Abstract
Purpose
We examined the prevalence and effect of smoking on cervical cancer recurrence and mortality in patients undergoing definitive treatment with radiation.
Methods and Materials
Between July 2007-September 2013, 96 locally advanced cervical cancer patients received definitive radiation or chemoradiation followed by brachytherapy. Smoking status was obtained from prospective intake questionnaires and quantified by pack-years. Pelvic control (PC), disease free survival (DFS), and overall survival (OS) were analyzed by multivariable Cox proportional hazards models.
Results
Smoking history included 51 (53.1%) non smokers, 45 active and former smokers: 20 (20.8%) with 1-20 pack-years and 25 (26%) with 21+ pack-years. With a median follow up of 2 years on univariate analysis, the impact of 1-20 pack-years on PC, DFS, and OS relative to nonsmokers was HR 4.29 (CI:1.36-14.1; P=0.014), 4.99 (CI:1.21-22.4; P=0.027), and 4.77 (CI:1.34-17.8; P=0.017) respectively. For patients with 21+ pack-years, the impact on PC, DFS, and OS was HR 6.13 (CI: 2.29-18.6; P<0.001), 7.24 (CI: 2.28-29.1; P=0.001), and 4.21(CI: 1.26-15.4; P=0.02). On multivariate analysis, there remained a significant difference of 1 to 20 pack-years smoking history on OS relative to nonsmokers, HR 4.68 (CI:1.02-29; P=0.047). For patients with 21 or over pack-years smoking history, there continued to be a negative impact on PC and DFS, HR 5.66 (CI: 1.7-22.18; P=0.004), and 6.89 (CI:1.54-42; P=0.011), respectively.
Conclusions
Former and active tobacco smoking during radiation therapy for cervical cancer is associated with unfavorable pelvic control, disease free and overall survival outcomes. The increased number of smoking pack years conferred a worse outcome effect in those treated with radiation.
Introduction
In the five decades since the Surgeon General's January 1964 landmark pronouncement on the negative impact of tobacco on health, the reduction in smoking among the world's population has averted millions of deaths. Key efforts have included taxes on tobaccos, clean air laws, and mass public education. From the 1960s to today, the prevalence of adult smokers in the United States has fallen significantly. By the second quarter of this century, it is estimated that the number of smokers in the United States will fall to an all-time low of 15% of the adult population [15]. However, amongst female smokers, the relative and absolute risk of death from smoking is continues to increase. Smoking remains the number one preventable cause of premature death in women, and accounts for over 30% of all-cause mortality [29]. Other common health risks associated with smoking includes chronic obstructive lung diseases, stroke, coronary artery disease, and peripheral vascular disease. Smoking has also been linked to other types of malignancies such as cancer of the lung, colorectum, bladder, kidney, stomach, pancreas, and the cervix [9].
Studies have shown a potential link between smoking and incidence of cervical cancer, especially in setting of concurrent human papillomavirus (HPV) infection [4,24]. The exact mechanism is unknown, although it is believed to stem from several factors including carcinogens in cigarette smoke, immunosuppression, and poor compliance of smokers to cervical cancer screening [6].
Less is known about the effect of smoking on disease specific mortality and overall survival after a diagnoses of cervical cancer in those treated with brachytherapy as part of their care. Recent publications have suggested that smoking may lead to greater mortality in those patients diagnosed with cervical cancer [6,30]. On the other hand, other survival analysis have not demonstrated such relationship [11]. In the era of treatment and novel therapeutics to improve cervical cancer outcomes, we sought to investigate the effect of former and active smoking, on cervical cancer outcomes. Our study examines the prevalence of smoking among our locally advanced cervical cancer population receiving definitive radiation or chemoradiation therapy followed by brachytherapy, and the impact of smoking on pelvic, control, disease free and overall survival.
Methods and Materials
Patient Characteristics
Between July 2007 and September 2013, 96 patients received radiation and chemotherapy for definitive treatment of locally advanced cervical cancer. Patients were identified as former or active smokers based on their completion of a prospective intake questionnaire at the time of the radiation oncology consultation. Smoking habits were quantified based on the number of packs smoked per day multiplied by the number of years the patient smoked (pack-years).
Tumor Characteristics
Tumor characteristics included stage, histology, and nodal status. Cervical cancers were staged based on the International Federation of Gynecology and Obstetrics (FIGO) staging system. The histology was based on pathology reports and categorized as squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. Positive pelvic and paraaortic lymph nodes were based on radiographic imaging by PET/CT, or lymph node surgical staging.
Treatment Procedure
Patients were treated with concurrent chemotherapy consisting of weekly cisplatin 40mg/m2 or 5-FU 1000mg/m2. They also received external beam radiation therapy (EBRT) to the entire pelvis using a 4 field conformal technique, or if there were positive para-aortic lymph nodes involved, an extended field radiation technique was used. Radiation was delivered in five fractions per week for a total of 45 Gy in 25 fractions. A few patients received a parametrial or lymph node boost, typically consisting of 3 to 5 additional fractions. All patients subsequently underwent intracavitary high-dose-rate (HDR) brachytherapy using a tandem and ring applicator.
Three-Dimensional (3D) Treatment Planning
We used Varian Eclipse BrachyVision (v 8.1) for 3D treatment planning following brachytherapy. The following reference points were identified: International Commission on Radiation Units and Measurements (ICRU) rectal points and Manchester's A and B points. The ICRU rectal point was defined as the point on the lateral plain film 5mm behind the packing at the level of the tandem ring. A manual optimization was carried out using the planning software with a traditional loading pattern of the tandem and ring. The dose was normalized to point A with the HDR prescription dose of 6 Gy for 5 fractions or 8 Gy for 3 fractions. From 2007 to the beginning of 2011, the brachytherapy prescriptions were normalized to ICRU point A. Since 2011, our current practice is to normalize to ICRU point A and then using CT based imaged guided techniques, manually adjust the dwell times to account for volumetric dose constraints to the high-risk clinical target volume, rectum, sigmoid, and bladder.
Follow Up
Patients were recommended to follow up in 3 month intervals after finishing radiation therapy. During follow up patients were asked questions about side effects and underwent a pelvic exam. Patients underwent a post radiation 12 week PRT scan, and further imaging, such as CT of the abdomen, in setting of symptom concerning for cervical cancer such as weight loss and abdominal fullness. They also underwent further imaging in setting of positive exam findings such as palpable lymph nodes or mass per clinical examination.
Statistical methods
Continuous variables were compared among smoking categories using analysis of variance, except for analyses of number of chemotherapy cycles, which used the Kruskal-Wallis test. The distributions of categorical variables were compared among smoking categories using chi-square tests.
Medians for time to event outcomes (OS, CSS, DFS, and pelvic control) were estimated using the Kaplan-Meier method.
Time to event outcomes were analyzed by patient characteristics using Cox proportional hazards models. In order to obtain estimates of hazard ratios when there were no events in a given covariate category, estimation was conducted using penalized likelihood (Heinze and Schemper, 2001). First, univariate analyses were conducted for each baseline characteristic and time-to-event outcomes; multivariable Cox models for each time to event outcome included covariates with a p-value less than 0.2 in univariate Cox analyses that also had a p-value less than 0.2 for an association with smoking.
Analyses were conducted using the statistical software environment R, version 3.0.1. [28]. Penalized likelihood estimation for Cox models was implemented using the R package coxphf, version 1.10, (Ploner and Heinze, 2013), and penalized likelihood estimation for logistic regression models was conducted using the R package logistf, version 1.21 (Heinze et al, 2013).
Time to event endpoints (overall survival, disease free survival, and pelvic control) were analyzed by patient and disease characteristics using multivariable Cox proportional hazards models. Pelvic control was defined as time from diagnosis to last follow up when the patient was free from any recurrence of the cancer. DFS was defined as time from diagnosis to last follow up when the patient had local or distant metastasis. OS was defined as time from diagnosis to either death or last follow up.
Results
Patient Characteristics
The study population consisted of 96 patients with an average age of 54.8 years (range, 27-91 years). Smoking history at the start of radiation included 51 (53.1%) patients with no history of smoking, 20 (20.8%) patients with one to twenty pack-years, and 25 (26%) patients with twenty-one or more pack-years smoked. Mean BMI was 28.5 (range, 17.3-47.2).
Tumor Characteristics
Cervical cancer FIGO stages included the following: 10 with stage 1B1 (10.4%), 16 stage IB2 (16.7%), 3 stage 2A (3.1%), 49 stage 2B (51%), 1 stage 3A (1%), and 17 stage 3B (17.7%). Cervical cancer histology included 79 squamous cell carcinomas (82.3%), 14 adenocarcinomas (14.6%), and 3 adenosquamous carcinomas (3.1%). Of the study population, 67 (69.8%) had zero positive paraaortic or pelvic lymph nodes, 8 (8.3%) had one positive lymph node, 6 (6.2%) had two positive lymph nodes, 10 (10.2%) had three positive lymph nodes, and 5 (5.2%) had four or more positive lymph nodes.
There was no difference between the non smokers, or smokers by category (1-20 pack years, vs. 20 years plus) for BMI, performance status, chemotherapy regimen nor cycles, brachytherapy dose, total radiation time, comorbidity index, lymph node status, alcohol use, stage, histology, or use of a parametrial or lymph node boost as shown in Table 1. There was a statistically significant difference between the non smokers and smokers for age, illicit drug use, and ethnicity.
Table 1.
Patient and Treatment Characteristics By Smoking Pack Years
| 0 Pack Years (n = 51) | 1—20 Pack Years (n = 20) | 21 or More Pack Years (n = 24) | All Patients (n = 95) | P-Value | |
|---|---|---|---|---|---|
| Age (Years) | 0.019A | ||||
| Mean (SD) | 55.1 (13) | 48.6 (12.2) | 59.4 (11.2) | 54.9 (12.8) | |
| BMI | 0.201A | ||||
| Mean (SD) | 28.4 (6) | 30.9 (10.1) | 27.1 (5.9) | 28.6 (7.1) | |
| KPS | 0.369A | ||||
| Mean (SD) | 87.5 (6.7) | 89.5 (3.9) | 88.8 (5.4) | 88.2 (5.9) | |
| Number of Chemo Cycles | 0.509K | ||||
| Mean (SD) | 5.3 (1.8) | 5.5 (1.4) | 5.7 (1.2) | 5.5 (1.6) | |
| Brachytherapy Dose (cGy) | 0.114A | ||||
| Mean (SD) | 2729.4 (287.6) | 2875.8 (280.6) | 2819.3 (265.6) | 2782.9 (284.4) | |
| Total Days Radiation | 0.450A | ||||
| Mean (SD) | 50 (6) | 49.5 (6.2) | 51.6 (6.6) | 50.3 (6.2) | |
| Ethnicity (n, %) | <0.001C | ||||
| White | 21 (41.2%) | 17 (85%) | 21 (87.5%) | 59 (62.1%) | |
| Black | 3 (5.9%) | 2 (10%) | 1 (4.2%) | 6 (6.3%) | |
| Hispanic | 19 (37.3%) | 0 | 2 (8.3%) | 21 (22.1%) | |
| Asian/Pac. Islander | 8 (15.7%) | 1 (5%) | 0 | 9 (9.5%) | |
| Charlson Comorbidity Index | 0.303C | ||||
| (n, %) | |||||
| 2 | 38 (74.5%) | 18 (90%) | 18 (75%) | 74 (77.9%) | |
| 3 | 12 (23.5%) | 2 (10%) | 4 (16.7%) | 18 (18.9%) | |
| 4 | 1 (2%) | 0 | 2 (8.3%) | 3 (3.2%) | |
| Illicit Drugs (n, %) | 0.002C | ||||
| No | 51 (100%) | 19 (95%) | 19 (79.2%) | 89 (93.7%) | |
| Yes | 0 | 1 (5%) | 5 (20.8%) | 6 (6.3%) | |
| Excessive Alcohol (n, %) | 0.205C | ||||
| No | 48 (94.1%) | 16 (80%) | 21 (87.5%) | 85 (89.5%) | |
| Yes | 3 (5.9%) | 4 (20%) | 3 (12.5%) | 10 (10.5%) | |
| LN Status (n, %) | 0.459C | ||||
| None | 37 (72.5%) | 15 (75%) | 15 (62.5%) | 67 (70.5%) | |
| Positive Pelvic LN | 14 (27.5%) | 5 (15%) | 9 (29.2%) | 28 (24.2%) | |
| Stage (n, %) | 0.499C | ||||
| 1B1 | 5 (9.8%) | 3 (15%) | 2 (8.3%) | 10 (10.5%) | |
| 1B2 | 10 (19.6%) | 3 (15%) | 3 (12.5%) | 16 (16.8%) | |
| 2A | 1 (2%) | 2 (10%) | 0 | 3 (3.2%) | |
| 2B | 26 (51%) | 10 (50%) | 12 (50%) | 48 (50.5%) | |
| 3A | 0 | 0 | 1 (4.2%) | 1 (1.1%) | |
| 3B | 9 (17.6%) | 2 (10%) | 6 (25%) | 17 (17.9%) | |
| Histology (n, %) | 0.759C | ||||
| Squamous Cell Carcinoma | 39 (76.5%) | 18 (90%) | 21 (87.5%) | 78 (82.1%) | |
| Adeno-carcinoma | 9 (17.6%) | 2 (10%) | 2 (8.3%) | 13 (13.7%) | |
| Adeno-squamous | 2 (3.9%) | 0 | 1 (4.2%) | 3 (3.2%) | |
| Poorly Differ-entiated Neuro-endocrine | 1 (2%) | 0 | 0 | 1 (1.1%) | |
| Chemo Regimen (n, %) | 0.516C | ||||
| None | 5 (9.8%) | 1 (5%) | 1 (4.2%) | 7 (7.4%) | |
| Cisplatin | 46 (90.2%) | 18 (90%) | 22 (91.7%) | 86 (90.5%) | |
| 5-FU | 0 | 1 (5%) | 1 (4.2%) | 2 (2.1%) | |
| Parametrial or LN boost | 0.766C | ||||
| No | 24 (47.1%) | 11 (55%) | 13 (54.2%) | 48 (50.5%) | |
| Yes | 27 (52.9%) | 9 (45%) | 11 (45.8%) | 47 (49.5%) |
= P-value from ANOVA F-test
= P-value from Kruskal-Wallis test
= P-value from chi-square test
Patient Survival Characteristics
The median follow up time was approximately 2 years (range 0-6 years). At most recent follow up, 85 patients (88.5%) were free of local recurrence of cancer and 86 patients (89.6%) were free of distant metastasis. The details on the time to events is shown in table 2. On univariate analysis, as shown in table 3, the impact of 1 to 20 pack years smoking history on pelvic control, DFS, and OS relative to nonsmokers was hazards ratio 4.29 (CI:1.36-14.1; P=0.014), 4.99 (CI:1.21-22.4; P=0.027), and 4.77 (CI:1.34-17.8; P=0.017) respectively. For patients with 21 or over pack-years smoking history, the impact on pelvic control, DFS, and OS was hazards ratio 6.13 (CI: 2.29-18.6; P<0.001), 7.24 (CI: 2.28-29.1; P=0.001), and 4.21(CI: 1.26-15.4; P=0.02) respectively as shown in the univariate analysis in table 3. We then controlled for potential confounders in the model such as age, stage, ethnicity, race, BMI, KPS, lymph node status, chemotherapy receipt, radiation dose, brachytherapy dose, receipt of a parametrial boost, total time of completion of radiation for the multivariate analysis. On multivariate analysis, there remained a significant difference of 1 to 20 pack-years smoking history on OS relative to nonsmokers was hazards ratio 4.68 (CI:1.02-29; P=0.047). For patients with 21 or over pack-years smoking history, there continued to be a negative impact on on pelvic control and DFS, with hazards ratio 5.66 (CI: 1.7-22.18; P=0.004), and 6.89 (CI:1.54-42; P=0.011) respectively as shown in table 4. The Kaplan Meir survival curves for pelvic control, DFS, and OS are shown in figures 1, 2 and 3.
Table 2.
Summary of Time-to-Event Endpoints By Smoking Pack Years
| Time to Event (Months) | 0 Pack Years (n = 51) | 1—20 Pack Years (n = 20) | 21 or More Pack Years (n = 24) |
|---|---|---|---|
| Median OS (95% CI) | NA (NA, NA) | 45 (29, NA) | 34 (18, NA) |
| Number of OS Events | 4 | 5 | 6 |
| Median CSS (95% CI) | NA (NA, NA) | 45 (29, NA) | 34 (30, NA) |
| Number of CSS Events | 3 | 4 | 5 |
| Median DFS (95%) | NA (NA, NA) | NA (18, NA) | 33 (16, NA) |
| Number of DFS Events | 3 | 4 | 9 |
| Median Pelvic Control (95% CI) | NA (NA, NA) | 37 (19, NA) | 16 (12, NA) |
| Number of Failure of PC Events | 5 | 6 | 11 |
NA = median not reached or confidence bound not defined due to insufficient number of events.
Table 3.
A Univariate Cox Proportional Hazards Analysis by Baseline Characteristics
| Pelvic Control | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI for Hazard Ratio | P-Value | Hazard Ratio | 95% CI for Hazard Ratio | P-Value | Hazard Ratio | 95% CI for Hazard Ratio | P-Value |
| Smoking | |||||||||
| 1 to 20 Pack Years vs. 0 Pack Years | 4.29 | (1.36, 14.1) | 0.014 | 4.99 | (1.21, 22.4) | 0.027 | 4.77 | (1.34, 17.8) | 0.017 |
| 21 or More Pack Years | 6.13 | (2.29, 18.6) | <0.001 | 7.24 | (2.28, 29.1) | 0.001 | 4.21 | (1.26, 15.4) | 0.02 |
| BMI | 1 | (0.93, 1.06) | 0.939 | 1.02 | (0.95, 1.10) | 0.526 | 1 | (0.92, 1.08) | 0.938 |
| Age (Years) | 0.99 | (0.96, 1.03) | 0.668 | 0.99 | (0.95, 1.03) | 0.636 | 0.98 | (0.94, 1.02) | 0.452 |
| Ethnicity | |||||||||
| Black vs. White | 1.28 | (0.14, 5.18) | 0.78 | 1.85 | (0.20, 7.87) | 0.515 | 2.85 | (0.30, 12.9) | 0.298 |
| Hispanic vs. White | 0.44 | (0.11, 1.24) | 0.127 | 0.4 | (0.08, 1.35) | 0.15 | 0.25 | (0.03, 1.05) | 0.059 |
| Asian/Pac. Islander vs. White | 1.35 | (0.27, 4.37) | 0.675 | 1.11 | (0.12, 4.69) | 0.907 | 2.97 | (0.55, 11.1) | 0.179 |
| Charlson Comorbidity Index (3 or 4 vs. 2) | 0.51 | (0.13, 1.41) | 0.211 | 0.73 | (0.19, 2.13) | 0.584 | 0.48 | (0.09, 1.62) | 0.26 |
| KPS | 0.96 | (0.91, 1.03) | 0.227 | 0.97 | (0.91, 1.06) | 0.5 | 0.97 | (0.91, 1.05) | 0.454 |
| Lymph Node Status (Positive vs. Negative) | 2.4 | (1.04, 0.52) | 0.04 | 2.26 | (0.86-5.97) | 0.098 | 1.88 | (0.68, 5.14) | 0.219 |
| Stage | |||||||||
| 2A or 2B vs. 1B1 or 1B2 | 1.44 | (0.51, 4.82) | 0.505 | 2.62 | (0.70, 11.3) | 0.152 | 1.34 | (0.40, 5.54) | 0.65 |
| 3A or 3B vs. 1B1 or 1B2 | 2.79 | (0.89, 9.89) | 0.079 | 2.11 | (0.52, 19.2) | 0.335 | 2.6 | (0.69, 11.2) | 0.156 |
| Histology (Squamous Cell Carcinoma vs. Adenocarcinoma, Adenosquamous, or Poorly Differentiated Neuroendocrine) | 1.79 | (0.57, 8.92) | 0.35 | 2.91 | (0.39, 372) | 0.374 | 2.28 | (0.55, 21.0) | 0.288 |
| Chemotherapy (Yes vs. No) | 1.31 | (0.34, 11.8) | 0.741 | 1.1 | (0.88, 1.33) | 0.372 | 0.88 | (0.22, 8.01) | 0.879 |
| EBRT Dose (Gy) | 1.16 | (0.97, 1.35) | 0.093 | 1.25 | (1.01, 1.57) | 0.035 | 1.08 | (0.88, 1.31) | 0.467 |
| Brachytherapy Dose (Gy) | 1.18 | (1.00, 1.42) | 0.054 | 0.96 | (0.88, 1.05) | 0.391 | 1.16 | (0.96, 1.44) | 0.126 |
| Total Days Radiation | 0.97 | (0.90, 1.04) | 0.456 | 0.96 | (.88, 1.05) | 0.391 | 0.94 | (0.86, 1.02) | 0.169 |
| Booster Dose (Yes vs. No) | 1.41 | (0.62, 3.38) | 0.414 | 1.25 | (0.47, 3.39) | 0.655 | 2.05 | (0.74, 6.36) | 0.17 |
Table 4.
Multivariable Cox Proportional Hazards Analysis
| Pelvic Control | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI for Hazard Ratio | P-Value | Hazard Ratio | 95% CI for Hazard Ratio | P-Value | Hazard Ratio | 95% CI for Hazard Ratio | P-Value |
| Smoking | |||||||||
| 1 to 20 Pack Years vs. 0 Pack Years | 3.00 | (0.75-13.16) | 0.121 | 5.10 | (0.95-36.7) | 0.058 | 4.68 | (1.02- 29.0) | 0.047 |
| 21 or More Pack Years | 5.66 | (1.70 - 22.18) | 0.004 | 6.89 | (1.54-42.2) | 0.011 | 4.30 | (0.79- 26.8) | 0.090 |
Figure 1.
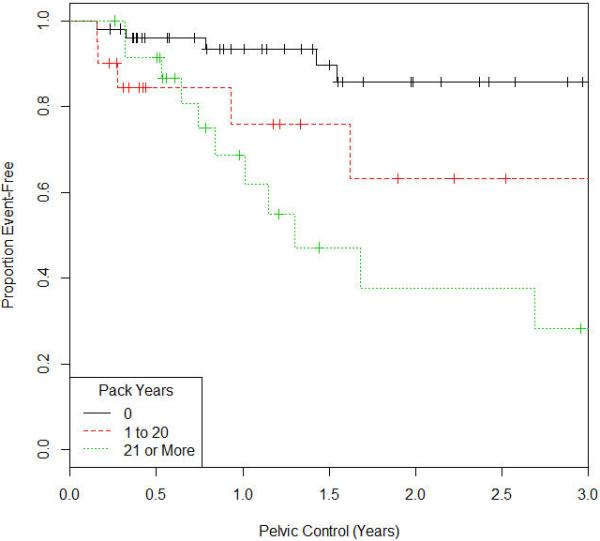
Kaplan Meir Survival Curve for Pelvic Control
Figure 2.
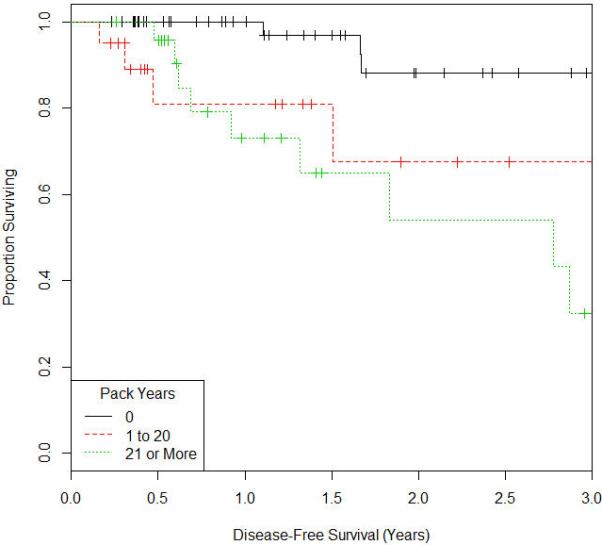
Kaplan Meir Survival Curve for Disease Free Survival
Figure 3.
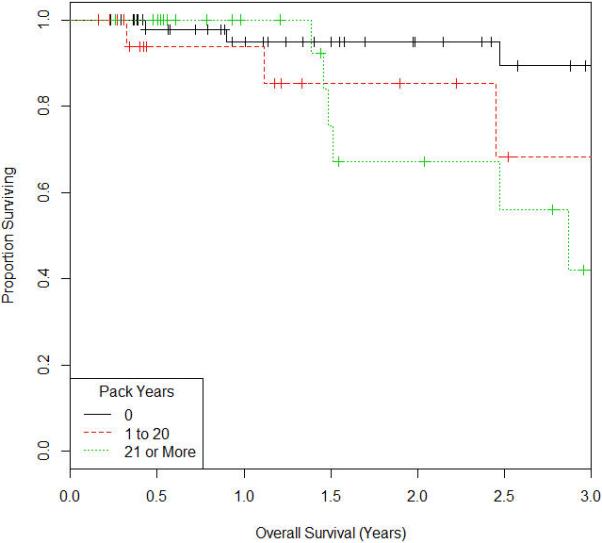
Kaplan Meir Survival Curve for Overall Survival
Discussion
Our study shows that active or having a history of smoking decreases pelvic control, disease free and overall survival in cervical cancer treated with radiation. Furthermore, in the era of treatment intensification and biologic therapeutic strategies to improve cervical cancer outcomes, our data suggest that lifestyle factors contribute significantly to long term survival. The detriment of smoking was seen in smokers prior to radiation therapy, and there was a relationship to worse survival in those smoking a higher number of cigarettes per day. This data provides the radiation oncologist with tangible data that patients who do not smoke during radiation will live longer and have less recurrences of their cervical cancer.
Throughout the last decade there continues to be strides to decrease the death rate in patients with locally advanced cervical cancer with the anticipated discovery and use of novel therapeutics in the field. The addition of systemic therapy to radiation for the treatment of invasive cervical cancer has revolutionized the management and clinical outcome of patients with locally advanced cervical cancer [17,22,25]. However, there continue to be subgroups of cervical cancer patients who do not experience a complete response and improved survival conferred by chemoradiation. Most noticeable among these patients are those with nodal metastases or those with advanced stage disease. Therefore, there is a high risk patient population that will further benefit from efforts aimed at either treatment intensification, or optimization of environmental factors that impact disease free survival. For example, the role of adjuvant chemotherapy after standard chemoradiation has been shown to improve overall survival in a recent phase 3 randomized trial in Mexico, and is currently the study arm of a global prospective randomized trial, the OUTBACK trial [7]. However, intensification of therapy is not without potential significant toxicity; patients on the Mexican trial who received adjuvant chemotherapy experienced a higher risk of Grade 3 and 4 toxicity, 86.5% v 46.3%, respectively, P = .001[5]. Due to the potential for adverse side effects from treatment intensification and the poor disease free survival of locally advanced cervical cancer, there have been increasing efforts to target lifestyle and alter environmental factors to improve patient outcomes. Our study shows patients who had a history of smoking at the time of radiation therapy consultation had a decrease in pelvic control, disease free and overall survival, allowing an opportunity for outcome improvement through lifestyle modification targeting those active smokers during radiation.
We currently know that smoking increases the risk of developing invasive cervical cancer. Winkelstein Jr, et al. first postulated smoking to be a risk factor for cervical cancer, publishing this work in 1977 [31]. Since then, several studies have confirmed the association between smoking and incidence of cervical cancer, but the exact mechanism by which smoking leads to increased risk for carcinogensis is unknown [4,24]. Studies have suggested that smoking increases the risk of HPV infection. For example, in a study by Simen-Kapeu, et al. (2008), smoking was shown to be associated with acquisition of high risk HPV [26]. This is partially explained by smokers being less compliant with cervical cancer screening and subsequent management [19]. Furthermore, smokers have delayed clearance of HPV infection [12,18].
Investigations have been performed to determine the effect of smoking on patients with concurrent HPV infection, and the increased risk of developing cervical cancer. One leading theory of enhanced carcinogenesis is that exposure of cervical epithelial cells to nicotine and cotinine causes DNA damage and subsequent increased risk of malignancy [14,27]. According to Alam, et al. , benzo[a]pyrene (BaP) is a major carcinogen found in cigarettes and may also play a significant role [1]. High levels of BaP were detected in cervical tissues of women smokers and believed to modulate the HPV lifecycle by increasing expression of oncogenes E6 and E7 [21]. Smoking is also believed to inhibit the immune system response to HPV, especially T help lymphocyte, natural killer cell, and immunoglobulin E activity [16]. It is postulated that smoking may lead to more aggressive forms of pre invasive cervical cancer. For example, in a study by McIntyre-Seltman et al., smokers were found to have more rapid development of CIN 3 [20].
It is uncertain why smoking may lead to increased cervical cancer mortality. Fyles et al. examined the effect of smoking on tumor hypoxia and cervical cancer outcomes since tumor hypoxia is associated with an increased risk of cervical tumor recurrence and death[11]. Tumor oxygenation was determined using the Eppendorf polarographic oxygen electrode and tumor oxygenation is represented by the hypoxic proportion HP5 (% of pO2 measurements <5 mmHg)[11]. There was no significant association between smoking and tumor hypoxia (P=0.3)[11], They conclude that there was no significant association between smoking and tumor hypoxia, treatment response or survival in this study of patients with cervix cancer. In addition, there is an association between p53 mutation and smoking. p53 mutations are frequent in tobacco-related cancers and the mutation load is often higher in cancers from smokers than from nonsmokers[23]. Carcinogens from tobacco smoke can also cause G to T transversion mutations, which occur when the substitution of a purine for a pyrimidine or vice versa. This type of mutation alters the chemical structure of DNA dramatically and is has increased potential for degeneracy due to the wobble position of DNA being less tolerant to transversions then, for example, transitions. In addition, data suggest that p53 mutations in certain cancers can be attributed to direct DNA damage from cigarette smoke carcinogens rather than to selection of pre-existing endogenous mutations[23].
The results of our study are consistent with those previously published in the literature, for smokers to have a statistically significant higher hazards ratio than nonsmokers for both DFS and OS in cervical cancer. In a study by Coker et al., women smokers were 35% more likely to die of any cause and 21% more likely to die of cervical cancer related cause [6]. In addition, in an analysis of the Gynecology Oncology Group trial 165, a phase III study (GOG 165) in which patients were randomly allocated to receive radiation plus either cisplatin or 5-Fluorouracil, smoking behavior was ascertained using an administered questionnaire and by quantifying urine cotinine concentration [30]. Compared with non-smokers, median survival was 15 months shorter for reported smokers and 20 months shorter for cotinine-derived smokers (p< 0.01) [30]. After adjusting for covariates, a significant increase in the risk of death (but not disease progression) was observed for reported smokers (hazard ratio [HR]: 1.51; 95% confidence interval [CI]: 1.01–2.27; P = 0.04) [30].
Our study findings augment these findings in the literature as we found a linear relationship between the number of smoking pack years and cervical cancer recurrence and mortality. Patients with 21 or more smoking pack-years had higher hazards ratio of DFS and OS compared to patients with 20 or less smoking pack-years, suggesting a role for intervention on the number of cigarettes consumed during the difficult process of smoking cessation.
The effect of smoking on detrimental outcomes exhibited in our data is not limited to cervical cancer. Head and neck cancer, a cancer which has many similarities to cervical cancer in the tempo of disease progression, and therapeutic options including definitive concurrent chemoradiotherapy, also has data in the literature that smoking decreases survival[2,3,5]. In a report of patients with head and neck squamous cell cancer, Chen et al., showed that active smokers had significantly inferior 5-year overall survival (23% vs. 55%), locoregional control (58% vs. 69%), and disease-free survival (42% vs. 65%) compared with the former smokers who had quit before radiation therapy (p< 0.05) [5].
The main limitation of our study is a retrospective cohort from a single institution. The primary aim of our study is to examine the effect of smoking status on outcomes, and offer an opportunity for cervical cancer mortality improvement. The questionnaires issued at the time of radiation oncology consultation were patient reported, and therefore could be subject to underreporting of the smoking status or pack years smoked. In addition, this study examined the effects of smoking on cervical cancer outcomes and did not specifically examine the effect of former vs active smoking as in the literature, but we know that few smokers with cervical cancer quit during treatment [31]. The mechanisms of effect on treatment outcome may be different between active and former smokers. Former smoking and carcinogenesis may lead to bulkier and more advanced stage tumors, whereas current smoking may increase radioresistance and hypoxia.
In smokers, competing causes of death are a confounding issue since smoking increases both cardiovascular death and further complications from oncology treatments [8]. According to Coker et al. the increased cervical cancer mortality associated with smoking may be attributed to causes separate from cervical cancer. For example, smoking has strong association with cardiovascular events and stroke [10]. We did, however, control our data for socioeconomic and treatment related factors that could influence outcomes, such as the concurrent use of chemotherapy, alcohol, drugs, and overall treatment time.
Although subject to the limitations of a retrospective review, our data shows that lifestyle habits influence cervical cancer outcomes. This finding allows an opportunity for improvement in patients who identify themselves as active smokers during radiation. Various methods to encourage smoking cessation are available, including contracts signed by the patient upon starting radiation [13]. There is a dirth of data on the effect of smoking intervention, and smoking cessation on cervical cancer morbidity and mortality. Indeed, more studies are needed to determine the effect and effectiveness of smoking cessation efforts on cervical cancer mortality. Novel smoking cessation intervention, effectiveness, and potential outcome improvement are being developed by our group.
Our data shows that smoking increases the risk of cancer recurrence and mortality in patients with locally advanced cervical cancer. Our data illustrates compelling evidence to support the role of increased efforts promoted at smoking cessation during radiation and brachytherapy in active smokers.
Acknowledgements
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial disclosures:
Conflict of interest: None
References
- 1.Alam S, et al. The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis. Journal of virology. 2008;82:1053–1058. doi: 10.1128/JVI.01813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Ad V, et al. Combination of p16 levels and pre-radiotherapy factors predicts outcome in patients treated for oropharyngeal carcinoma. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2013;18:982–988. [PubMed] [Google Scholar]
- 3.Browman GP, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. The New England journal of medicine. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 4.Castle PE, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. Journal of the National Cancer Institute. 2002;94:1406–1414. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 5.Chen AM, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. International journal of radiation oncology, biology, physics. 2011;79:414–419. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Coker AL, et al. Smoking and survival among kentucky women diagnosed with invasive cervical cancer: 1995-2005. Gynecologic oncology. 2009;112:365–369. doi: 10.1016/j.ygyno.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duenas-Gonzalez A, et al. Phase iii, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage iib to iva carcinoma of the cervix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 8.Eifel PJ, et al. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 9.Fagerstrom K. The epidemiology of smoking: Health consequences and benefits of cessation. Drugs. 2002;62(Suppl 2):1–9. doi: 10.2165/00003495-200262002-00001. [DOI] [PubMed] [Google Scholar]
- 10.Frey P, et al. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the treating to new targets [tnt] and the incremental decrease in end points through aggressive lipid lowering [ideal] trials). The American journal of cardiology. 2011;107:145–150. doi: 10.1016/j.amjcard.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Fyles A, et al. The effect of smoking on tumour oxygenation and treatment outcome in cervical cancer. Clinical oncology. 2002;14:442–446. doi: 10.1053/clon.2002.0116. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano AR, et al. Clearance of oncogenic human papillomavirus (hpv) infection: Effect of smoking (united states). Cancer causes & control : CCC. 2002;13:839–846. doi: 10.1023/a:1020668232219. [DOI] [PubMed] [Google Scholar]
- 13.Harari PM, et al. Radiation oncologists can assist head and neck cancer patients with smoking cessation. International journal of radiation oncology, biology, physics. 1995;31:645–649. doi: 10.1016/0360-3016(94)E0297-W. [DOI] [PubMed] [Google Scholar]
- 14.Hellberg D, et al. Smoking and cervical intraepithelial neoplasia: Nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. American journal of obstetrics and gynecology. 1988;158:910–913. doi: 10.1016/0002-9378(88)90093-2. [DOI] [PubMed] [Google Scholar]
- 15.Jha P, et al. 21st-century hazards of smoking and benefits of cessation in the united states. The New England journal of medicine. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JD, et al. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: A review. Critical reviews in toxicology. 1990;20:369–395. doi: 10.3109/10408449009089870. [DOI] [PubMed] [Google Scholar]
- 17.Keys HM, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage ib cervical carcinoma. The New England journal of medicine. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 18.Koshiol J, et al. Smoking and time to clearance of human papillomavirus infection in hiv-seropositive and hiv-seronegative women. American journal of epidemiology. 2006;164:176–183. doi: 10.1093/aje/kwj165. [DOI] [PubMed] [Google Scholar]
- 19.Marteau TM, Hankins M Collins B. Perceptions of risk of cervical cancer and attitudes towards cervical screening: A comparison of smokers and nonsmokers. Family practice. 2002;19:18–22. doi: 10.1093/fampra/19.1.18. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre-Seltman K, et al. Smoking is a risk factor for cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:1165–1170. doi: 10.1158/1055-9965.EPI-04-0918. [DOI] [PubMed] [Google Scholar]
- 21.Melikian AA, et al. Identification of benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues in humans by gas chromatography-mass spectrometry. Cancer letters. 1999;146:127–134. doi: 10.1016/s0304-3835(99)00203-7. [DOI] [PubMed] [Google Scholar]
- 22.Morris M, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. The New England journal of medicine. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer GP, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 24.Plummer M, et al. Smoking and cervical cancer: Pooled analysis of the iarc multi-centric case--control study. Cancer causes & control : CCC. 2003;14:805–814. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 25.Rose PG, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. The New England journal of medicine. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 26.Simen-Kapeu A, et al. Tobacco smoking and chewing as risk factors for multiple human papillomavirus infections and cervical squamous intraepithelial lesions in two countries (cote d'ivoire and finland) with different tobacco exposure. Cancer causes & control : CCC. 2009;20:163–170. doi: 10.1007/s10552-008-9230-x. [DOI] [PubMed] [Google Scholar]
- 27.Simons AM, Phillips DH, Coleman DV. Damage to DNA in cervical epithelium related to smoking tobacco. BMJ. 1993;306:1444–1448. doi: 10.1136/bmj.306.6890.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Team RDC. R: A language and environment for statistical computin. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 29.Thun MJ, et al. 50-year trends in smoking-related mortality in the united states. The New England journal of medicine. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waggoner SE, et al. Association between cigarette smoking and prognosis in locally advanced cervical carcinoma treated with chemoradiation: A gynecologic oncology group study. Gynecologic oncology. 2006;103:853–858. doi: 10.1016/j.ygyno.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Winkelstein W., Jr. Smoking and cancer of the uterine cervix: Hypothesis. American journal of epidemiology. 1977;106:257–259. doi: 10.1093/oxfordjournals.aje.a112460. [DOI] [PubMed] [Google Scholar]


