emotional face in the 50 mg condition (SD = 9.0%), compared to 50% in the placebo condition (SD = 5.5%; t[27] = 3.50, p = .002), as shown in Figure 2. The 25 mg dose had no significant effect on direction of first gaze, with 52% of gazes directed toward the emotional face in the 25 mg condition (SD = 6.6%; t[25] = 1.09, p = .284). The increased initial bias toward emotional faces at 50 mg was not specific to any particular type of emotional face. NTX did not affect total attentional bias, i.e., total time spent looking at emotional faces vs. neutral faces, for any type of emotional face.
Figure 2.
NTX (50 mg) increased initial attention, measured as initial direction of gaze, to emotional faces (vs. neutral faces) regardless of the emotion displayed. *p < .05 difference between 50 mg NTX and placebo.
Identification of and responses to emotional expressions
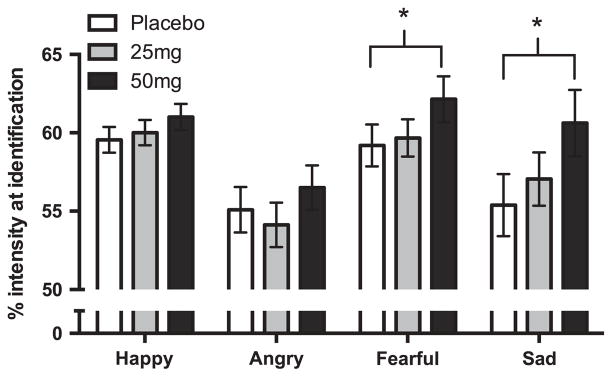
One participant failed to complete the identification task properly, waiting until the end of the video to identify all expressions. This person’s data was omitted from identification analyses. One participant was missing zygomatic data for one session due to equipment malfunction. There was a significant interaction between drug and emotion on identification of emotion (F[6, 3563] = 2.16, p = .04). Post hoc paired t-tests showed that 50 mg of NTX significantly slowed identification of fear and sadness, but not happiness or anger, as shown in Figure 3 and in Table 2. To further investigate the specificity of the NTX effect for fear and sadness, change scores between 50 mg and placebo were compared for each pair of emotions using paired t-tests. Fifty milligrams of NTX did not produce any differential slowing of identification of happiness vs. anger (happiness vs. anger t[33] = 0.15, p = .88). However, 50 mg NTX slowed identification of fear to a marginally greater extent than identification of happiness and anger (happiness vs. fear t[33] = −1.77, p = .08; anger vs. fear t[33] = −1.75, p = .08), and identification of sadness to a significantly greater extent than identification of happiness and anger (happiness vs. sadness t [33] = −2.16, p = .04; anger vs. sadness t[33] = −2.18, p = .04). Fifty milligrams of NTX did not produce any differential slowing of identification of sadness vs. fear (sadness vs. fear t[33] = −1.04, p = .31). The 25 mg dose did not significantly affect identification of any of the emotions (see Table 2). NTX did not significantly affect either corrugator or zygomatic responses to the emotional facial expressions.
Figure 3.
NTX (50 mg) increased the intensity required for identification of sad and fearful emotional expressions without significantly affecting identification of happy expressions. *p < .05 difference between 50 mg NTX and placebo.
Table 2.
Intensity of facial expression at the time of identification for placebo, 25 mg, and 50 mg doses of NTX.
| Emotion
|
Placebo
|
25 mg
|
50 mg
|
|||||
|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | t-test vs. placebo | M | (SD) | t-test vs. placebo | |
| Happy | 59.55 | (4.72) | 60.01 | (4.64) | t(32) = 0.74, p = .46 | 60.69 | (4.41) | t(32) = 1.91, p = .07 |
| Angry | 55.08 | (8.32) | 54.13 | (8.16) | t(32) = 0.87, p = .39 | 56.50 | (8.16) | t(32) = 1.87, p = .07 |
| Fearful | 59.19 | (7.70) | 59.67 | (6.81) | t(32) = 0.60, p = .55 | 62.14 | (8.36) | t(32) = 3.35, p = .002 |
| Sad | 55.39 | (11.40) | 57.05 | (9.77) | t(32) = 1.13, p = .27 | 60.62 | (12.18) | t(32) = 4.37, p < .001 |
Responses to emotional scenes
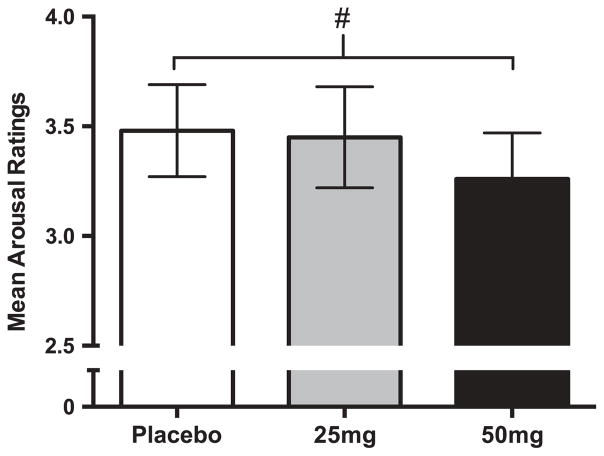
One participant was missing zygomatic data for one session due to equipment malfunction. NTX had no significant effect on ratings of picture valence for any picture type. There was a main effect of drug on arousal ratings (F[2, 5457] = 8.69, p < .001). However, post hoc paired t-tests found only a marginal decrease in overall arousal ratings between placebo (M = 3.48, SD = 1.22) and 50 mg (M = 3.26, SD = 1.23), as shown in Figure 4 (t[33] = 1.95, p = .06). The 25 mg dose had no significant effect on arousal ratings (M = 3.44, SD = 1.31; t[33] = 0.27, p = .78). NTX did not alter either corrugator responses to any picture type. There was an interaction between NTX and social content on zygomatic responses (F[2, 4539] = 3.01, p = .05). However, post hoc tests found no significant differences between placebo and NTX for either social or nonsocial stimuli at either dose, making this effect difficult to interpret.
Figure 4.
NTX (50 mg) marginally decreased ratings of arousal for all types of emotional pictures, regardless of social vs. nonsocial content. #p = .06 difference between 50 mg NTX and placebo.
Discussion
NTX at 50 mg increased initial attentional capture by all facial expressions of emotion (relative to neutral expressions), but slowed identification, specifically of sadness and fear. NTX at 50 mg also slightly decreased ratings of arousal for all types of social and nonsocial emotional scenes. These effects were not evident at the lower dose of 25 mg. These results suggest a complex effect of NTX on social stimuli, and are not fully consistent with either a simple “social deficit” result of mu-opioid antagonism, or with a state-dependent effect as hypothesized by the SOMSOM theory. Rather, they point to possible kappa-opioid anxiolytic-like effects of NTX. NTX is a nonselective opioid antagonist and acts at multiple types of receptors. It is therefore impossible to determine the specific contributions of each receptor type in the behaviors we observed. Nonetheless, in the following sections, we speculate based on the existing literature as to the possible mu-opioid and kappa-opioid explanations of these findings.
Our primary prediction was that NTX would produce a decrease in responses to positive social stimuli, but we saw no evidence for a decrease in either subjective or psychophysiological responses to either social or nonsocial positive stimuli. This is puzzling given that NTX and the very similar opiate antagonist naloxone have reduced neural, self-report, and behavioral responses to other positive social and nonsocial stimuli, such as attractive faces (Chelnokova et al., 2014) and gambling wins (Petrovic et al., 2008), in healthy adults. There are several possible reasons for this discrepancy. First, it may be that NTX’s effects are specific to certain types of positive stimuli, and were not evident with the pictorial stimuli used in the current study. However, it should be noted the same type of images used in this study have previously been affected by opioid manipulations, thereby arguing against an overall lack of relevance for pictorial stimuli to the opioid system. For example, the mu agonist remifentanil increased ratings of positivity for neutral pictures (Gospic et al., 2008), the partial mu-opioid antagonist buprenorphine increased ratings of positivity for positive pictures, with specifically social content (Bershad et al., 2016), and NTX decreased ratings of liking for attractive faces (Chelnokova et al., 2014) in previous studies. Second, it is possible that effects of NTX on our positive stimuli were present, but were not captured by our measures. Although these and similar measures have been sensitive to a variety of pharmacological manipulations (Wardle & de Wit, 2014; Wardle et al., 2012, 2014), including opiate manipulations (Bershad et al., 2016; Gospic et al., 2008; Ipser et al., 2013), it is possible that the effects of acute NTX on positive stimuli are subtle enough that they are better captured with neural measures like EEG and fMRI (e.g., Murray et al., 2014; Petrovic et al., 2008; Schweiger et al., 2013). Last, it may be that the dosing regimen was not sufficient to produce effects on positive stimuli. This dose was equivalent to the dose used in most other acute studies, including the most similar study, documenting reduced wanting and liking for attractive faces (Chelnokova et al., 2014). However, in some other previous studies, NTX has been given at the same 50 mg dose, but chronically over 4 days (Inagaki et al., 2015). Chronic dosing may be necessary to reveal consistent negative effects of NTX on social pleasure.
NTX did produce some unexpected effects on social processing, including generally increasing attention to all emotional expressions relative to neutral ones. There are few other pharmacological manipulations or clinical conditions that produce a similar generalized effect across all emotions on this type of task, making this finding difficult to interpret. For example, anxiety disorders increase attention to “threat-related” angry and fearful faces, (Bradley, Mogg, & Millar, 2000; Garner et al., 2006; Mogg, Garner, & Bradley, 2007; Mogg, Millar, & Bradley, 2000), but not happy faces; thus, the observed NTX effect does not seem clearly anxiogenic, nor does it fit perfectly with the “social deficit” hypothesis of increased seeking of socially positive stimuli. However, one possible interpretation is that emotional faces are more socially informative than nonemotional faces, and NTX increased seeking of social information, potentially with the end goal of increasing social comfort. This could be partially consistent with the predictions of either the “social deficit” theory for mu-opioid antagonism, or the SOMSOM theory, provided the laboratory setting is assumed to induce a state of mild distress for the participants. Further tests involving more direct measures, such as trading money for social contact (e.g., Higgins, Hughes, & Bickel, 1989; Higgins & Stitzer, 1988; Kirkpatrick, Lee, Wardle, Jacob, & de Wit, 2014), could confirm whether the observed attentional effects of NTX indeed represent “social seeking.” Combining these measures of social seeking with manipulations of baseline state would also allow a more direct test of the “social deficit” vs. SOMSOM predictions.
NTX also produced some effects on social and emotional processing that are not consistent with either of the models of mu-opioid blockade, but might be explained by a kappa-antagonist effect. We found that NTX selectively slowed identification of sadness and fear. Here, the effects of NTX most closely resemble the “pro-social” drug MDMA and anxiolytics, which selectively impair identification of negative emotional expressions (Bedi, Hyman, & de Wit, 2010; Hysek, Domes, & Liechti, 2012; Hysek et al., 2014; Kirkpatrick et al., 2014; Wardle & de Wit, 2014; Zangara, Blair, & Curran, 2002). Interestingly, decreased fear perception has also been seen with the partial mu-opioid agonist and kappa-antagonist buprenorphine (Ipser et al., 2013), raising two possible explanations. First, accurate recognition of negative faces may relate to mu-opiate functioning via an inverted U, such that either increased or decreased mu functioning disrupts this ability. Second, this effect may be due to the anxiolytic effects of kappa-opioid antagonism, a mechanism NTX and buprenorphine share. This finding is interesting in the context of studies in prairie voles, suggesting that kappa antagonism reduces aversive social motivation (Resendez & Aragona, 2013; Resendez et al., 2012) in addition to exerting more general anxiolytic effects (Falcon, Maier, Robinson, Hill-Smith, & Lucki, 2015). Currently, there are not selective kappa antagonists available for use in humans; however, some have suggested that combining buprenorphine and NTX can effectively produce a selective kappa antagonist, as their mu effects are opposing (Rothman et al., 2000). This combination might allow future studies to determine whether the observed anxiolytic-like effects on emotional processes seen with both buprenorphine and NTX are kappa-opioid-related. NTX also produced a small overall decrease in arousal for all types of pictures, which could also be interpreted as sedative or anxiolytic in nature. Although NTX has not often been tested in rodent anxiolytic paradigms, due to its non-specific actions, it has shown anxiolytic properties in some tests in rodents (Zurita, Martijena, Cuadra, Brandão, & Molina, 2000) although c.f. (Lee & Rodgers, 1990). Together, these observed anxiolytic and sedative-like effects of NTX on emotional and social processing open interesting questions as to the role that anxiolytic kappa-antagonist effects of NTX may play in its utility in alcohol and opiate use treatment.
There are several limitations of the current study. First, the use of healthy adults as participants limits the extent to which these findings can be generalized to clinical use of NTX. Second, the tasks used did not include any neural measures of NTX’s effects, potentially limiting sensitivity. They also did not include tests of actual social interactions, limiting the conclusions that can be drawn about effects of NTX on “real-world” social behavior. Third, the dose range was limited to clinical and subclinical doses for addiction, and some authors have argued that much higher doses may be needed to observe all of the effects of NTX, and particularly kappa effects (Jonas & Gold, 1988). Fourth, as can be observed from Figure 1, although we timed the social processing tasks to what we expected to be the peak effects of the drug, peak subjective effects took place slightly later in the session, so this timing issue may have diminished the effects of NTX that we were able to observe.
In summary, in the first examination of the effect of NTX on both positive and negative social stimuli in healthy humans across several stages of social processing, we failed to observe a mu-opioid antagonist-like effect of dampened positive responses to positive social stimuli. We did, however, observe possible enhancement of “seeking” of social stimuli, which could be consistent with either the “social deficit” or SOMSOM theory of mu-opioid effects. NTX also produced effects that were broadly more consistent with kappa-opioid antagonist effects, slowing identification of negative emotions, and dampening arousal to all types of emotional stimuli. These findings are puzzling from the perspective of theories on the role of the mu-opiate system in social engagement and pleasure in humans, but open interesting questions as to the possible role of kappa antagonism in the clinical effects of NTX in humans.
Acknowledgments
The authors would like to thank Aoibhin Curran, Matt Pulaski, Kevin Yan, Sarah Ellefson, Jacob Seiden and Tim O’Neal for help with data collection and scoring.
Funding
This work was supported by the National Institute on Drug Abuse [R01 DA002812].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arbisi PA, Billington CJ, Levine AS. The effect of naltrexone on taste detection and recognition threshold. Appetite. 1999;32(2):241–249. doi: 10.1006/appe.1998.0217. [DOI] [PubMed] [Google Scholar]
- Bates DM, Meachler M, Bolker B. lme4: Linear mixed-effects model using S4 classes. 2011. [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is Ecstasy an “Empathogen”? Effects of ±3,4-Methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological Psychiatry. 2010;68(12):1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CP, Etchells PJ, Porter G, Clark AP, Penton-Voak IS, Nikolov SG. Turning the other cheek: The viewpoint dependence of facial expression after-effects. Proceedings of the Royal Society B: Biological Sciences. 2007;274(1622):2131–2137. doi: 10.1098/rspb.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Seiden JA, de Wit H. Effects of buprenorphine on responses to social stimuli in healthy adults. Psychoneuroendocrinology. 2016;63:43–49. doi: 10.1016/j.psyneuen.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cognition & Emotion. 2000;14(6):789–808. doi: 10.1080/02699930050156636. [DOI] [Google Scholar]
- Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Research Reviews. 2009;62(1):127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience. 2009;21(1):83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-Like Effects of -Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology. 2010;35(3):752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology. 2010;210(2):295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B. Review of naltrexone, a long-acting opiate antagonist. Clinical Pharmacy. 1983;3(3):273–280. [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Eikemo M, Riegels J, Loseth G, Maurud H, Leknes S. Rewards of beauty: The opioid system mediates social motivation in humans. Molecular Psychiatry. 2014;19(7):746–747. doi: 10.1038/mp.2014.1. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Karlsson B. Facial reactions to different emotionally relevant stimuli. Scandinavian Journal of Psychology. 1997;38(4):297–303. doi: 10.1111/1467-9450.00039. [DOI] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11(1):86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacology Biochemistry and Behavior. 1982;16(4):653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2015;232(5):907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. American Journal of Physiology. 1986;251(1 Pt 2):R91–96. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. British Journal of Addiction. 1991;86(12):1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115(4):760–770. doi: 10.1037/0021-843x.115.4.760. [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska Directed Emotional Faces: A validation study. Cognition & Emotion. 2008;22(6):1094–1118. doi: 10.1080/02699930701626582. [DOI] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P. Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology. 2008;197(2):295–307. doi: 10.1007/s00213-007-1032-4. [DOI] [PubMed] [Google Scholar]
- Green A, Kaul A, O’Shea J, Sharma E, Bennett L, Mullings EL, Donaldson LF. Opiate agonists and antagonists modulate taste perception in opiate-maintained and recently detoxified subjects. Journal of Psychopharmacology. 2013;27(3):265–275. doi: 10.1177/0269881112472567. [DOI] [PubMed] [Google Scholar]
- Gros DF, Hawk LW, Jr, Moscovitch DA. The psychophysiology of social anxiety: Emotional modulation of the startle reflex during socially-relevant and -irrelevant pictures. International Journal of Psychophysiology. 2009;73:207–211. doi: 10.1016/j.ijpsycho.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008;55(6):1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Havassy BE, Hall SM, Wasserman DA. Social support and relapse: Commonalities among alcoholics, opiate users, and cigarette smokers. Addictive Behaviors. 1991;16(5):235–246. doi: 10.1016/0306-4603(91)90016-B. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Hughes JR, Bickel WK. Effects of d-amphetamine on choice of social versus monetary reinforcement: A discrete-trial test. Pharmacology, Biochemistry and Behavior. 1989;34(2):297–301. doi: 10.1016/0091-3057(89)90315-8. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Stitzer ML. Time allocation in a concurrent schedule of social interaction and monetary reinforcement: Effects of d-amphetamine. Pharmacology, Biochemistry and Behavior. 1988;31(1):227–231. doi: 10.1016/0091-3057(88)90338-3. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012:1–10. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Liechti ME. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. The International Journal of Neuropsychopharmacology. 2014;17(03):371–381. doi: 10.1017/S1461145713001132. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Irwin MR, Eisenberger NI. Blocking opioids attenuates physical warmth-induced feelings of social connection. Emotion. 2015;15(4):494. doi: 10.1037/emo0000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Terburg D, Syal S, Phillips N, Solms M, Panksepp J, van Honk J. Reduced fear-recognition sensitivity following acute buprenorphine administration in healthy volunteers. Psychoneuroendocrinology. 2013;38(1):166–170. doi: 10.1016/j.psyneuen.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Jonas JM, Gold MS. The use of opiate antagonists in treating bulimia: A study of low-dose versus high-dose naltrexone. Psychiatry Research. 1988;24(2):195–199. doi: 10.1016/0165-1781(88)90062-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Research. 1988;440(2):285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129(1):15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Gunduz M, O’Brien CP, Kreek MJ. Naltrexone biotransformation and incidence of subjective side effects: A preliminary study. Alcoholism, Clinical and Experimental Research. 1997;21(5):906–909. 100000374-199708000-00020[pii] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and Intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. 2014;39(7):1654–1663. doi: 10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1998;285(2):518–526. [PMC free article] [PubMed] [Google Scholar]
- Kusnetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixed effect models. 2014 Retrieved from https://cran.r-project.org/web/packages/lmerTest/index.html.
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: NIMH Center for the study of emotion and attention, University of Florida; 1999. [Google Scholar]
- Langleben DD, Busch EL, O’Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology. 2012;220(3):559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40(5):776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: A single-item measure of positivity and negativity. Cognition & Emotion. 2009;23(3):453–480. doi: 10.1080/02699930801994054. [DOI] [Google Scholar]
- Lee C, Rodgers RJ. Antinociceptive effects of elevated plus-maze exposure: Influence of opiate receptor manipulations. Psychopharmacology. 1990;102(4):507–513. doi: 10.1007/BF02247133. [DOI] [PubMed] [Google Scholar]
- Lewis BA, O’Neill HK. Alcohol expectancies and social deficits relating to problem drinking among college students. Addictive Behaviors. 2000;25(2):295–299. doi: 10.1016/S0306-4603(99)00063-5. [DOI] [PubMed] [Google Scholar]
- López-Martínez AE, Esteve-Zarazaga R, Ramírez-Maestre C. Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. The Journal of Pain. 2008;9(4):373–379. doi: 10.1016/j.jpain.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Loseth GE, Ellingsen DM, Leknes S. State-dependent μ-opioid modulation of social motivation. Frontiers in Behavioral Neuroscience. 2014;8:430. doi: 10.3389/fnbeh.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Simpson MJA, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Developmental Psychobiology. 1995;28(2):71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2005;31(6):1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mogg K, Garner M, Bradley BP. Anxiety and orienting of gaze to angry and fearful faces. Biological Psychology. 2007;76(3):163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109(4):695–704. doi: 10.1037/0021-843X.109.4.695. [DOI] [PubMed] [Google Scholar]
- Moody EJ, McIntosh DN, Mann LJ, Weisser KR. More than mere mimicry? The influence of emotion on rapid facial reactions to faces. Emotion. 2007;7(2):447–457. doi: 10.1037/1528-3542.7.2.447. [DOI] [PubMed] [Google Scholar]
- Murray E, Brouwer S, McCutcheon R, Harmer CJ, Cowen PJ, McCabe C. Opposing neural effects of naltrexone on food reward and aversion: Implications for the treatment of obesity. Psychopharmacology. 2014;231(22):4323–4335. doi: 10.1007/s00213-014-3573-7. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, DeVries AC, Berntson GG. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology. 2011;25(10):1313–1319. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews. 1981;4(4):473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Pleger B, Seymour B, Klöppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. The Journal of Neuroscience. 2008;28(42):10509–10516. doi: 10.1523/jneur-osci.2807-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Reviews in the Neurosciences. 2013;24:51. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. -Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. The Journal of Neuroscience. 2012;32(20):6771–6784. doi: 10.1523/jneurosci.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DL. A longitudinal study of life stress and social support among drug abusers. Substance Use & Misuse. 1983;18(2):195–222. doi: 10.3109/10826088309027352. [DOI] [PubMed] [Google Scholar]
- Roche DJO, King AC. Sex differences in acute hormonal and subjective response to naltrexone: The impact of menstrual cycle phase. Psychoneuroendocrinology. 2015;52(0):59–71. doi: 10.1016/j.psyneuen.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. Journal of Substance Abuse Treatment. 2000;18(3):277–281. doi: 10.1016/S0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Schino G, Troisi A. Opiate receptor blockade in juvenile macaques: Effect on affiliative interactions with their mothers and group companions. Brain Research. 1992;576(1):125–130. doi: 10.1016/0006-8993(92)90617-I. [DOI] [PubMed] [Google Scholar]
- Schweiger D, Stemmler G, Burgdorf C, Wacker J. Opioid receptor blockade and warmth-liking: Effects on interpersonal trust and frontal asymmetry. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S, Rist F, Gerlach AL. Influence of on the processing of emotional facial expressions in individuals with social phobia. British Journal of Clinical Psychology. 2009;48(2):125–140. doi: 10.1348/014466508x368856. [DOI] [PubMed] [Google Scholar]
- Stevens S, Rist F, Gerlach AL. Eye movement assessment in individuals with social phobia: Differential usefulness for varying presentation times? Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(2):219–224. doi: 10.1016/j.jbtep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. Selective natrexone-derived opioid receptor antagonists. Annual Review of Pharmacology and Toxicology. 1992;32:239–269. doi: 10.1146/annurev.pa.32.040192.001323. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends in Pharmacological Sciences. 2010;31(10):463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus Accumbens -Opioid Receptors Mediate Social Reward. The Journal of Neuroscience. 2011;31(17):6362–6370. doi: 10.1523/jneurosci.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229(3):435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wardle M, Garner M, Munafò M, de Wit H. Amphetamine as a social drug: Effects of d-amphetamine on social processing and behavior. Psychopharmacology. 2012:1–12. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, De Wit H. MDMA alters emotional processing and facilitates positive social interaction. Psychopharmacology. 2014;231(21):4219–4229. doi: 10.1007/s00213-014-3570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Kirkpatrick MG, de Wit H. ‘Ecstasy’ as a social drug: MDMA preferentially affects responses to emotional stimuli with social content. Social Cognitive and Affective Neuroscience. 2014;9:1076–1081. doi: 10.1093/scan/nsu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JI, Stein JA, Grella CE. Role of social support and self-efficacy in treatment outcomes among clients with co-occurring disorders. Drug and Alcohol Dependence. 2007;89(2–3):267–274. doi: 10.1016/j.drugalcdep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiology & Behavior. 1996;60(2):439–446. doi: 10.1016/S0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Effects of naltrexone on food intake and changes in subjective appetite during eating: Evidence for opioid involvement in the appetizer effect. Physiology & Behavior. 1997;62(1):15–21. doi: 10.1016/S0031-9384(97)00101-7. [DOI] [PubMed] [Google Scholar]
- Zangara A, Blair RJR, Curran HV. A comparison of the effects of a β-adrenergic blocker and a benzodiazepine upon the recognition of human facial expressions. Psychopharmacology. 2002;163(1):36–41. doi: 10.1007/s00213-002-1120-4. [DOI] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandão ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: Reversal by naltrexone pretreatment. Behavioural Brain Research. 2000;117(1–2):163–171. doi: 10.1016/S0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]