Abstract
Objectives
With recent approval of standalone HPV testing and increasing uptake of HPV vaccination, some have postulated that we are moving towards a “post-Pap” era of cervical cancer prevention. However, the total number cases that have been prevented by Pap smear screening as well as its impact on racial disparities are unknown.
Methods
We estimated national cervical cancer incidence from 1976 to 2009 using the Surveillance, Epidemiology, and End Result (SEER) database. Screening data were obtained from literature and National Cancer Institute Progress Reports. We examined early, late, and race-specific trends in cancer incidence, and calculated the estimated number of cancers prevented over the past three decades.
Results
From 1976 to 2009, there was a significant decrease in the incidence of early-stage cervical cancer, from 9.8 to 4.9 cases per 100,000 women (p < .001). Late-stage disease incidence also decreased, from 5.3 to 3.7 cases per 100,000 women (p < .001). The incidence among black women decreased from 26.9 to 9.7 cases per 100,000 women (p < .001), a greater decline compared to that of white women and women of other races. After adjusting for “pre-screening era” rates of cervical cancer, we estimate that Pap smears were associated with a reduction of between 105,000 to 492,000 cases of cervical cancer over the past three decades in the U.S.
Conclusions
A large number of early and late-stage cervical cancers were prevented and racial disparity in cancer rates were reduced during an era of widespread Pap smear screening.
Keywords: Cancer Screening, Incidence, Cervical Neoplasms, Neoplasms, Papanicolaou Test, HPV DNA Test
Introduction
Screening for cervical cancer is currently recommended by the United States Preventive Services Task Force as part of routine health maintenance for women ages 21 through 65.1 Current recommendations for cervical cancer screening are based on efficacy of the Pap smear,2,3 and more recently, HPV testing.4,5 Over the past 30 years, the relatively widespread utilization of Pap smear screening is thought to be largely responsible for reducing the incidence of invasive cervical cancer in the United States.6,7 However, despite the historical trends of increased screening utilization, the net benefit of screening at the population level remains to be quantified. Furthermore, there have been long-standing disparities in cervical cancer incidence between different racial groups in the U.S. Multiple studies have shown a much higher8–10 incidence of cervical cancer among black and Hispanic women compared to non-Hispanic white women, although this has been improving.11,12 It is unclear if screening may have had a disparate effect on cervical cancer incidence by race.
Given the advent of HPV vaccination and HPV DNA-based testing, the landscape of cervical cancer screening and prevention is poised to undergo significant changes. U.S. women who have received HPV vaccination are thought to be protected against high-risk HPV infection and the subsequent development of cervical cancer.13,14 It is likely that this will have a discernable impact on the incidence of abnormal Pap smear findings and cervical cancer in the coming years.15 Additionally, HPV test screening has come into clinical practice in recent years as an adjuvant to Pap smears, and most recently in 2014, HPV-testing was approved for primary screening of cervical cancer.16 As cervical cancer remains a public health issue that potentially contributes to healthcare disparities, even as we move into the vaccination era and new models of HPV screening, examining the impact of screening in the current era provides an important baseline for continued assessment of existing cancer disparities and cervical cancer screening and prevention efforts. A thorough understanding of the progress made to date is even timelier now that there are new approaches available for cervical cancer prevention, as the experience of the past 30 years can highlight areas of success, and opportunities for improvement.
Our study seeks to provide such an analysis by estimating the net impact of cervical cancer screening in terms of the number of cancer cases prevented over the past three decades in association with Pap smear screening. Moreover, given that screening is expected to be more effective in populations with higher incidence of the disease,17 we seek to quantify how screening may have driven disparate changes in the incidence of cervical cancer between different racial groups in the U.S.
Materials and Methods
Overview
We obtained population-level data on Pap smear utilization and cervical cancer incidence from the National Cancer Institute (NCI) progress reports, the Surveillance, Epidemiology, and End Results (SEER) database, and literature searches. We calculated an estimated baseline cervical cancer incidence at the beginning of our period of analysis. Next, we determined the reduction of cervical cancer cases from baseline for each subsequent calendar year. We then scaled this change to the U.S. population to estimate the number of cases prevented at the national level. Finally, we summed the change from baseline for every year from 1979 to 2009 to estimate the net impact of screening.
Data sources
We obtained Pap smear utilization rates for all U.S. women age 18 or older for years 1987 to 2010 from the NCI Cancer Trends Progress Report,18 which is based on National Health Interview Survey (NHIS) data. Pap smear screening rates for earlier years (1963 to 1982) were obtained from survey studies19,20 and earlier reports from NHIS.21,22 We obtained cervical cancer incidence rates for the years 1973 to 2009 from the SEER*Stat Database.23 The SEER database pools data from 17 tumor registries and represents over 6 million cancer cases total. The database’s comprehensive and robust nature allows it to be considered somewhat representative of the U.S. population as a whole.24 Additionally, population counts for U.S. women, delimitated by age and race, were downloaded from the SEER website.25 The Yale Human Investigations Committee considered this study exempt from review.
Classification of cancer stages
Consistent with prior studies,26,27 we classified cervical cancer cases into early- and late-stage cancers based on SEER historical stage A. Cases with localized staging at the time of diagnosis were classified as early-stage cancers. Cases with regional or distant staging at the time of diagnosis were classified as late-stage cancers. Due to the lack of complete data on cases with in situ staging at the time of diagnosis, we excluded in situ cases from our analysis. Unstaged cervical cancer cases, which accounted for approximately 1% of all cases in our analysis, were also excluded from our calculations.
Estimate of baseline and current incidence rates
We calculated the baseline incidence for cervical cancer by taking the three year average incidence from 1976 to 1978 for U.S. women age 18 to 65. Consistent with previous studies, we excluded the initial years (1973 to 1975) for which SEER data was available due to the spurious variability in incidence data for those years.26,27 In our analysis, the baseline incidence is indicative of cervical cancer rates at the initial time period when cervical cancer screening was widely utilized. By this time, screening rates have risen from 37.8% in 1963 and stabilized at over 70% in the 1980s. We calculated the current incidence for cervical cancer by taking the three year average incidence from 2006 to 2009. The current incidence is indicative of cervical cancer rates in the U.S. after over 30 years of widespread screening.
Estimate of cancer cases prevented
We took two similar approaches in estimating a lower and upper bound for the total number of cancer cases prevented. In this study, we refer to these to these approaches as the “baseline incidence” approach and the “pre-screening incidence” approach, respectively.
In the baseline incidence approach, we assumed that the underlying rates of cancer remained constant at the baseline incidence rate, that is, the three year average incidence from 1976 to 1978. For each subsequent year, we found the reduction of cervical cancer incidence from the baseline incidence, and multiplied it by the number of women age 18 to 65 in the U.S. to find the number of cases reduced for each year. Then, we summed the reduced cases from all years over 1979 to 2009 to estimate the total number of cases prevented in association with screening (see Table, Supplemental Digital Content 1, which shows the number of cases reduced from baseline for each year from 1979 to 2009).
However, given that Pap smear screening had been in clinical practice since the 1950s, and given the likely long duration of the preclinical phase of cervical cancer,28 we also calculated the number of cancer cases prevented using a pre-screening incidence approach. In this approach, we estimated the pre-screening incidence of cervical cancer rates prior to the availability of Pap smear screening. While evidence from the 1950s and earlier is sparse, multiple reports indicate that the cervical cancer incidence in the U.S. prior to the wide availability of Pap smears is likely to be in the 30s per 100,000 women.29–31 One report found the incidence among white women to be 32.4 in the late 1940s31, while another report found the overall incidence to be 33.8 in the early 1950s29. A national survey found cervical cancer incidence to be 38.4 among Whites and 74.6 among Blacks in 1947.30 As such, in our pre-screening incidence approach, we took 30.0 per 100,000 women to be a conservative baseline incidence, and in repeating the same analysis as described in our “baseline incidence” approach, we were able to calculate a conservative upper bound of cervical cancer cases prevented in association with Pap smear screening.
Race-specific estimates of cancer cases prevented
Race-specific incidence of cervical cancer from 1976 to 2009 was obtained according to the SEER race recode classification, which describes race as white, black, or other (American Indian/Alaskan Native, Asian/Pacific Islander). For each of the three race groups in SEER, we calculated a baseline incidence by taking the three-year average race-specific incidence from 1976 to 1978. We then found the race-specific reduction from baseline for each subsequent year from 1979 to 2009, scaled to the race-specific U.S. population as previously described, and summed the reduction for all years to find the race-specific estimates of cervical cancer cases prevented in association with Pap smear screening.
Statistical analysis
Our study population is limited to U.S. women age 18 to 65. The rates of cervical cancer were age-adjusted based on the 2000 U.S. Standard Population. Cervical cancer incidence rates were rounded to the nearest tenth. Estimation of the number of cancer cases prevented were rounded to the nearest thousand. Tests of significance for differences in cancer incidence rates were performed in SEER*Stat using methods described by Tiwari et al.32 All analyses in our study were performed using SEER*Stat and Microsoft Excel software.
Results
Trends in Pap smear screening utilization
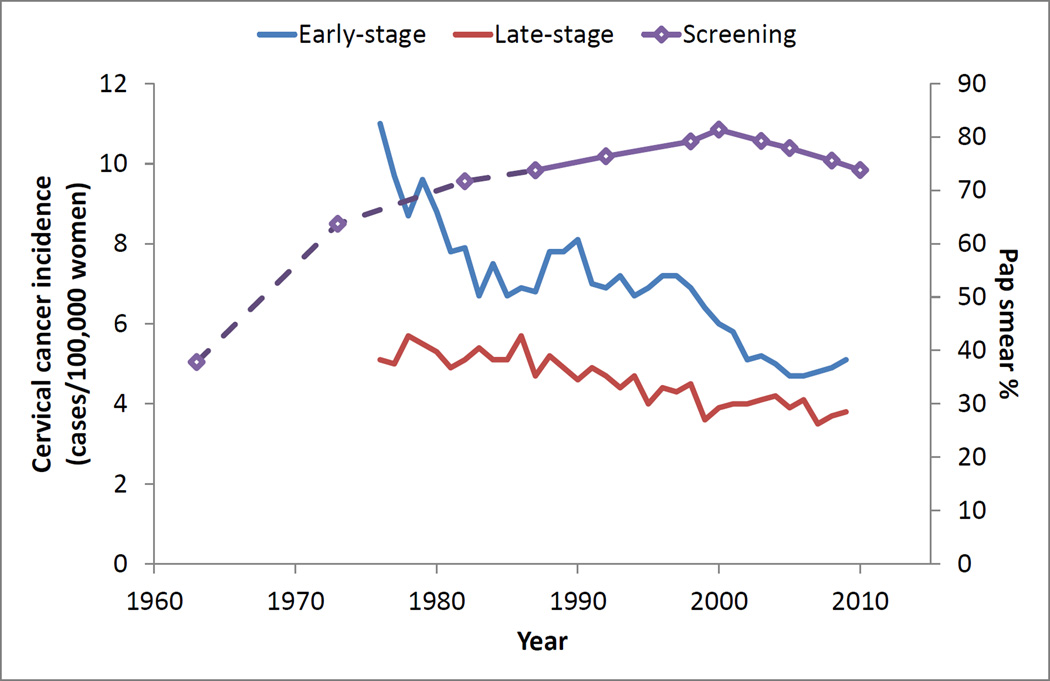
Pap smear screening rates, measured as the percentage of U.S. women age 18 and older who reported having had a Pap test within the past three years, remained high and relatively constant over the past three decades (Figure 1). According to the NCI Cancer Progress Report, in 1987 the percentage of women age 18 or older who have had a Pap test in the past three years was 73.7%. This increased slightly through the year 2000 (81.4%), before falling slightly in 2010 (73.8%).
Fig. 1.
Pap smear utilization rates and associated changes in early- and late-stage cervical cancer incidence
Changes in cervical cancer incidence
Concurrent with an era of widespread and increasing screening, there was a significant decrease in early and late-stage cervical cancer incidence among U.S. women age 18 to 65 from the years 1976 through 2009 (Table 1, Figure 1). The incidence of early-stage cancers decreased from 9.8 to 4.9 cases per 100,000 women (p < .001), and the incidence of late-stage cancers decreased from 5.3 to 3.7 cases per 100,000 women (p < .001). Overall combined incidence decreased from 15.1 to 8.6 cases per 100,000 women (p < .001).
Table 1.
Summary of overall changes in cervical cancer incidence and cases prevented
| Annual cancer incidence (cases per 100,000 women) |
Estimated cases prevented | ||||
|---|---|---|---|---|---|
| Three decades ago (1976–1978) |
Current (2007–2009) |
Change | From baseline incidence |
From pre- screening incidence |
|
| Early-stage cancer incidence | 9.8 | 4.9 | −4.9 | 84,544 | N/A |
| Late-stage cancer incidence | 5.3 | 3.7 | −1.6 | 20,647 | N/A |
| Cases prevented over 3 decades: | 105,000 | 492,000 | |||
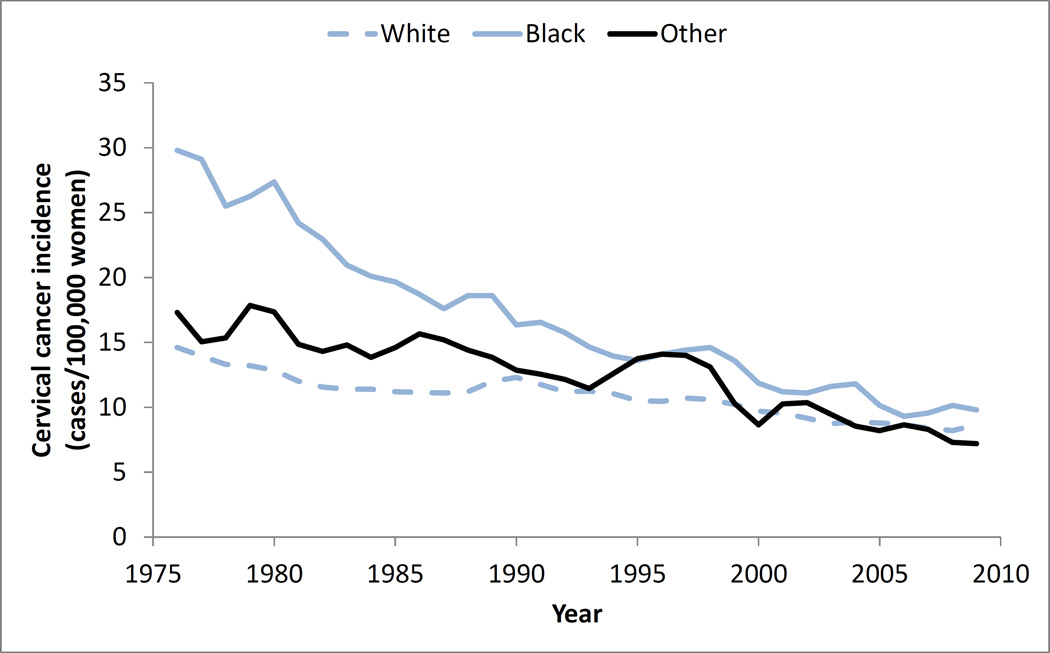
Notably, during this period, there were also differences in changes in race-specific cervical cancer incidence (Table 2, Figure 2), with an overall reduction in the disparity of cervical cancer incidence over time. The incidence among black women decreased from 26.9 to 9.7 cases per 100,000 women (p < .001), whereas the incidence among white women decreased from 13.7 to 8.5 cases per 100,000 women (p < .001), and the incidence among women of other races decreased from 16.0 to 7.4 cases per 100,000 women (p < .001).
Table 2.
Summary of race-specific incidence changes and number of cancer cases prevented
| Annual cancer incidence (cases per 100,000 women) |
Estimated number of cases prevented |
|||
|---|---|---|---|---|
| Three decades ago (1976– 1978) |
Current (2007–2009) |
Change | ||
| Total incidence (all races) | 15.1 | 8.6 | −6.5 | 105,000 |
| White | 13.7 | 8.5 | −5.2 | 71,000 |
| Black | 26.9 | 9.7 | −17.2 | 40,000 |
| Other* | 16 | 7.4 | −8.6 | 6,000 |
Includes American Indian/Alaskan Native, Asian/Pacific Islander
Fig. 2.
Trends in race-specific cervical cancer incidence among U.S. women age 18 to 65
Number of cancer cases prevented
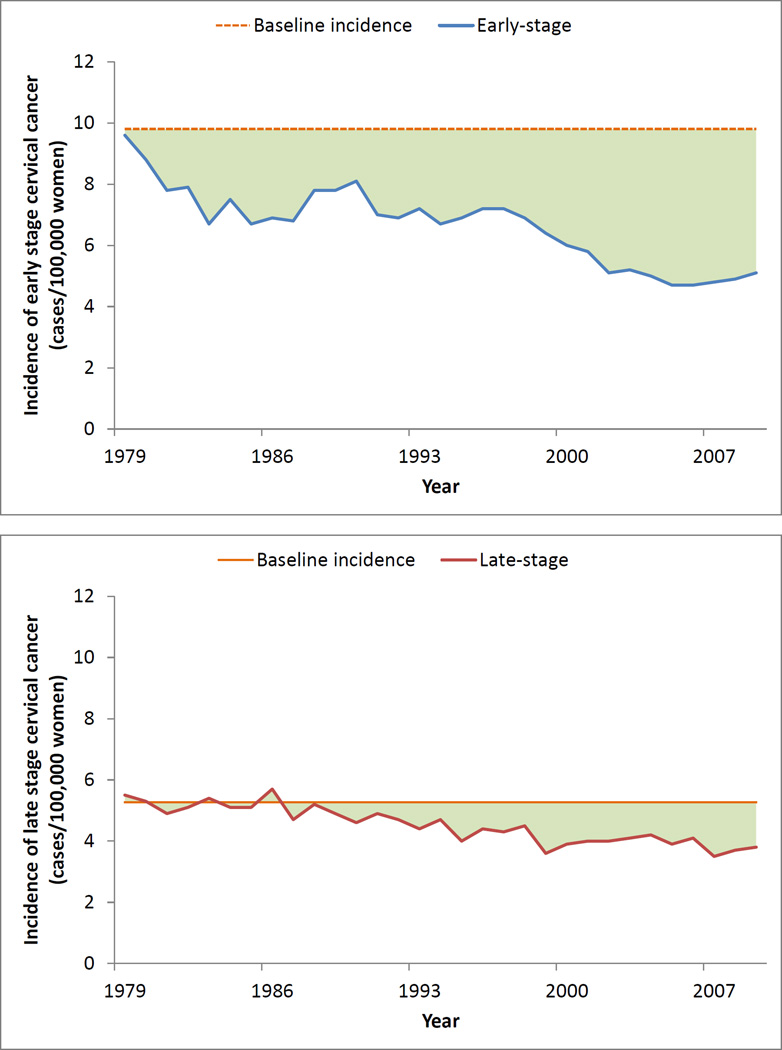
In our baseline incidence approach, we assumed that the underlying cancer incidence remained constant at the 1976–1979 baseline incidence rate of 15.1 per 100,000 women. This relatively conservative estimate demonstrated a reduction of 84,544 early-stage cancers and 20,647 late-stage cancers, leading to an overall reduction of approximately 105,000 cases of cervical cancer (Table 1, Figure 3). In contrast, in the pre-screening incidence approach, in which we assumed the baseline incidence was 30.0 per 100,000 women, there was a more generous reduction of approximately 492,000 cases of cervical cancer.
Fig. 3.
Actual cancer incidence compared to baseline incidence. Area under curve represents the number of cancer cases reduced from baseline
The number of cancer cases prevented for each race group were also different. Using a race-specific baseline incidence approach, there were at least 71,000 cases prevented among white women, 46,000 cases among black women, and 6,000 cases among women of other races (Table 2).
Discussion
In association with widespread Pap smear screening, there has been a significant decline in both early and late-stage cervical cancer incidence over the past three decades. We estimate that 105,000 to 492,000 cases of cervical cancer have been prevented as a result of screening, reflecting the large impact of screening at the population level. Moreover, the impact of screening was different among the different racial groups examined in our study. Consistent with the belief that screening is most effective in high-risk populations, we found that the greatest number of cancer cases prevented were among black women in proportion to the underlying population, and that disparities in terms of race-specific incidences were reduced.
Our study describes a substantial decline in both early- and late-stage cervical cancer incidence over time. These declines occurred after national cervical cancer screening rates increased. We find it plausible that these two observations are connected – that the wide dissemination of cervical cancer screening (i.e. of Pap test utilization and treatment of pre-cancerous lesions) led to both the effective secondary prevention of early invasive cervical cancer, as well as the detection of early-stage cancer before it grew to late-stage.8,33,34 Notably, a large portion of cancers prevented were early-stage cancers. This marked decrease in early invasive cervical cancer despite a concomitant decline in late-stage disease (Figure 3) suggests that a greater percentage of cancer prevented were due to secondary prevention of early invasive disease, compared to detection of early-stage cancer before its progression to late-stage. However, such an observation is ecological in nature. Despite the strong temporal relationship between Pap test utilization and cervical cancer incidence, other factors may also have contributed to the decline in cervical cancer incidence. Secular changes in sexual behavior (e.g. number of sexual partners and contraceptive use),35 HPV prevalence,36 and immunocompetence status of the host (e.g. HIV infection or post-transplant immunosuppression),37,38 as well as decreasing rates of cigarette smoking39 may also have accounted for some of the observed changes in cervical cancer incidence. Most recently, the advent and dissemination of HPV vaccination since 200640 is also likely to reduce invasive cervical cancer incidence in the coming years. However, given the relatively long pre-malignant period of HPV infection, primary prevention via vaccination is unlikely to have had a significant effect during the time period examined in our study.
Additionally, our study indicates that while disparities were persistent, the incidence rates of cervical cancer appear to be converging across different race groups (blacks, whites, and other). While previous studies have demonstrated similar trends,8,10–12 our analysis uniquely estimates the net impact of screening by calculating the magnitude of cancers prevented by race. As we progress towards an era of HPV vaccination and HPV-based screening,41,42 such race-specific trends will become of especially important concern. Given the current disparities in HPV vaccination use,43 differences in race-specific cervical cancer incidence may again magnify in the coming years, especially as black and lower socioeconomic status women are at greater risk of being diagnosed with late-stage cervical cancers.44–46 Moreover, with the recent 2014 FDA approval of the use of the HPV test alone for primary cervical cancer screening,16 it remains to be seen what changes in screening utilization and cervical cancer incidence will occur as a result of this approval.47,48 While racial breakdown of participants in the ATHENA HPV trial was comparable to that of the United States population as a whole,49,50 subsequent studies indicate that HPV test utilization patterns have thus far and will likely continue to differ by practice setting and population demographics.51,52 The changing landscape of cervical cancer screening will likely present challenges in terms of equal access to the increasing number of possible screening approaches.53,54 Therefore, it is necessary that we continue to monitor rates of cervical cancer screening and the impact of screening on cancer rates in the current and future eras, especially for at risk populations such as minorities and women of lower socioeconomic status.
In recent years, there has also been increasing concern that widespread cancer screening can lead to over-diagnosis and over-treatment,55–57 and the realization that the benefits of cancer screening depend on the type of cancer and the population screened. As a result, cancer screening may be increasingly viewed as a matter of choice rather than a routine part of health maintenance. Our findings of a greater decline in early stage cancers and a smaller but still significant decline in late stage cancers indicates that though there may be increasing diagnosis of early stage cancers that may otherwise have gone undetected, we feel that the significant overall decline in late stages cancers justifies the current screening regimen.
There are several important limitations to our study. First, we were unable to account for factors outside of screening. As previously alluded, while the Pap smear test is most likely the primary cause of decreased cervical cancer incidence, it is likely that other factors may have also played a role. As such, our results should be interpreted as an association between screening and the number of cancer cases prevented, rather than a causal effect. Second, our analysis did not account for the underlying rates of hysterectomy, which may be higher in blacks than whites.58,59 This underlying statistic may skew our findings since hysterectomy can be indicated by factors in addition to cervical malignancy. Since women who have undergone hysterectomy and are not at risk for cervical cancer were included in the denominator of our incidence rate calculations, the incidence of cervical cancer may in fact be higher in blacks than as reported in our study. Third, we were unable to take into cytopathologic innovation in examining cervical cytology such as the introduction of the Bethesda system,60 or other incremental changes to medical technology over time that may have influenced trends in the diagnosis of cervical cancer. Finally, our study is ecological and has limitations inherent to such studies.61 For example, there is lack of complete data on race and ethnicity of the underlying U.S. population, and it is important to recognize that the racial groups we have specified in our study are heterogeneous and consist of a number of unique racial and ethnic populations. The categorization of “Race” as Black/White/Other leaves out subtleties in ethnicity and self-identification, including the potential for convergence of racial identification over time. The impact of more nuanced racial identification should be the subject of future study as registry data and understanding of the impact of racial self-identification becomes more sophisticated.
In conclusion, we estimate that a large number of early and late-stage cervical cancers were prevented, and that racial disparity in cervical cancer rates were reduced during an era of widespread Pap test screening. Our study is unique in that we sought to quantify the impact of screening at the population level by estimating the number of cancers prevented in association with Pap smear utilization. Furthermore, our analysis demonstrates that the impact of screening has likely differed by race in the U.S., with the at-risk populations most preferentially affected. Given disparate access to HPV vaccination,34 it will be important to continually assess national cervical cancer incidence to measure the additional benefit of the vaccine against the known benefit of screening, and to ensure equal access and outcomes for all women.
Supplementary Material
Acknowledgments
James B. Yu is supported by CTSA grant KL2 RR024138 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. James B. Yu and Cary P. Gross have research funding from 21st Century Oncology LLC.
Footnotes
Disclosure statements:
None of the authors has any affiliation, financial agreement, or other involvement with any company whose product figures prominently in the submitted manuscript. We are not using any copyrighted information, nor are we using any identifiable patient photographs or other patient identifiers in this paper.
This work was presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in June of 2014, and at the American Society of Radiation Oncology (ASTRO) Annual Meeting in September of 2014.
References
- 1.Moyer VA Force USPST. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;156(12):880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 2.Clarke EA, Anderson TW. Does screening by "Pap" smears help prevent cervical cancer? A case-control study. Lancet. 1979;2(8132):1–4. doi: 10.1016/s0140-6736(79)90172-7. [DOI] [PubMed] [Google Scholar]
- 3.Vesco KK, Whitlock EP, Eder M, et al. Screening for Cervical Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): 2011. [PubMed] [Google Scholar]
- 4.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. The New England journal of medicine. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 5.Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. The New England journal of medicine. 2007;357(16):1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 6.Akers AY, Newmann SJ, Smith JS. Factors underlying disparities in cervical cancer incidence, screening, and treatment in the United States. Current problems in cancer. 2007;31(3):157–181. doi: 10.1016/j.currproblcancer.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Janerich DT, Hadjimichael O, Schwartz PE, et al. The screening histories of women with invasive cervical cancer, Connecticut. American journal of public health. 1995;85(6):791–794. doi: 10.2105/ajph.85.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce Campbell CM, Menezes LJ, Paskett ED, Giuliano AR. Prevention of invasive cervical cancer in the United States: past, present, and future. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(9):1402–1408. doi: 10.1158/1055-9965.EPI-11-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDougall JA, Madeleine MM, Daling JR, Li CI. Racial and ethnic disparities in cervical cancer incidence rates in the United States, 1992–2003. Cancer causes & control : CCC. 2007;18(10):1175–1186. doi: 10.1007/s10552-007-9056-y. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 11.Simard EP, Naishadham D, Saslow D, Jemal A. Age-specific trends in black-white disparities in cervical cancer incidence in the United States: 1975–2009. Gynecologic oncology. 2012;127(3):611–615. doi: 10.1016/j.ygyno.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Changing demographics of cervical cancer in the United States (1973–2008) Gynecologic oncology. 2012;126(3):330–333. doi: 10.1016/j.ygyno.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR. Morbidity and mortality weekly report. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn JA. HPV vaccination for the prevention of cervical intraepithelial neoplasia. The New England journal of medicine. 2009;361(3):271–278. doi: 10.1056/NEJMct0806938. [DOI] [PubMed] [Google Scholar]
- 15.Chesson HW, Flagg EW, Koutsky L, et al. Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine. 2013;31(29):3019–3024. doi: 10.1016/j.vaccine.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA approves first human papillomavirus test for primary cervical cancer screening [press release] http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm2014.
- 17.Nielsen C, Lang RS. Principles of screening. The Medical clinics of North America. 1999;83(6):1323–1337. v. doi: 10.1016/s0025-7125(05)70169-3. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Percent of women aged 18 years and older who had a pap smear test within the past 3 years by race/ethnicity: 1987–2010. Cancer Trends Progress Report – 2011/2012 Update. [Google Scholar]
- 19.Dickinson LE. Control of cancer of the uterine cervix by cytologic screening. Gynecologic oncology. 1975;3(1):1–9. doi: 10.1016/0090-8258(75)90001-3. [DOI] [PubMed] [Google Scholar]
- 20.Kegeles SS, Kirscht JP, Haefner DP, Rosenstock IM. Survey of beliefs about cancer detection and taking Papanicolaou tests. Public health reports. 1965;80(9):815–823. [PMC free article] [PubMed] [Google Scholar]
- 21.Moss AJ, Wilder MH. Use of selected medical procedures associated with preventive care: United States-1973. National Health Survey. 1977 [PubMed] [Google Scholar]
- 22.Bloom B. Use of selected preventive care procedures: United States, 1982. U.S. Department of Health and Human Services; 1986. [PubMed] [Google Scholar]
- 23.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub, Vintage 2009 Pops (1973–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission
- 24.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Current problems in cancer. 2012;36(4):183–190. doi: 10.1016/j.currproblcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 25.US Population Data-- 1969–2011. Surveillance, Epidemiology, and End Results (SEER) Program 2013. [Accessed February 1, 2014]; http://seer.cancer.gov/popdata/.
- 26.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. The New England journal of medicine. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 27.Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014 doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]
- 28.Plaxe SC, Saltzstein SL. Estimation of the duration of the preclinical phase of cervical adenocarcinoma suggests that there is ample opportunity for screening. Gynecologic oncology. 1999;75(1):55–61. doi: 10.1006/gyno.1999.5524. [DOI] [PubMed] [Google Scholar]
- 29.Lundin FE, Jr, Christopherson WM, Mendez WM, Parker JE. Morbidity from cervical cancer: effects of cervical cytology and socioeconomic status. Journal of the National Cancer Institute. 1965;35(6):1015–1025. [PubMed] [Google Scholar]
- 30.Cramer DW. The role of cervical cytology in the declining morbidity and mortality of cervical cancer. Cancer. 1974;34(6):2018–2027. doi: 10.1002/1097-0142(197412)34:6<2018::aid-cncr2820340621>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Devesa SS, Silverman DT, Young JL, Jr, et al. Cancer incidence and mortality trends among whites in the United States, 1947–84. Journal of the National Cancer Institute. 1987;79(4):701–770. [PubMed] [Google Scholar]
- 32.Tiwari Rc Fau - Clegg LX, Clegg Lx Fau - Zou Z, Zou Z. Efficient interval estimation for age-adjusted cancer rates. doi: 10.1177/0962280206070621. (0962-2802 (Print)). [DOI] [PubMed] [Google Scholar]
- 33.Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116(11):2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinton LA, Hamman RF, Huggins GR, et al. Sexual and reproductive risk factors for invasive squamous cell cervical cancer. Journal of the National Cancer Institute. 1987;79(1):23–30. [PubMed] [Google Scholar]
- 36.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Koutsky L. Epidemiology of genital human papillomavirus infection. The American journal of medicine. 1997;102(5A):3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 38.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA : the journal of the American Medical Association. 2000;283(8):1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 39.Slattery ML, Robison LM, Schuman KL, et al. Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA : the journal of the American Medical Association. 1989;261(11):1593–1598. [PubMed] [Google Scholar]
- 40.Baylor NW. SERVICES DOHAH, editor. Approval Letter - Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm111283.htm2006.
- 41.Franco EL, Cuzick J, Hildesheim A, de Sanjose S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24(Suppl 3):S3/171–S3/177. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 42.Isidean SD, Franco EL. Embracing a new era in cervical cancer screening. Lancet. 2014;383(9916):493–494. doi: 10.1016/S0140-6736(13)62028-0. [DOI] [PubMed] [Google Scholar]
- 43.Dempsey A, Cohn L, Dalton V, Ruffin M. Worsening disparities in HPV vaccine utilization among 19–26 year old women. Vaccine. 2011;29(3):528–534. doi: 10.1016/j.vaccine.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. American journal of public health. 1991;81(5):646–649. doi: 10.2105/ajph.81.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrante JM, Gonzalez EC, Roetzheim RG, Pal N, Woodard L. Clinical and demographic predictors of late-stage cervical cancer. Archives of family medicine. 2000;9(5):439–445. doi: 10.1001/archfami.9.5.439. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Medical care. 1997;35(12):1220–1224. doi: 10.1097/00005650-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Feldman S. Human Papillomavirus Testing for Primary Cervical Cancer Screening: Is It Time to Abandon Papanicolaou Testing? JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2014.4021. [DOI] [PubMed] [Google Scholar]
- 48.Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Archives of internal medicine. 2010;170(11):977–985. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 49.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. American journal of obstetrics and gynecology. 2012;206(1):46 e41–46 e11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecologic oncology. 2015;136(2):189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 51.Weinmann S, Williams AE, Kamineni A, et al. Cervical cancer screening and follow-up in 4 geographically diverse US health care systems, 1998 through 2007. Cancer. 2015 doi: 10.1002/cncr.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuzick J, Myers O, Hunt WC, et al. A population-based evaluation of cervical screening in the United States: 2008–2011. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(5):765–773. doi: 10.1158/1055-9965.EPI-13-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiffman M, Wentzensen N. Transitioning to a new era in cervical cancer screening. Gynecologic oncology. 2015;136(2):175–177. doi: 10.1016/j.ygyno.2015.01.538. [DOI] [PubMed] [Google Scholar]
- 54.Sauer AG, Jemal A, Simard EP, Fedewa SA. Differential uptake of recent Papanicolaou testing by HPV vaccination status among young women in the United States, 2008–2013. Cancer epidemiology. 2015 doi: 10.1016/j.canep.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Woolf SH, Harris R. The harms of screening: new attention to an old concern. JAMA : the journal of the American Medical Association. 2012;307(6):565–566. doi: 10.1001/jama.2012.100. [DOI] [PubMed] [Google Scholar]
- 56.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement emerging overdiagnosis and overtreatment viewpoint. JAMA : the journal of the American Medical Association. 2013 doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 57.Gross CP. Cancer Screening in Older Persons: A New Age of Wonder. JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2014.3901. [DOI] [PubMed] [Google Scholar]
- 58.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. American journal of public health. 2009;99(2):300–307. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. American journal of obstetrics and gynecology. 2008;198(1):34, e31–e37. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 60.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA : the journal of the American Medical Association. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 61.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annual review of public health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.