Abstract
Background
Pathology generated equations have been introduced to predict Oncotype DX recurrence score (ORS) in breast cancer. The purpose of the study is to improve these equations.
Material and Methods
Slides from 416 (test set) consecutive breast cancers with available Oncotype DX were reviewed. A validation set (n=91) was prospectively scored using the generated formulas from the test set. The following histopathologic features were graded: Nottingham grade (designated as current Nottingham grade), necrosis, and degree of tumor infiltrating lymphocytes (TIL). The following data were extracted from the pathology report: Nottingham grade (designated as reported Nottingham grade), tumor size, ER/PR Allred scores, HER2 status, and ORS. Equations were calculated, one included the reported Nottingham grade, one included the current Nottingham grade, and one included the current Nottingham grade with the other significant histopathologic variables.
Results
In the equation that included the reported Nottingham grade, ER, PR and HER2, the overall concordance with the ORS was 64.86%. After excluding the intermediate category detected by the formula, the concordance rate was 95.28%. When the current Nottingham grade was included, the concordance rate became 69.61% and 98.62%, respectively. When necrosis and the degree of TIL were added to the previous equation, these rates became 70.1% and 98.63%, respectively.
Conclusions
Our equation has better correlation to ORS than previously published results.
Keywords: Breast Cancer, Oncotype Recurrence Score, Histomorphology
INTRODUCTION
Traditionally, the surgical pathology evaluation of the tumor provides prognostic information, including tumor size, histologic type, Nottingham grade, lymph node status and biomarkers profile [estrogen receptor (ER), progesterone receptor (PR), and HER2] (1,2). While HER2 positive patients are offered anti-HER2 targeted therapy, patients with triple negative cancers are usually treated with chemotherapy. Estrogen receptor positive breast cancer is normally treated with hormonal therapy. However, subset of these patients who have more aggressive disease benefit from adjuvant chemotherapy. There have been many attempts to recognize this subset, Oncotype DX assay provided by Genomic Health (Redwood City, CA) is the most widely used test to answer that specific question
Oncotype DX assay is a quantitative reverse transcription-polymerase chain reaction based assay usually offered to patients who have node-negative and ER-positive invasive breast cancer. The assay calculates the Oncotype recurrence score (ORS) based on the expression of 21 genes, 16 of which are cancer-related. The score ranges from 0 to100 and the results are divided into 3 risk categories, low <18, intermediate 18–30, and high >30. These scores were validated using formalin fixed paraffin embedded tissue blocks from the tamoxifen-treated arm of the National Surgical Adjuvant Breast and Bowel Project clinical trial B-14 (3). Tissue blocks from the National Surgical Adjuvant Breast and Bowel Project clinical trial B-20 were used to perform the Oncotype DX assay and calculate the ORS for each patient. Patients with high ORS were shown to benefit from adjuvant chemotherapy while patients with low-ORS were shown to derive minimal or no benefit (4). It has been validated by experience from multiple studies which included several thousand patients (5–8).
Although this assay seems ideal for recognizing the subset of patients who can benefit from adjuvant chemotherapy, there have been multiple issues related to its high cost ($4000) which can only be afforded by a subset of patients and its unavailability in poor countries. Moreover, a small fraction of cases get rejected by Genomic Health due to either insufficient tissue or degraded RNA. There have been multiple studies that evaluated the histomorphology and immunohistochemistry (IHC) to predict the ORS with variable success (9,10). These studies extracted the histologic and IHC variables from the pathology reports with a non-uniform interpretation. In addition, other studies showed that tumors enriched with stroma, even though they were traditionally known to have low risk of recurrence, had unexpectedly high ORS (11). Therefore, we intended to uniformly review the histologic slides and evaluate multiple histomorphologic parameters in order to improve the already published equations with resulting benefit to patients with ER positive breast cancers.
MATERIAL AND METHODS
Cases and histologic evaluation
A total of 416 (test set) consecutive breast carcinoma cases with available ORS were reviewed. The cases were from Roswell Park Cancer Institute files between 2005 and 2013. A validation set of 91 cases was prospectively reviewed at the time of assay order between 2013 and 2014. The cases were scored using the generated formulas from the test set. These cases had Oncotype DX assay based on clinical request by the treating breast oncologist. The following variables were abstracted from the pathology report: tumor size, Nottingham grade (designated as reported Nottingham grade, see below), ORS, ER and PR Allred scores, and HER2 status. Estrogen and progesterone receptors were assessed semiquantitatively by using the Allred scoring method. This method incorporates intensity and distribution of reactivity (12). HER2 immunohistochemical results were reported according to the College of American Pathologist/ American Society of Clinical Oncology guidelines (13).
The corresponding Hematoxylin and Eosin slide to the tested tissue block for each patient were reviewed and scored jointly by two pathologists (TK and XH). The following variables were graded and recorded: tumor necrosis, degree of tumor infiltrating lymphocytes (TIL), and percentage of ductal carcinoma in situ. Tumor necrosis was graded from 0 to 4: grade 0 = no necrosis, grade 1 = one focus (a cluster of more than 3 cells), grade 2 = two or more non-connected foci of necrosis, grade 3 = two or more connected foci but not geographic, and grade 4 = geographic necrosis. The degree of TIL was graded from 0 to 4: grade 0 = virtually no lymphocytes, grade 1 = sparse non-aggregated, grade 2 = clustered, grade 3 = sheets not obscuring the tumor, and grade 4 = sheets obscuring the tumor. Tumor/stroma ratio was visually estimated as a percentage (0% to 100%). The percentage of ductal carcinoma in situ was visually estimated (from 0% to 100%).
We intended to evaluate if uniform interpretation of Nottingham grade has better correlation with the ORS than just abstracting the reported Nottingham grade. For that we uniformly followed the Nottingham grading system. For tubular formation, a score of 1 was given when the tubular structures composed >75% of the tumor, grade 3 was given when <10% of tumor had tubular structures and grade 2 for any value between 10% and 75%. For nuclear grading, grade 1 small monomorphic cell, grade 2 moderate nuclear variation, and grade 3 marked nuclear pleomorphism. For mitotic count; a score of 1 was given for mitotic count of 0 to 8/10-HPF, a score of 2 was given for a mitotic count of 8 to 17/10-HPF, and a score of 3 was given for mitotic count of >17/10-HPF with area measuring 0.237mm2 (Olympus BX 45 microscope, Tokyo Japan). Then to obtain the overall tumor grade the scores of each category were added together, giving a possible total of 3 to 9. Tumor grade was then allocated on the following basis, 3–5 points grade I, 6 to 7 points grade II, and 8 to 9 grade III (14). The study is conducted after the approval of RPCI Institutional Review Board (IRB) # EDR 241113. Since the study included only pre-exciting data, no patient’s consent was required.
Statistical analysis
Equations were calculated including all significant variables, one with reported Nottingham grade, one with current Nottingham grade and one with current Nottingham grade and other histopathologic variables. Pearson correlation coefficient r and coefficient of determination r2 were used for continuous variables, while Spearmen rank correlation coefficient was used for categorical variables.
RESULTS
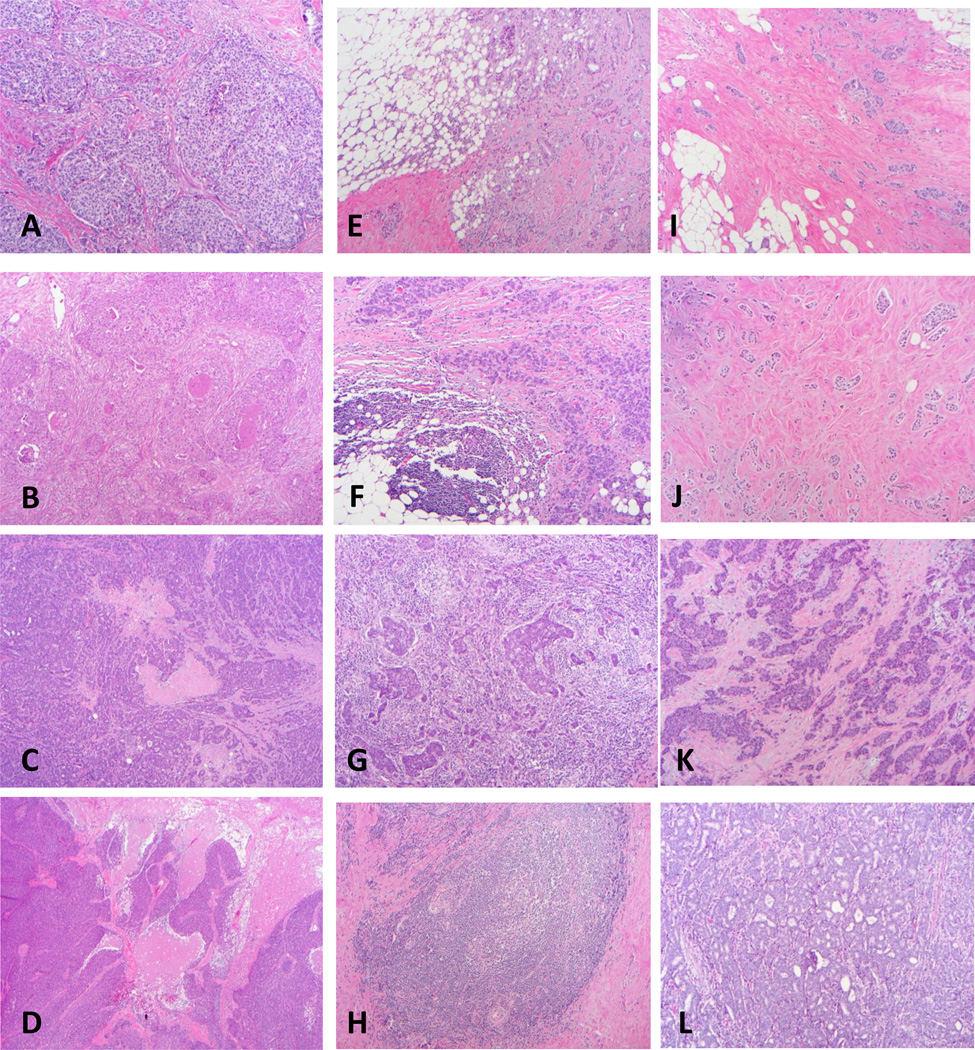
Table 1 illustrates the distribution of clinicopathologic variables in the test set according to ORS. The following variables were significantly correlated with high ORS, histologic type with no special type having higher risk than lobular, higher Nottingham grade, lower ER and PR expression, amplified HER2, higher degree of necrosis (figure 1A–D), and higher degree of TIL (figure 1E–1H). Also, higher tumor/stroma ratio correlated with higher ORS (figure 1I–1L). When we performed the regression analysis, we found that all of the above variables significantly correlated with the ORS. However, tumor/stroma ratio did not improve the equations. Our Nottingham grading had better predictability for the ORS than the reported Nottingham grade with "r" value of 0.14 vs. 0.32 respectively (table 2).
Table 1.
Recurrence score categories vs. clinical and pathologic.
| ORS<18 | ORS (18–30) | ORS>30 | P value | ||
|---|---|---|---|---|---|
| Age (years, median, mean+/− s.e.m) | 56,56.32+/−0.67 | 56,57.41+/−0.77 | 57.5,56.84+/−1.59 | NS | |
| Tumor size (mm, median, mean+/− s.e.m) | 1.4,1.56+/−0.05 | 1.4,1.56+/−0.07 | 1.5,1.61+/−0.11 | NS | |
| Menopause | |||||
| Pre- | 80(37.04) | 39(27.27) | 11(25) | NS | |
| Post- | 136(62.96) | 104(72.73) | 33(75) | ||
| Race | |||||
| Caucasian | 202(89.78) | 134(91.16) | 37(86.05) | NS | |
| African American | 16(7.11) | 9(6.12) | 3(6.98) | ||
| Other | 7(3.11) | 4(2.72) | 3(6.98) | ||
| Histologic type | |||||
| No special type | 179(83.64) | 118(81.94) | 43(97.73) | 0.02 | |
| Lobular | 35(16.36) | 26(18.06) | 1(2.27) | ||
| Tubular formation | |||||
| 1 | 38(16.89) | 14(9.52) | 3(6.82) | 0.01 | |
| 2 | 72(32) | 46(31.29) | 7(15.91) | ||
| 3 | 115(51.11) | 87(59.18) | 34(77.27) | ||
| Nuclear grade | |||||
| 1 | 33(14.67) | 11(7.48) | 1(2.27) | <0.01 | |
| 2 | 171(76) | 107(72.79) | 11(25) | ||
| 3 | 21(9.33) | 29(19.73) | 32(72.73) | ||
| Mitotic count | |||||
| 1 | 199(88.44) | 111(75.51) | 11(25) | <0.01 | |
| 2 | 17(7.56) | 21(14.29) | 9(20.45) | ||
| 3 | 9(4) | 15(10.2) | 24(54.55) | ||
| No. mitotic figures/10HPF (median, mean+/− s.e.m) | 1,3.43+/−0.47 | 3,5.97+/−0.72 | 17,20.3+/−2.69 | <0.01 | |
| Nottingham Grade | |||||
| 1 | 109(48.44) | 53(36.05) | 5(11.36) | <0.01 | |
| 2 | 105(46.67) | 72(48.98) | 9(20.45) | ||
| 3 | 11(4.89) | 22(14.97) | 30(68.18) | ||
| ER (median, mean+/− s.e.m) | 8,7.75+/−0.04 | 8,7.38+/−0.09 | 7.5,6.18+/−0.37 | <0.01 | |
| PR (median, mean+/− s.e.m) | 8,7.25+/−0.1 | 7,5.86+/−0.2 | 3,3.05+/−0.46 | <0.01 | |
| HER2 | |||||
| Positive | 3(1.33) | 3(2.04) | 5(11.36) | <0.01 | |
| Negative | 222(98.67) | 144(97.96) | 39(88.64) | ||
| Necrosis | 0,0.05+/−0.02 | 0,0.12+/−0.04 | 0,0.68+/−0.16 | <0.01 | |
| TIL | 1,0.86+/−0.03 | 1,0.99+/−0.05 | 1,1.2+/−0.11 | <0.01 | |
| Tumor/stroma (median, mean +/− s.e.m) | 70,60.93+/−1.31 | 60,60.41+/−1.62 | 70,70.91+/−2.56 | <0.01 | |
| Tumor/stroma (median, mean +/− s.e.m) | 70,60.93+/−1.31 | 60,60.41+/−1.62 | 70,70.91+/−2.56 | <0.01 | |
| DCIS (median, mean+/− s.e.m) | 2,9.69+/−1.25 | 1,5.54+/−0.88 | 1,7.36+/−2.53 | 0.051 | |
NS: Not significant; TIL, tumor infiltrating lymphocytes
Figure 1.
Significantly predicting histologic variables; A–D, necrosis [A: grade 1 with one focus of necrosis (10×), B: grade 2 with multiple non-connected foci of necrosis (10×), C: grade 3 with interconnected necrosis but not geographic (4×); D: grade 4 with geographic necrosis (4×)]; E–H, lymphocytic infiltrate [E: grade 1 sparse non-aggregated (10×), F: grade 2 clustered (10×), G: grade 3 sheets not obscuring the tumor (10×), H: grade 4 sheets obscuring the tumor (10×)]. I–L, tumor/stroma ratio [I: ratio 10% (10×), J: ratio 30% (10×), K: ratio 60% (10×), L: ratio 90% (10×)], note low grade tumors in I and J, and high grade tumor in K and L
Table 2.
The correlation between each of the clinical variables and ORS
| Variable | r | r2 | p-value |
|---|---|---|---|
| Tumor size | 0.04 | 0 | 0.38 |
| ER Allred score | −0.46 | 0.21 | <0.01 |
| PR Allred score | −0.58 | 0.34 | <0.01 |
| Her2* | 0.16 | 0.03 | <0.01 |
| Tubular formation* | 0.12 | 0.01 | 0.01 |
| Nuclear grade* | 0.15 | 0.02 | <0.01 |
| Mitotic count* | 0.38 | 0.14 | <0.01 |
| Current NG* | 0.32 | 0.1 | <0.01 |
| Reported NG* | 0.14 | 0.02 | <0.01 |
| Necrosis | 0.36 | 0.13 | <0.01 |
| TIL | 0.22 | 0.05 | <0.01 |
| DCIS% | −0.09 | 0.01 | 0.08 |
indicate categorical variable,
NG, Nottingham Grade, TIL, tumor infiltrating lymphocytes
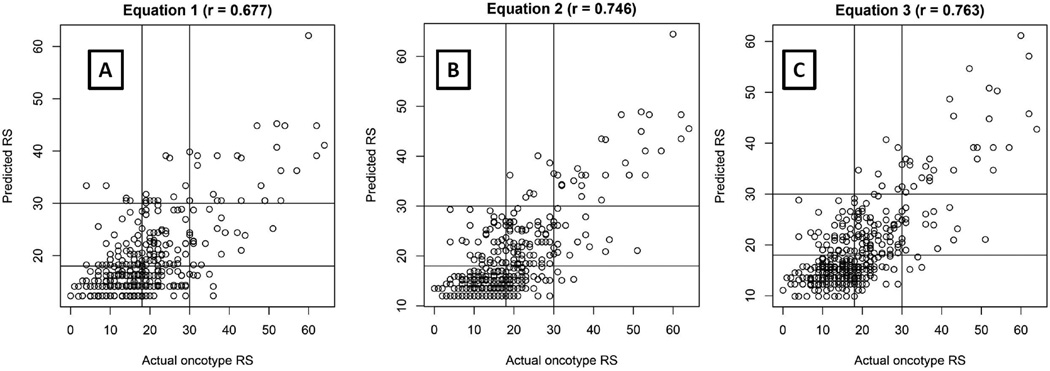
In the equation using the reported Nottingham grade, ER, PR and HER2, the overall concordance with the ORS was 64.86%. After excluding the intermediate category detected by the formula, the concordance rate was 95.28% (figure 2A). When the current Nottingham grade was used instead of the reported Nottingham grade, the concordance rate became 69.61% and 98.62%, respectively (figure 2B). When the histologic variables were added to the previous equation, we found tumor necrosis and TIL improved this equation. The rates became 70.1% and 98.63%, respectively (figure 2C).
Figure 2.
Graphical representation of scores estimated/predicted by using three different equations [(Y axis) versus actual ORS (X axis)]. Intercepts are drawn at ORS 18 and 30. Equation 1 represents reported Nottingham grade with no other histologic variables; Equation 2 represents current Nottingham grade with no other histologic variables; Equation 3 includes current Nottingham grade with other histologic variables; Note increasing r values.
This equation was produced: ORS=49.409 − 2.226xER − 1.633xPR + (−8.655 for Her2 negative, 0 for Her2 positive) + (0 for Nottingham Grade 1, 1.232 for Nottingham Grade 2, 9.346 for Nottingham Grade 3) + 2.205xNecrosis + 2.423x TIL.
The total number of patients who had either low or high RS using our formula was 291 of 408 (71.3%) (table 3). Only 3 patients were misclassified as having low RS using our formula instead of high RS using Oncotype DX assay. The characteristics of these three patients are listed in table 4. In the equation using ER, PR, HER2 and Nottingham grade, the concordance rate for the validation set was 72.53% and 100%, respectively (table 5). Adding necrosis and TIL did not improve the concordance rate.
Table 3.
Comparison of recurrence score (RS) categories and the estimated risk using our formula in the test set
| ORS>30 | ORS (18–30) | ORS<18 | Total | |
|---|---|---|---|---|
| Estimate RS-High | 28 | 8 | 0 | 36 |
| Estimate RS-Intermediate | 13 | 71 | 33 | 117 |
| Estimate RS-low | 3 | 64 | 188 | 255 |
| Total | 44 | 143 | 221 | 408* |
Eight patients with missing values; the total number of cases with low or high RS using our formula is 291
Table 4.
Characteristics of three patients who had high ORS but low RS using our formula
| Patient #1 | Patient #2 | Patient #3 | ||
|---|---|---|---|---|
| Age | 63 | 67 | 63 | |
| Surgery | Excision ALD | Excision ALD | Excision | |
| ORS | 35 | 34 | 38 | |
| RS using our formula | 15.57 | 17.6 | 15.2 | |
| ER | IHC (Allred score) | 8 | 6 | 8 |
| RT-PCR | Positive (8.5)* | Positive (11.1) | Positive (9.8) | |
| PR | IHC (Allred score) | 6 | 4 | 0 |
| RT-PCR | Positive (5.7) | Negative (3.2) | Negative (3.2) | |
| Tumor size (mm) | 9 | 8 | 38 | |
| Node status positive/total (N-stage) | 1/8 (1mi) | 1/3 (1mi) | 0/9 | |
| Histology | IC NST | IC NST | ILC | |
| Nottingham grade | II | II | III | |
| TIL | 1 | 0 | 1 | |
| Necrosis | 0 | 0 | 0 | |
| Therapy | Chemotherapy | Cytoxan/Taxotere | None** | Cytoxan/Taxotere |
| Hormonal therapy | Arimidex | Arimidex | Arimidex | |
| Radiation therapy | Post excision | Post excision | Post excision | |
| Survival | Alive and disease free (21 months) | Alive and disease free (24 months) | Bone metastases (28 months) Alive after 57 months |
Interpretation (ER Negative < 6.5, Positive ≥ 6.5; PR Negative < 5.5, Positive ≥ 5.5);
Declined chemotherapy;
ALD, axillary lymph node dissection; mi, micrometastases (>0.2-mm and <2-mm); IC NST, invasive carcinoma no special type; ILC, invasive lobular carcinoma; TIL, tumor infiltrating lymphocytes
Table 5.
Comparison of recurrence score (RS) categories and the estimated risk using ER, PR, HER2 and Nottingham grade in the validation set
| ORS>30 | ORS (18–30) | ORS<18 | Total | |
|---|---|---|---|---|
| Estimate RS-High | 4 | 1 | 0 | 5 |
| Estimate RS-Intermediate | 3 | 19 | 13 | 35 |
| Estimate RS-low | 0 | 8 | 43 | 51 |
| Total | 7 | 28 | 56 | 91 |
Comparing with new Magee’s equation
In order to compare with Magee’s equation we referred to their equation that included tumor size, Nottingham grade, and ER/PR/HER2. The correlation with ORS was 59.4% (overall) and 100% (after excluding the intermediate category) (10). The distribution of cases in our cohort in terms of ORS low, intermediate, and high was 54%, 35%, 10%, and in Klein's study was 46%, 41% 11%, respectively. Therefore, there were less cases with intermediate ORS and more cases with low ORS in our data than in Klein’s data. In order to examine this factor, we randomly select cases to match Klein's data. We included 351 cases from the original data with the ORS distribution for low, intermediate and high ORS, 165 (47%), 147 (41.9%), and 39 (11.1%), respectively. Then we calculated the concordance rate. They became 68.6% and 98.19%, respectively.
Subset analysis based on Nottingham grade and PR levels
We divided the data (test set) into six subgroups based on Nottingham grade (1, 2, and 3) and PR Allred score (≥5 and <5). We found that 3 of 144 (2.08%) cases with Nottingham grade 1 and PR≥ 5 had high ORS. All 26 cases with Nottingham grade 3 and PR <5 had intermediate (15.38%) or high (84.62%) ORS (table 6). Two of the three patients who had high ORS with low Nottingham grade and high PR score had HER2 gene amplification and treated with Herceptin. All three patients had positive lymph node and axillary lymph node dissection. One patient had low PR using RT-PCR (5.7) (table 7). In the validation set, all cases (n=15) that had Nottingham grade 1 and PR≥5 had low or intermediate ORS. All cases (n=6) that had Nottingham grade 3 and PR <5 had intermediate or high ORS.
Table 6.
Recurrence score categories based on Nottingham grade and PR levels (Allred score <5 vs. ≥5)
| ORS<18 | ORS (18–30) | ORS>30 | % of total cases (N=408)* |
|
|---|---|---|---|---|
| NG 1 + PR ≥ 5 | 103(71.53) | 38(26.39) | 3(2.08) | 144(35.29) |
| NG 1 + PR < 5 | 4(21.05) | 13(68.42) | 2(10.53) | 19(4.66) |
| NG 2 + PR ≥ 5 | 97(62.18) | 55(35.26) | 4(2.56) | 156(38.24) |
| NG 2 + PR < 5 | 6(23.08) | 15(57.69) | 5(19.23) | 26(6.37) |
| NG 3 + PR ≥ 5 | 11(29.73) | 18(48.65) | 8(21.62) | 37(9.07) |
| NG 3 + PR < 5 | 0(0) | 4(15.38) | 22(84.62) | 26(6.37) |
Eight patients with missing values;
NG, Nottingham grade; ORS, Oncotype DX recurrence score
Table 7.
Characteristics of three patients who had Nottingham grade 1, PR≥5 and high ORS
| Patient #1 | Patient #2 | Patient #3 | ||
|---|---|---|---|---|
| Age | 71 | 38 | 63 | |
| Surgery | Excision ALD | Excision ALD | Excision ALD | |
| ORS | 43 | 44 | 35 | |
| RS using our formula | 15.57 | 15.77 | 13.5 | |
| ER | IHC (Allred score) | 8 | 7 | 8 |
| RT-PCR | 10.7* | 8.9 | 8.5 | |
| PR | IHC (Allred score) | 8 | 8 | 6 |
| RT-PCR | 8.1 | 6.1 | 5.7 | |
| Tumor size (mm) | 15 | 11 | 9 | |
| Node status positive/total (N-stage) | 2/17 (1a) | 1/22 (1mi) | 1/8 (1mi) | |
| Histology | IC NST | IC NST | IC NST | |
| TIL | 1 | 1 | 2 | |
| Necrosis | 0 | 0 | 0 | |
| Therapy | Chemotherapy | Cytoxan/Taxotere **Herceptin |
**Herceptin | Cytoxan/Taxotere |
| Hormonal therapy | Arimidex | ***None | Arimidex | |
| Radiation therapy | Post excision | ***None | Post excision | |
| Survival | Alive and disease free after 41 months | Alive and disease free after 12 months | Alive and disease free after 17 months |
Interpretation (ER Negative < 6.5, Positive ≥ 6.5; PR Negative < 5.5, Positive ≥ 5.5);
HER2 positive by FISH;
patient refused therapy;
ALD, axillary lymph node dissection; mi, micrometastases (>0.2-mm and <2-mm); IC NST, invasive carcinoma no special type, TIL, tumor infiltrating lymphocyte
DISCUSSION
A large subset of patients with ER positive, node negative breast cancer do not benefit from chemotherapy. In order to identify this subset, multiple assays have been introduced into the market, with most widely used being Oncotype DX. This assay has been shown to correlate with the clinical outcome through multiple prospective studies (3–8). We recently found good correlation between Oncotype DX assay and IHC in detecting ER and PR status (15). Other studies have shown considerable discordance particularly for HER2 amplification (16). For many patients the information about benefits of chemotherapy can be obtained by merely examining multiple histopathology variables which would allow many more women to avoid unnecessary and toxic treatments. There have been multiple studies showing good correlation between ORS and the pathologic-generated equations in the past although in this study we present improvement over (9,10).
One of these equations is the new Magee equation. The correlation with ORS was 59.4% (overall) and 100% (after excluding the intermediate category) (10). When we compared our equation including the same variables the rate of concordance was 69.61% and 98.62%, respectively. There was significant difference in the rate of concordance between the two equations for the overall cases (59.4% vs. 69.61%). There are two possible reasons for this difference. The first reason is possibly due to our consistent review of the histologic slides and grading the tumor rather than extracting the Nottingham grade from the pathology report. In fact when we included the reported Nottingham grade in the equation, the concordance rate was 64.86% and 95.28% respectively. The other reason is possibly due to the difference in the distribution of the ORS (low, intermediate, high) between the two studies. After randomly selecting cases from our cohort to match Klein’s data, the rates became 68.6% and 98.19%, respectively. These rates were slightly less than the rates when all cases were included but our equation continued to perform significantly better than the new Magee’s equation. Therefore, we conclude that consistent review of the histologic slides could improve the existing equations.
It is known that there is a degree of interobserver variability in interpreting Nottingham grading. Fireson et al found a substantial agreement for tubule formation, moderate agreement for mitotic count and near moderate agreement for nuclear pleomorphism (17). We found variability between the current and reported Nottingham grade. While the “r” value for the first was 0.32, it was 0.14 for the second (table 2). This is considered a disadvantage of this equation or any equation that Nottingham grading is part of. Oncotype DX assay has the advantage of being more reproducible.
To use this formula in clinical practice, we recommend excluding cases that have intermediate RS. The total number of patients who had low or high RS using our formula was 291. Currently the cost of one Oncotype DX assay is about $4000 (18). Therefore, in the period of the study of 8 years, by using our formula we could have saved 291 × $4000 = $1.164.000.00 for the healthcare industry. However, the down side of this application is that a very small fraction of patients [3 of 291 (<1%)] could have been misclassified as having low RS while actually they have high RS. It is also worth noting that two patients (#1 and #2, table 4) had positive lymph nodes. The Oncotype DX was validated for node negative patients, making the ORS questionable. The third patient (#3, table 4) did not respond to chemotherapy and developed distant metastases., PR is one of the major genes in Oncotype DX recurrence score. We found discordance between these two assays in one patient (#2) and one patient (#1) had intermediate Allred score of 6 and low RT-PCR result (5.7). We have found previously that PR had higher discordance (positive vs. negative) between these two assays than ER does (15). This discordance might have played a role in the overall RS. This low cost formula would also allow clinicians in other parts of the world where Oncotype DX assay is not available, to provide their patients with the information to make best clinical decisions.
Acs et al found that cellular stroma and/or inflammatory cells associated with the tumor cells may contribute to intermediate or high ORS in low-grade invasive breast carcinomas (11). It is known that when the tissue undergoes Oncotype DX assay the samples are macrodissected which includes stroma and intimate inflammatory cells. When the stroma and/or inflammatory cells are mitotically active, the ORS would be falsely higher (19–21). In our study we separated the degree of TIL from the proportion of tumor/stroma. The reason for this separation was that the stroma of many cases with high stromal proportion was paucicellular. Tumors with paucicellular stroma - mitotically inactive- would not contribute in increasing the ORS. Therefore, this separation would help identifying each component’s (inflammatory cells vs. stroma) contribution in the ORS. We found that low stromal component being predictor of higher ORS. That is possibly due to the fact that many of the cases that had higher stromal proportion were paucicellular and therefore mitotically inactive which would not contribute to a high ORS (Fig 1I and 1J). We noticed that many of the tumors that had high tumor/stroma ratio also had high Nottingham grade and tumors with low tumor/stroma ratio had low Nottingham grade. When we performed Pearson correlation between tumor/stroma ratio and Nottingham grade, we found strong correlation (r=0.32, r2=0.11, p value <0.01). There was no correlation between tumor/stroma ratio with other adverse factors such as ER and PR expression. That is why when we performed the regression analysis we found that tumor/stroma ratio did not improve our equation.
We also found that higher degree of TIL and tumor necrosis predicted higher ORS. TIL has been found to indicate a better prognosis for many solid organ malignancies (22). The possible reason for this discordance is that the macrodissected tumor tested with Oncotype DX is contaminated with mitotically active lymphocytes consistent with what Acs et al found (11). However, the more likely explanation is that presence of TIL is indicative of genetic instability. Therefore, it is indicative of higher proliferation rate of the tumor itself and presence of multiple tumor antigens (23). This finding correlates with the high ORS and benefit from chemotherapy. We believe as the TIL scoring methods become standardized it will become a valuable tool in helping clinicians make treatment decisions. In order to minimize intra-observer and inter-observer variability we used infiltration pattern rather than TIL percentage. The latter has been suggested by the International TILs Working Group 2014 (24). It has been proven that tumor necrosis is an independent prognostic predictor for early recurrence and death in breast cancer (25). Therefore, we thought of examining its role in independently predicting the ORS. We found that not only the presence of necrosis predicting high ORS but also the degree of necrosis. In the validation set, necrosis and TIL did not improve the concordance rate. We think that is due to the small number of cases with high OSR (n=7). We conclude that adding both tumor necrosis and TIL improve the equation.
Allison et al investigated into the possibility of using subset analysis rather than a new equation to predict the ORS. They divided their data into six subgroups based on Nottingham grade (1, 2, and 3) and PR Allred score (≥5 and <5). They found that all cases (n=26) with Nottingham grade 1 and PR Allred score ≥5 had intermediate (n= 7) or low ORS (n= 19). Similarly, all cases (n=5) with Nottingham grade 3 and PR Allred score <5 had intermediate (n=1) or high (n=4) ORS (26). We performed similar analysis. We found that 3 of 144 (2.08%) cases had high ORS in the first category. We also found that none of the 26 patients had low ORS in the second category (table 6). The partial discordance between Allison's and our study is possibly due to our higher numbers of included cases in each category (144 vs. 26 and 26 vs. 5). The vast majority of patients with Nottingham grade 1 and PR Allred score ≥5 had low or intermediate ORS, while all patients who had Nottingham grade 3 and PR Allred score <5 had intermediate or high ORS. It is worth noting that the categorization suggested by Allison et al misclassified 3 of 144 (2.08%). Using our formula and including all cases, we misclassified 3 of 291 (<1%) of the patients. Therefore, we conclude that our formula is better than subcategorizing patients based on Allison’s et al proposal.
We conclude that our equation validated the Magee equation. Moreover, consistently interpreting Nottingham grade and incorporating tumor necrosis and TIL have shown to improve our equation. However, it should be noted that when more variables are added to any given equation, the reproducibility becomes weaker, which may negate this improvement. Our study is limited by the fact that the validation set was too small and had small number of cases with necrosis or high TIL. Therefore, additional studies with larger number of cases are needed to test the benefit of adding tumor necrosis and TIL to the equation.
REFERENCES
- 1.Pereira H, Pinder SE, Sibbering DM, et al. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathol. 1995;27:219–226. doi: 10.1111/j.1365-2559.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathol. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 5.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteva FJ, Sahin AA, Cristofanilli M, et al. Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res. 2005;11(9):3315–3319. doi: 10.1158/1078-0432.CCR-04-1707. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23(29):7265. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 8.Toi M, Iwata H, Yamanaka T, et al. Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer. 2010;116(13):3112–3118. doi: 10.1002/cncr.25206. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan MB, Dabbs DJ, Brufsky AM, Beriwal S, Bhargava R. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol. 2008;21(10):1255–1261. doi: 10.1038/modpathol.2008.54. [DOI] [PubMed] [Google Scholar]
- 10.Klein ME, Dabbs DJ, Shuai Y, et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol. 2013;26(5):658–664. doi: 10.1038/modpathol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acs G, Esposito NN, Kiluk J, Loftus L, Laronga C. A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX Recurrence Scores in low-grade invasive breast carcinomas. Mo. Pathol. 2012;25(4):556–566. doi: 10.1038/modpathol.2011.194. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J C Oncol. 2013;32:1–18. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 14.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathol. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Khoury T, Yan L, Liu S, Bshara W. Oncotype DX® RT-qPCR assay for ER and PR correlation with IHC: a study of three different clones. App Immunohistochem & Molec Morphol. 2015;23(3):178–187. doi: 10.1097/PAI.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 16.Dabbs DJ, Klein ME, Mohsin SK, Tubbs RR, Shuai Y, Bhargava R. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol. 2011;29(32):4279–4285. doi: 10.1200/JCO.2011.34.7963. [DOI] [PubMed] [Google Scholar]
- 17.Fireson HF, Jr, Wolber RA, Berean KW, et al. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. A J Clin Pathol. 1995;103(2):195–198. doi: 10.1093/ajcp/103.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J. Pathol. 2010;220:263–280. doi: 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- 20.Baehner F, Quale C, Pomeroy C, et al. Biopsy cavities in breast cancer specimens: their impact on quantitative RT-PCR gene expression profiles and recurrence risk assessment (abstract) Mod Pathol. 2008;22:28A–29A. [Google Scholar]
- 21.Diab SG, Clark GM, Osborne CK, et al. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clil Oncol. 1999;17:1442–1448. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 22.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumor infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SD, Warren RL, Gibb EA, et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. An Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilchrist KW, Gray R, Fowble B, Tormey DC, Taylor SG., 4th Tumor necrosis is a prognostic predictor for early recurrence and death in lymph node-positive breast cancer: a 10-year follow-up study of 728 Eastern Cooperative Oncology Group patients. J Clin Oncol. 1993;11:1929–1935. doi: 10.1200/JCO.1993.11.10.1929. [DOI] [PubMed] [Google Scholar]
- 26.Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, Gown AM. Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing? Breast Cancer Res Treat. 2012;131(2):413–424. doi: 10.1007/s10549-011-1416-3. [DOI] [PubMed] [Google Scholar]