Abstract
Background
Magnetic resonance imaging (MRI) and targeted biopsies (TB) have shown potential to more accurately detect significant prostate cancer (PC) compared to prostate-specific antigen (PSA) and systematic biopsies (SB).
Objective
To compare sequential screening (PSA + MRI) with conventional PSA screening.
Design, Setting and Participants
Of 384 attendees in the 10th screening round of the Göteborg randomised screening trial, 124 men, median age 69.5, had a PSA of ≥1.8 ng/ml and underwent a prebiopsy MRI. Men with suspicious lesions on MRI and/or PSA ≥3.0 ng/ml were referred for biopsy. SB was performed blinded to MRI results and TB was performed in men with tumour-suspicious findings on MRI. Three screening strategies were compared (PSA≥3.0+SB; PSA≥3.0+MRI+TB and PSA≥1.8+MRI+TB).
Outcome Measurements and Statistical Analysis
Cancer detection rates, sensitivity and specificity were calculated per screening strategy and compared using McNemar´s test.
Results and Limitations
In total, 28 PC were detected, of which 20 were diagnosed in biopsy-naïve men. Both PSA≥3.0+MRI and PSA≥1.8+MRI significantly increased specificity compared with PSA≥3.0+SB (0.92 and 0.79 vs. 0.52; p<0.002 for both), while sensitivity was significantly higher for PSA≥1.8+MRI compared with PSA>=3.0+MRI (0.73 vs. 0.46, p=0.008). The detection rate of significant cancer was higher with PSA≥1.8+MRI compared to PSA≥3.0+SB (5.9 vs. 4.0%), while the detection rate of insignificant cancer was lowered by PSA≥3.0+MRI (0.3 vs. 1.2%). The primary limitation of this study is the small sample of men.
Conclusion
A screening strategy with a lowered PSA cut-off followed by TB in MRI-positive men seems to increase the detection of significant cancers while improving specificity. If replicated, these results may contribute to a paradigm shift in future screening.
Patient Summary
Major concerns in prostate-specific antigen screening are overdiagnosis and underdiagnosis. We evaluated whether prostate magnetic resonance imaging could improve the balance of benefits to harm in prostate cancer screening, and we found promising potential of using magnetic resonance imaging in addition to prostate-specific antigen.
Introduction
Prostate-specific antigen (PSA)-based screening has proved effective in reducing prostate cancer (PC)-specific mortality(1, 2), but is associated with overdiagnosis of low-malignant cancers, and underdiagnosis of potentially lethal cancers(3, 4). There is a need for a more effective screening strategy that improves upon the benefits in terms of increased sensitivity and reduces harm by avoiding unnecessary biopsies and overdiagnosis.
The current diagnostics for PC (PSA, digital rectal examinations and systematic transrectal ultrasound (TRUS)-guided biopsy) are far from perfect. Firstly, the specificity of PSA is low, no cut-off value can rule out PC and only about 25% of men with PSA 3–10 ng/ml have cancer on TRUS-guided biopsy(2, 5, 6). Secondly, the systematic biopsy (SB)-approach is a limitation for diagnostic accuracy. TRUS-guided SB are upgraded in as much as 35–46% of cases after radical prostatectomy(7–9), with anterior and transition-zone cancers frequently being missed(10). Overdiagnosis is another problem as 30–60% of men in the ages 50–70 years harbour histologically evident PC(11–13). Screen-detected cancers are predominately (60%) low-risk cancers with a Gleason score (GS) ≤6 and PSA <10 ng/ml, and a large proportion (about 33%) are clinically insignificant according to Epstein criteria(14, 15).
Multiparametric magnetic resonance imaging (MRI) combining T2-weighted, dynamic contrast-enhanced and diffusion weighted imaging (DWI) has been shown to be accurate in detecting significant PC(16–18). To our knowledge, no randomised study has evaluated MRI as an up-front tool in population-based PC-screening. The aim of this pilot study was to explore the efficacy of current screening compared with novel screening strategies including using MRI and PSA at different cut-offs. We are launching a large randomised trial to evaluate the potential benefit of MRI in PSA-screening. The results presented are from the pilot study conducted during 2013–2014.
Methods
Patients
The pilot study was nested within the 10th and last screening round of the Göteborg randomised screening trial, in which 20,000 men aged 50–64 years were randomised to a screening and a control group in 1995. Men in the screening group received invitations to PSA-screening biennially until an upper age limit (average 69 years). A PSA ≥3.0 ng/ml (corresponding to a PSA of ≥2.54 ng/ml if calibrated to the World Health Organisation) was considered an indication for TRUS-guided SB. Controls were not invited. The design of the Göteborg randomised screening trial has been described previously(2).
MRI
All examinations were performed using a 3Tesla system (Philips Achieva 3.0, Philips Healthcare, Best, the Netherlands). During the first part of the study, a SENSE Cardiovascular Array Coil with 32 overlapping elements was used. During the study period the system was upgraded and a digital coil system (dStream Torso with integrated anterior and posterior coils) was used (no endorectal coil). Three sequences were used: T2-weighted, dynamic contrast-enhanced and DWI. For DWI, b-values 0–1000 were used. Apparent diffusion coefficient-maps were calculated and qualitatively assessed. MR-spectroscopy was not performed. Suspicious lesions were pointed out in a diagram by region in the transversal and sagittal plane and scored according to the validated Prostate Imaging Reporting and Data System for each sequence, ranging from 1 to 5 according to the likelihood of significant PC presence(16, 19–21). A score in any sequence of ≥3 (equivocal) was regarded as positive. All images were read in consensus by three radiologists of whom two had several years’ experience of MRI-reading.
Study algorithm
Men with PSA ≥1.8 ng/ml were referred for evaluation with MRI (sequential testing). Men with a positive MRI and/or those with PSA of ≥3.0 ng/ml were referred for biopsy at a second visit. One urologist (J.H.) performed all biopsies. The 10-core TRUS-guided SB was sampled first, blinded to MRI results, using a scheme with 12 anterior and 12 posterior sectors of which 10 posterior were sampled routinely. The MRI results were then revealed, and MRI-targeted biopsy (TB) was performed on men with cancer-suspicious findings on MRI through three additional cores sampled per suspicious region by means of “cognitive” targeting. Men with negative MRIs and PSA <3.0 ng/ml were assumed cancer-free and released without further work-up.
Through this algorithm, three different screening strategies were identified:
PSA ≥3.0 ng/ml followed by SB (“reference strategy”)
PSA ≥3.0 ng/ml+MRI followed by TB in MRI-positive men (no SB)
PSA ≥1.8 ng/ml+MRI followed by TB in MRI-positive men (no SB)
Each man was defined as screen-positive or screen-negative according to each of these strategies. Similarly, cancers were defined as screen-detected or missed by each respective strategy, including whether detected at SB, TB, or both.
Classification of cancers
Cancers were classified according to the modified Epstein criteria for clinically insignificant PC (clinical stage (digital rectal examinations only) T1c, PSA density <0.15, GS <7, ≤2 positive cores, and unilateral cancer)(15, 22). Due to sampling differences between MRI-positive (10 SB + minimum three TB) and MRI-negative men (10 SB), we translated number of “positive cores” into number of “positive sectors” in order not to overestimate cancer extent in men who underwent TB.
Statistics
The main outcome measurement was cancer detection rates, defined as the proportion of men screened that had screen-detected PC according to screening strategy 1, 2 and 3. Strategy 1 was regarded as a reference. We observed a higher MRI-attendance among men with PSA ≥3.0 ng/ml than among men with PSA 1.8–2.99 ng/ml. Therefore, in comparisons between the screening strategies, we corrected for this imbalance by calculating the cancer yield with Strategy 2 and 3 as if all men with an indication for MRI consequently underwent MRI, followed by TB if indicated. Similarly, we calculated the outcome for Strategy 1 as if all men with an indication for SB actually underwent SB.
Point estimates for the statistics sensitivity, specificity, and positive and negative predictive values were calculated as row or column percentages of the two-by-two tables. The binominal option provided exact confidence intervals. Analyses were made using the free statistical software R, utilizing the package DTComPair(23, 24). A p value for comparing sensitivities and specificities were calculated with McNemar's test. A p value for comparing positive predictive value and negative predictive value were calculated using the method described by Moskowitz and Pepe(25).
The number needed to biopsy to detect one PC was calculated, as well as number needed to undergo MRI+TB, to detect one PC. Biopsy modes were compared (SB vs. TB) with regards to cancer yield by using the Z-Test for population proportions.
Ethical committee
The ethical committee at the Göteborg University (approval number 130408) approved the study.
Results
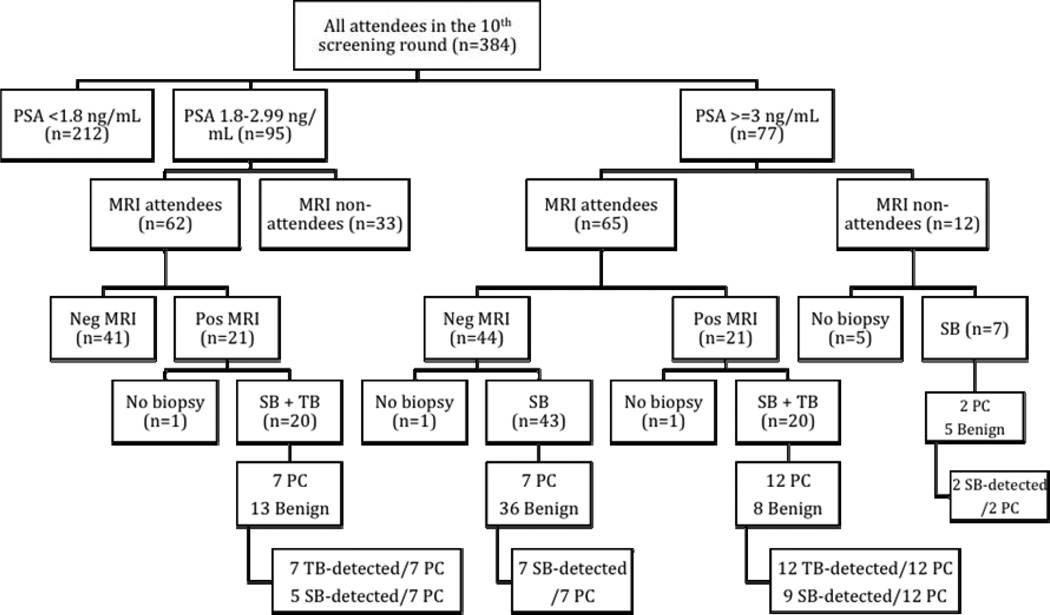
The 10th screening round of the Göteborg screening trial took place during 2013–2014, and of the 596 men invited, 384 (64%) attended. The median age was 69.3 years (interquartile range (IQR) 69.0–69.6) and the median PSA was 1.6 ng/ml (IQR 0.9–2.7). Of the 172 men with a PSA ≥1.8 ng/ml (median 2.9, IQR 2.3–3.8), 127 underwent a prebiopsy MRI. In total, one third (42/127) of MRIs were positive, and in almost half (19/40) of those cancer was detected at TB. Nine additional cancers were detected in 56 men undergoing SB only (following a negative MRI or nonattendance at MRI). The overall cancer detection rate was 7.3% (28/384)(Figure 1).
Figure 1.
Diagram of the pilot study conducted within the 10th screening round of the Göteborg randomised screening trial.
* PSA = prostate specific antigen, PC = prostate cancer, Pos = positive, Neg = negative, SB = systematic biopsy, TB = targeted biopsy,
Of the three screening strategies analysed, the most effective in terms of cancer detection rate was Strategy 3. Compared with the reference, this strategy yielded a 48% higher detection rate of significant PC (5.9 vs. 4.0%), and a 38% higher detection rate of GS≥7 PC (3.7 vs. 2.6%). The cancer detection rate with Strategy 2 was lower than with the reference. However, Strategy 2 detected the fewest insignificant cancers and was most efficient in terms of number needed to biopsy (Table 1 and 4).
Table 1.
Cancer detection rates in the 10th screening round of the Göteborg randomised screening trial, by three different screening strategies.
| Attendees in the 10th screening round (n=384) |
PSA ≥3 (SB) | PSA ≥3 + MRI (TB) |
PSA ≥1.8 + MRI (TB) |
|---|---|---|---|
| No. of men with elevated PSA | 77 | 77 | 172 |
| No. of men with MRI indication (proportion) | 0 | 77/384 (20%) | 172/384 (45%) |
| No. of men undergoing MRI (proportion) | 0 | 65/77 (84%) | 127/172 (74%) |
| No. of men with positive MRI (proportion) | 0 | 21/65 (32%) | 42/127 (33%) |
| No. of men with bx indication (proportion†) | 77 (20%) | 21 (6.5%) | 42 (15%) |
| No. of men biopsied (proportion) | 70/77 (91%) | 20/21(95%) | 40/42 (95%) |
| No. of PC detected (rate††) | 18 (5.2%) | 12 (3.9%) | 19 (7.0%) |
| No. of significant PC (rate) | 14 (4.0%) | 11 (3.6%) | 16 (5.9%) |
| No. of insignificant cancer (rate) | 4 (1.2%) | 1 (0.32%) | 3 (1.1%) |
| No. of GS ≥7 PC (rate) | 9 (2.6%) | 7 (2.3%) | 10 (3.7%) |
| No. of GS 6 PC (rate) | 9 (2.6%) | 5 (1.6%) | 9 (3.3%) |
Proportions calculated with the following formula: number of men with a positive MRI, divided by number of men attending MRI, multiplied by proportion of men with elevated PSA.
Rates calculated with the following formula: number of cancers detected, divided by number of men biopsied, multiplied by proportion of men with a biopsy indication.
Cancers classified as significant or insignificant according to the modified Epstein criteria (insignificant cancer = Clinical stage T1c, Gleason score ≤6, PSA density ≤0.15 ng/ml/m3, ≤2 sectors with cancer, unilateral cancer).
PC = prostate cancer, bx = biopsy, GS = Gleason score, PSA = prostate-specific antigen, MRI = magnetic resonance imaging, TB = targeted biopsy, SB = systematic biopsy
Table 4.
Estimated test performance for prostate cancer detection of three different screening strategies.
| 1. PSA≥3 (SB) | 2. PSA≥3 + MRI (TB) | 3. PSA ≥1.8 + MR (TB) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | |
| Sensitivity | 0.64 | 0.47 – 0.82 | 0.46 | 0.27 – 0.65 | 0.73 | 0.56 – 0.90 | |||
| Specificity | 0.52 | 0.43 – 0.62 | 0.92 | 0.86 – 0.97 | 0.79 | 0.70 – 0.87 | |||
| PPV | 0.27 | 0.16 – 0.37 | 0.60 | 0.39 – 0.81 | 0.48 | 0.32 – 0.63 | |||
| NPV | 0.84 | 0.75 – 0.93 | 0.87 | 0.80 – 0.93 | 0.92 | 0.86 – 0.98 | |||
| NNB per PC | 4 | 2 | 2 | ||||||
| NNB per sign PC | 5 | 2 | 3 | ||||||
| NNB per GS ≥7 PC | 8 | 3 | 4 | ||||||
| NNMRI + TB per PC | - | 5 | 7 | ||||||
| NNMRI + TB per sign PC | - | 6 | 8 | ||||||
| NNMRI + TB per GS ≥7 PC | - | 9 | 12 | ||||||
PC = prostate cancer, PSA = prostate-specific antigen, MRI = magnetic resonance imaging, TB = targeted biopsy, SB = systematic biopsy, CI = confidence interval, sign = significant, n = number, NNB = number needed to biopsy, NNMRI = number needed to undergo MRI
The proportion of men with an indication for biopsy decreased from 20% with the reference strategy to 6.5% with Strategy 2 and 15% with Strategy 3. In absolute numbers, these proportions corresponded to 70 SB with the reference, 20 TB with Strategy 2, and 40 TB with Strategy 3. Of the 40 TBs performed, 35 (88%) only required three cores (corresponding to one MRI-lesion). Apart from the 70 SB performed in men with PSA ≥3.0 ng/ml, another 20 SB were performed in men with a positive MRI and PSA 1.8–2.99 ng/ml, so, in total, 90 SB were performed within this study. TB was significantly more effective than SB on a per-patient basis, with a cancer-positivity rate of 48% (19/40) vs. 26% (23/90), p=0.014 and a cancer-positivity rate for significant PC of 40% (16/40) vs. 20% (18/90), p=0.017. The cancer-positivity correlating with Prostate Imaging Reporting and Data System 3/4/5 were 37%, 75%, and 100%. Corresponding rates for significant PC was 23%, 75%, and 100% (Table 6).
Table 6.
Correlation between Prostate Imaging Reporting and Data System score and cancer at the following targeted biopsy in men with positive magnetic resonance imaging.
| PIRADS | 3 | 4 | 5 |
|---|---|---|---|
| Positive MRI* | 30 | 4 | 5 |
| Cancer at TB (proportion) | 11 | 3 | 5 |
| Gleason ≥7 PC at TB (proportion) | 4 (13%) | 1 (25%) | 5 (100%) |
| Significant PC (proportion) | 7 (23%) | 3 (75%) | 5 (100%) |
One man missing compared with Figure 1 and Table 1 due to a missing PIRADS score. He had an MRI classified as positive and underwent TB but the lesion was located in the left vesicula seminalis and hence, no PIRADS were determined. The biopsies turned out benign.
PIRADS = Prostate Imaging Reporting and Data System, TB = targeted biopsy, MRI = magnetic resonance imaging, PC = prostate cancer
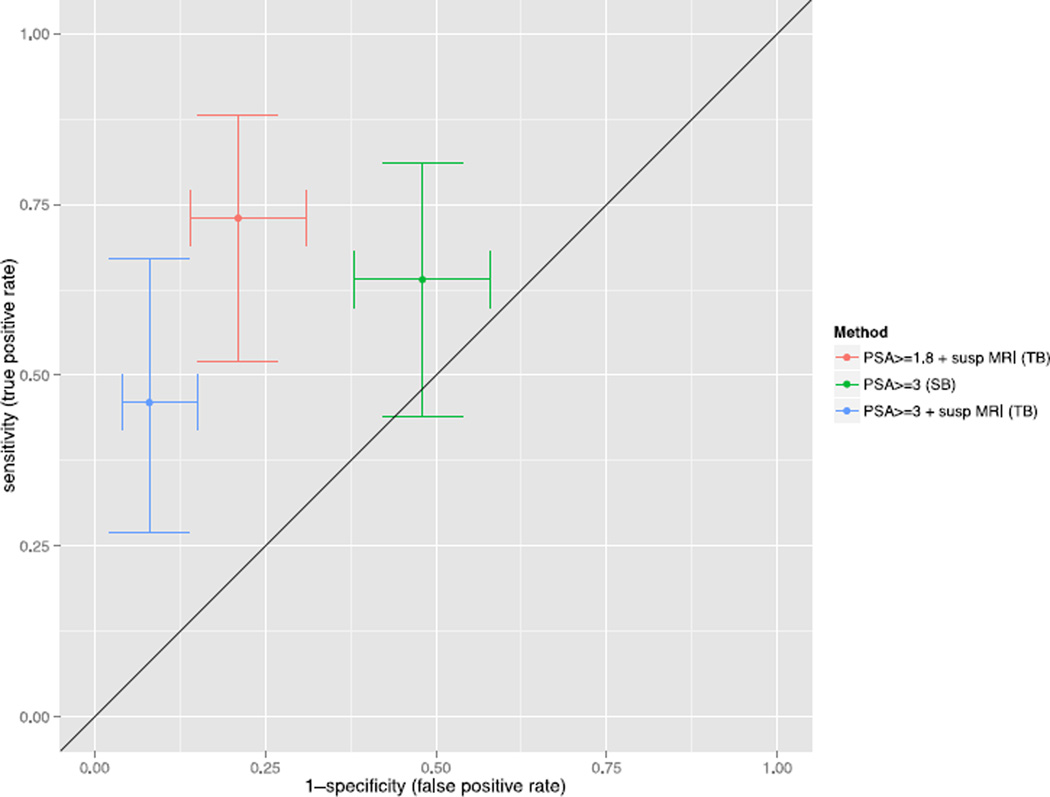
Seven PC were detected at TB among men with a positive MRI and PSA 1.8–2.99 ng/ml (Table 2). Three of those were GS 3+4, and, in total, four were significant. In total, seven cancers were not depicted by MRI, of which the majority were low-risk cancers; although two were GS 3+4, and, in total, three were significant (Table 3). Test performances of the three screening strategies are given in Figure 2, Table 4 and Table 5.
Table 2.
Characteristics of seven cases of prostate cancer detected by magnetic resonance imaging + targeted biopsy in men with PSA 1.8–2.99 ng/ml
| PSA | T-stage | Gleason | Biopsy mode | No. of sectors with cancer |
Modified Epstein criteria |
|
|---|---|---|---|---|---|---|
| 1 | 2.32 | T2a | 3+4=7 | SB + TB | 5/ 10 | S |
| 2 | 2.57 | T2a | 3+4=7 | SB + TB | 6/10 | S |
| 3 | 1.82 | T1c | 3+4=7 | SB + TB | 5/10 | S |
| 4 | 1.94 | T2a | 3+3=6 | SB + TB | 2/10 | S |
| 5 | 2.04 | T1c | 3+3=6 | SB + TB | 2/10 | IS |
| 6 | 2.94 | T1c | 3+3=6 | TB | 2/10 | S |
| 7 | 2.89 | T1c | 3+3=6 | TB | 1/10 | IS |
Table 3.
Characteristics of seven cases of prostate cancer detected by systematic biopsy with no abnormality on magnetic resonance imaging and no indication for targeted biopsy.
| PSA | T-stage | Gleason | Biopsy mode | No. of sectors with cancer |
Modified Epstein criteria |
|
|---|---|---|---|---|---|---|
| 1 | 3.47 | T1c | 3+3=6 | SB | 1/10 | IS |
| 2 | 4.05 | T1c | 3+3=6 | SB | 3/10 | S |
| 3 | 3.53 | T1c | 3+3=6 | SB | 1/10 | IS |
| 4 | 3.32 | T1c | 3+4=7 | SB | 1/10 | S |
| 5 | 6.83 | T1c | 3+4=7 | SB | 4/10 | S |
| 6 | 4.04 | T1c | 3+3=6 | SB | 1/10 | S |
| 7 | 3.03 | T1c | 3+3=6 | SB | 1/10 | IS |
Modified Epstein criteria for insignificant cancer (IS) = Clinical stage T1c, Gleason score ≤6, PSA density ≤0.15 ng/ml/m3, ≤2 sectors with cancer, unilateral cancer. PSA = Prostate-specific antigen, SB=Systematic biopsy, TB=Targeted biopsy, S=Significant, IS=Insignificant
Figure 2.
Estimated sensitivity and specificity of prostate cancer detection depending on screening strategy (prostate-specific antigen (PSA ≥3 ng/ml followed by systematic biopsy, PSA ≥3.0 ng/ml followed by targeted biopsy, and PSA ≥1.8 ng/ml followed by targeted biopsy). Bars indicate 95% confidence intervals for sensitivity (y-axis) and 1-specificity (x-axis).
* PSA = prostate specific antigen, Susp MRI = suspicious magnetic resonance imaging (see ‘Methods’ for details), SB = systematic biopsy, TB = targeted biopsy
Table 5.
Comparison between screening strategies for significant differences,
| Strategy 1 vs. 2 | Strategy 1 vs. 3 | Strategy 2 vs. 3 | |
|---|---|---|---|
| Sensitivity | 0.21 | 0.47 | 0.008 |
| Specificity | <0.001 | 0.001 | <0.001 |
| PPV | <0.001 | 0.006 | 0.09 |
| NPV | 0.55 | 0.17 | 0.03 |
PPV = positive predictive value, NPV = negative predictive value
Previous biopsy
Attendees in the 10th round were previously screened to a large extent. Only 2.0% were first-time screenees; the remaining 98% were PSA-screened one to nine times before. Of the 90 men referred for biopsy, 57 (63%) were biopsy-naïve and 33 (37%) previously biopsied. Of men attending MRI, 53 were biopsy-naïve and 29 were previously biopsied. The biopsy-naïve were three times more likely to have a positive MRI (64%; 34 of 53 men) than those previously biopsied (21%; six of 29 men). However, the risk that the MRI lesion revealed cancer at TB was similar in biopsy-naïve (47%; 16 cancers found at TB in 34 men) and previously biopsied men (50%; three cancers found at TB in six men).
Discussion
There is an urgent need for a better PC-screening strategy that can circumvent the low specificity of PSA. This pilot study shows promising potential of using MRI in screening to identify harmful cancers and minimize unnecessary biopsies. Instead of referring all men with PSA ≥3.0 ng/ml for SB, sequential screening with MRI as an indication for TB (without SB) in men with PSA ≥1.8 ng/ml reduced the proportion of men biopsied by 26% while increasing the detection rate of significant cancers by 48% (from 4.0 to 5.9%) and the detection of GS≥7 cancers by 43% (from 2.6 to 3.7%). The full-sized trial, Göteborg-2, has started to further establish the role of MRI in screening for PC.
Imaging with MRI plays two roles in PC-screening. Firstly, it functions as a secondary screening test, exempting men with non-suspicious tests from biopsy. Sequential screening is a strategy that can increase specificity and, according to the World Health Organisation, is a recommended option in cervical cancer screening(26). However, sequential testing might jeopardise sensitivity(27). In this study, MRI reduced the need for biopsy by 68% in men with PSA ≥3.0 ng/ml (common cut-off for biopsy), but at a cost of lowered sensitivity. PSA ≥3.0 ng/ml+MRI missed three significant PCs (of which two were GS 3+4 cancers). However, two significant cancers were detected by MRI-TB only in those men (PSA ≥3.0 ng/ml), while missed by the standard SB approach.
The second function of MRI is to provide an image of the lesion(s) so that sampling can be more precise. In this study, TB was significantly more accurate than SB on a per-patient basis in terms of cancer-positivity rate (48 vs. 26%, p=0.014). More importantly, TB outperformed SB in detecting clinically significant PC (40 vs. 20%, p=0.017). These findings are in line with several studies showing a greater accuracy of MRI-TB than SB in the diagnosis of PC. Hambrock et al (17) reported a significantly improved overall accuracy of TB (88%) over SB (55%) in determining Gleason grade. Haffner et al (28) reported a significantly improved overall accuracy of 98% with TB vs. 88% with SB in biopsy-naïve men referred for prebiopsy MRI, and that TB detected 16% more GS≥7 cancers. Puech et al (29) reported a 67% yield of significant PC with TB vs. 52% with SB in 67 men with suspicious prebiopsy MRIs out of 95 men with a clinical suspicion of localised PC.
We analyzed three different screening strategies, of which Strategy 2 was most accurate taking both sensitivity and specificity into account (Table 4). MRI seems to be of great value but the optimal cut-off for PSA to select men for MRI is not clear, as a lowered PSA cut-off was required to maintain sensitivity. Although the cut-off 1.8 ng/ml was arbitrarily chosen in this study, it was based on previous studies indicating that the risk of significant cancer increases gradually from levels around 1–3 ng/ml(30–32). The sample size in this study was too small to analyze, for example, a PSA cut-off at 2.5 ng/ml, but such calculations will be possible in the full-sized Göteborg-2 study.
One of the major concerns in PC screening is overdiagnosis. Although imaging with MRI is resource-consuming the cost of overdiagnosis and biopsy complications is alarming. The high proportion of men with an indication for MRI in this study (45% had a PSA ≥1.8 and 20% PSA≥3 ng/mL) is explained by the high age (median 69 years). A modelling study recently demonstrated that costs with MRI equaled that of standard care but improved quality of life (33). According to our results, MRI can be used to avoid unnecessary biopsies and to potentially reduce overdiagnosis but whether it is costeffective in routine screening remains to be evaluated. This question is also evaluated in ongoing studies in patients with a clinical suspicion of PC (PROMIS and PRECISION)(34, 35).
It is worth considering that men in this study had been invited to PSA-screenings at as many as nine times during 19 years. Repeated screening reduces cancer incidence(36) and advanced disease(37). Yet, MRI proved to be of great value even with repeatedly screened men with a similar PPV of MRI in biopsy-naïve and previously biopsied men (48 vs. 50%). This indicates that MRI may be valuable even in follow-up screenings. Also, the negative predictive value was very high (87–92%) and in concordance with previous studies(38).
As stated above, men with PSA <3.0 ng/ml and a negative MRI were assumed cancer-free. Verification bias may occur if disease verification differs according to test results. In an attempt to test for this, we performed a separate sensitivity analysis, where five hypothetical cancers were simulated to be present among men with nonsuspicious MRIs and PSA values between 1.8–2.99 ng/ml (data not shown). The choice of picking five cancers was based on findings from the Prostate Cancer Prevention Trial, which demonstrated a PC-prevalence of 19% (285/1480) in men with PSA 1.1–3.0 ng/ml(6). This analysis revealed that the significant differences between the three strategies remained with unchanged specificity but at a reduced sensitivity.
Another study limitation is that we lack information regarding interobserver variability among MRI readers. Another potential limitation is that fusion technology was not used to target biopsies (required equipment unavailable). In the large-scaled trial, comparisons between fusion and cognitive biopsies will be performed.
In summary, despite a heavily prescreened population, we found MRI to be a useful tool in detecting more significant PC compared with standard PSA-screening. Whether the extra cost associated with MRI outbalances the burden of unnecessary biopsies and overdiagnosis needs to be assessed in larger, prospective studies, but this pilot study indicates a plausible way out of the dilemma in PC screening.
Conclusion
A new screening strategy for PC involving imaging with MRI appears to be highly accurate in detecting significant cancer and minimising unnecessary biopsies. If verified in the full-sized trial, these pilot results may contribute to a paradigm shift in the approach to early detection of PC.
Acknowledgments
Funding: The Swedish Research Council, Swedish Cancer Society, University of Gothenburg, Biocare, Druid Ordern, Knut and Alice Wallenberg’s Foundation, Anna-Lisa and Bror Björnssons foundation. Carlsson’s work on this paper was supported in part by a Cancer Centre Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30 CA008748). Carlsson is also supported by a postdoctoral grant from AFA Insurance.
The authors thank Helén Ahlgren, data manager and responsible for the study secretary, and Maria Nyberg, study nurse for excellent contribution in collection of data for this study.
Footnotes
Disclosures: None.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 doi: 10.1016/S0140-6736(14)60525-0. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. The lancet oncology. 2010;11(8):725–732. doi: 10.1016/S1470-2045(10)70146-7. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA : the journal of the American Medical Association. 2005;294(1):66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Bak JB, Landas SK, Haas GP. Characterization of prostate cancer missed by sextant biopsy. Clinical prostate cancer. 2003;2(2):115–118. doi: 10.3816/cgc.2003.n.019. [DOI] [PubMed] [Google Scholar]
- 5.Holmstrom B, Johansson M, Bergh A, Stenman UH, Hallmans G, Stattin P. Prostate specific antigen for early detection of prostate cancer: longitudinal study. BMJ. 2009;339:b3537. doi: 10.1136/bmj.b3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level< or =4.0 ng per milliliter. The New England journal of medicine. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 7.Kvale R, Moller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU international. 2009;103(12):1647–1654. doi: 10.1111/j.1464-410X.2008.08255.x. Epub 2009/01/22. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. European urology. 2012;61(5):1019–1024. doi: 10.1016/j.eururo.2012.01.050. Epub 2012/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. The Journal of urology. 2001;166(1):104–109. discussion 9–10. [PubMed] [Google Scholar]
- 10.Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78(6):1356–1362. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In vivo. 1994;8(3):439–443. [PubMed] [Google Scholar]
- 12.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. Journal of the National Cancer Institute. 2013;105(14):1050–1058. doi: 10.1093/jnci/djt151. [DOI] [PubMed] [Google Scholar]
- 13.Soos G, Tsakiris I, Szanto J, Turzo C, Haas PG, Dezso B. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. European urology. 2005;48(5):739–744. doi: 10.1016/j.eururo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. European urology. 2013;63(1):101–107. doi: 10.1016/j.eururo.2012.08.066. Epub 2012/09/18. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA : the journal of the American Medical Association. 1994;271(5):368–374. Epub 1994/02/02. [PubMed] [Google Scholar]
- 16.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. European radiology. 2012;22(4):746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. European urology. 2012;61(1):177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Sciarra A, Barentsz J, Bjartell A, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. European urology. 2011;59(6):962–977. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU international. 2013;112(8):1080–1087. doi: 10.1111/bju.12259. [DOI] [PubMed] [Google Scholar]
- 20.Kayat Bittencourt L, Litjens G, Hulsbergen-van de Kaa CA, Turkbey B, Gasparetto EL, Barentsz JO. Prostate Cancer: The European Society of Urogenital Radiology Prostate Imaging Reporting and Data System Criteria for Predicting Extraprostatic Extension by Using 3-T Multiparametric MR Imaging. Radiology. 2015;276(2):479–489. doi: 10.1148/radiol.15141412. [DOI] [PubMed] [Google Scholar]
- 21.Roethke MC, Kuru TH, Schultze S, et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. European radiology. 2014;24(2):344–352. doi: 10.1007/s00330-013-3017-5. [DOI] [PubMed] [Google Scholar]
- 22.Kryvenko ON, Carter HB, Trock BJ, Epstein JI. Biopsy criteria for determining appropriateness for active surveillance in the modern era. Urology. 2014;83(4):869–874. doi: 10.1016/j.urology.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Christian Stock TH. DTComPair: comparison of binary diagnostic tests in a paired study design. R package version 1.0.3. R package version 1.0.3. ed2014 [Google Scholar]
- 24.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25.Moskowitz CS, Pepe MS. Comparing the predictive values of diagnostic tests: sample size and analysis for paired study designs. Clin Trials. 2006;3(3):272–279. doi: 10.1191/1740774506cn147oa. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva: 2013. [PubMed] [Google Scholar]
- 27.Tebeu PM, Fokom-Domgue J, Crofts V, et al. Effectiveness of a two-stage strategy with HPV testing followed by visual inspection with acetic acid for cervical cancer screening in a low-income setting. International journal of cancer Journal international du cancer. 2014 doi: 10.1002/ijc.29250. [DOI] [PubMed] [Google Scholar]
- 28.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU international. 2011;108(8 Pt 2):E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 29.Puech P, Rouviere O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268(2):461–469. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 30.Randazzo M, Beatrice J, Huber A, et al. A "PSA Pyramid" for Men with Initial Prostate-specific Antigen</=3 ng/ml: A Plea for Individualized Prostate Cancer Screening. European urology. 2014 doi: 10.1016/j.eururo.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugosson J, Aus G, Becker C, et al. Would prostate cancer detected by screening with prostate-specific antigen develop into clinical cancer if left undiagnosed? A comparison of two population-based studies in Sweden. BJU international. 2000;85(9):1078–1084. doi: 10.1046/j.1464-410x.2000.00679.x. [DOI] [PubMed] [Google Scholar]
- 33.de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. European urology. 2014;66(3):430–436. doi: 10.1016/j.eururo.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 34.El-Shater Bosaily A, Parker C, Brown LC, et al. PROMIS--Prostate MR imaging study: A paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp Clin Trials. 2015;42:26–40. doi: 10.1016/j.cct.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baco E, Rud E, Eri LM, et al. A Randomized Controlled Trial To Assess and Compare the Outcomes of Two-core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-core Systematic Biopsy. European urology. 2015 doi: 10.1016/j.eururo.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Godtman RA. Institute of clinical sciences at Sahlgrenska Academy UoG, Gthenbrg, Sweden, editor. The effect of start and stop age on the risk of being diagnosed with prostate cancer. 2015 [Google Scholar]
- 37.Schroder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC) European urology. 2012;62(5):745–752. doi: 10.1016/j.eururo.2012.05.068. Epub 2012/06/19. [DOI] [PubMed] [Google Scholar]
- 38.Futterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. European urology. 2015 doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]