Abstract
Black youth are at higher risk for type 2 diabetes than their white peers. Previously we demonstrated that for the same degree of insulin sensitivity, black youth have an upregulated β-cell function and insulin hypersecretion, in response to iv glucose, compared with whites. To investigate if the same holds true during an oral glucose challenge and because of the important role of GLP-1 and GIP in augmenting insulin secretion, we examined β-cell function and incretin hormones in 85 black and 78 white obese adolescents, with normal glucose tolerance (NGT), during a 2-hr OGTT with mathematical modeling of plasma glucose and C-peptide concentrations to assess β-cell glucose sensitivity (βCGS), rate sensitivity, potentiation factor and insulin sensitivity. Incretin, pancreatic polypeptide and glucagon concentrations were measured during the OGTT. Black obese youth had a heightened early insulin secretion together with significantly greater βCGS, rate sensitivity, and potentiation factor compared with whites, with no differences in incretin and glucagon concentrations. Basal and stimulated insulin clearance was lower (p=0.001) in black vs. white youth. In conclusion, during an OGTT black obese youth with NGT demonstrate a pronounced early insulin secretion jointly with heightened β-cell glucose sensitivity, rate sensitivity, and potentiation factor. These racial disparities in β-cell function and the pathophysiological components of type 2 diabetes are unlikely to be attributed to incretin hormones and remain to be investigated further to explain the metabolic basis for the enhanced risk of type 2 diabetes in back youth.
Keywords: Youth, Modelling, β-cell function, Insulin secretion, Race
Introduction
Black children have a fourfold higher incidence of developing type 2 diabetes (T2D) compared with their white peers (1, 2). The root cause of these disproportions are incompletely understood, and not completely accounted for by differences in obesity, diet, or levels of physical activity (3–5). Previously, we (6, 7) and others (8) demonstrated that for the same degree of insulin sensitivity, black youth have profound insulin hypersecretion compared with white youth in response to intravenous (iv) glucose challenge, partly explained by decreased insulin clearance (9) and an upregulated β-cell activity (10, 11). However, investigations examining racial disparities in insulin secretion in response to oral glucose administration yielded inconsistent results. In obese adults, blacks exhibit hyperinsulinemia and decreased insulin clearance compared with their white counterparts (12). On the other hand, black youth have reduced insulin concentrations compared with Latino youth (13), whereas black girls and women demonstrate higher acute insulin response and lower insulin sensitivity compared with whites, independent of age (14). To further explore the racial disparities in insulinemia in response to oral glucose, and examine the underlying mechanisms responsible for the observed contrast, we used an established mathematical model, elaborated in detail under calculations/methods, that considers the dynamic relationship between glucose concentration and insulin secretion from C-peptide during an OGTT.
Gut derived incretin hormones, glucagon-like peptide 1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), play an important role in augmenting glucose-stimulated insulin secretion (15) making them potential candidates for the observed racial disparity in insulinemia/insulin secretion. However, racial differences in the incretin response to oral glucose are conflicting. In obese adults, blacks had significantly higher fasting and postchallenge GLP-1 concentrations compared with whites (12), but black youth were reported to have higher (12) or lower (16, 17) fasting GLP-1 and GLP-1 AUC compared with whites. These investigations (12, 17) included youth with impaired glucose metabolism (IGM) and normal glucose tolerance (NGT) which may contribute to the aforementioned inconsistencies; as youth with impaired glucose tolerance (IGT) have impairments in the incretin effect compared with NGT youth (18). In an effort to explain the upregulated β-cell function and insulin hypersecretion in otherwise healthy normoglycemic (6, 7, 11) black youth, we investigated insulin secretion during a 2-hr OGTT, using mathematical modeling, and incretin hormones in black vs. white obese youth with normal glucose tolerance.
Research Design and Methods
Data were analyzed from 85 black and 78 white obese adolescents, with normal glucose tolerance, who had an OGTT performed as part of their participation in the National Institutes of Health-funded studies of “Childhood Metabolic Markers of Adult Morbidity in Blacks” (6, 18–20). This was a subset of our previously published data examining β-cell function and incretin effect across the spectrum of dysglycemia from normal glucose tolerance to pre diabetes to type 2 diabetes (18). The current subset was analyzed because they had normal glucose tolerance since our aim was to assess black/white contrast under normal physiological conditions excluding dysglycemia. All studies were performed at the Pediatric Clinical and Translational Research Center (PCTRC) at Children’s Hospital of Pittsburgh after parental consent and child assent were obtained. All participants were pubertal (Tanner II – V) and had exogenous obesity with no clinical evidence of endocrinopathy associated with obesity. Participants were advised to refrain from any physical activity, besides their daily routine and any dietary modification. None of the participants were involved in any exercise programs or weight modifying diets.
Fat free mass (FFM), fat mass (FM), and percent body fat (%BF) were evaluated with dual-energy x-ray absorptiometry (DEXA) as described previously (21). Abdominal subcutaneous (SAT) and visceral adipose tissue (VAT) were assessed by magnetic resonance imaging (N=125) (MRI) or computed tomography (N=34) (CT) at L4-5 intervertebral space (22, 23). Three white participants are missing DEXA data and 4 are missing abdominal adiposity data (3 black and 1 white) due to technical difficulties and weight exceeding the limit of measurement. Clinical characteristics of the study participants are summarized in Table 1.
Table 1.
Participant Characteristics
| Black | White | P | |
|---|---|---|---|
| N | 85 | 78 | |
| Age (yrs) | 14.6 ± 0.2 | 14.8 ± 0.2 | NS |
| Gender (N) (male/female) | 32/53 | 35/43 | NS |
| Tanner (N) (II–III/IV–V) | 8/77 | 16/62 | 0.008 |
| BMI (kg/m2) | 34.1 ± 0.6 | 34.1± 0.7 | NS |
| BMI percentile | 97.6 ± 0.3 | 97.2 ± 0.4 | NS |
| FM (kg) | 38.8 ± 1.3 | 40.4 ± 1.5 | NS |
| FFM (kg) | 51.7 ± 1.0 | 51.0 ± 1.3 | NS |
| %BF | 41.5 ± 0.9 | 42.6 ± 0.8 | NS |
| VAT (cm2) | 49.1 ± 3.1 | 74.9 ± 3.5 | <0.001 |
| SAT (cm2) | 458.1 ± 20.5 | 476.3 ± 21.8 | NS |
| VAT-to-SAT ratio | 0.12 ± 0.06 | 0.17 ± 0.07 | <0.001 |
| HbA1c (%) | 5.5 ± 0.1 | 5.3 ± 0.04 | 0.014 |
| OGIS (ml.min−1.m−2)* | 361.4 ± 7.3 | 349.6 ± 7.8 | NS |
FM = fat mass; FFM = fat-free mass; VAT = Visceral adipose tissue;
SAT = subcutaneous adipose tissue; NS = not significant.
OGIS= oral glucose insulin sensitivity, corrected for Tanner Stage
Oral glucose tolerance test
Participants underwent a 2-hr OGTT (1.75 g/kg, maximum 75g) after a 10–12 hr overnight fast (21, 24). Blood samples were obtained at −15, 0, 15, 30, 60, 90 and 120 min for measurement of glucose, insulin, C-peptide, glucagon, total GLP-1, GIP, pancreatic polypeptide (PP) and free fatty acids (FFA). The latter was to assess for black/white differences in FFA as a potential explanation for differences in insulin secretion.
Biochemical measurements
For the determination of insulin and C-peptide measurements, blood was collected at each sampling point and chilled in aprotinin/EDTA tubes as before (18). Plasma glucose was determined, at the bed-side, by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, Ohio), and plasma insulin and C-peptide by commercially available radioimmunoassay (Millipore, St. Charles, MO), as reported by us (24). FFA were determined using enzymatic colorimetric methods with a Wako nonesterified fatty acid (NEFA)-HR (2) test kit (Wako, Osaka, Japan). HbA1c was measured by high performance liquid chromatography (Tosoh Medics). Total GLP-1 was measured on a microplate reader (BioTek, Winooski, VT) using a multi species total GLP-1 ELISA Kit (catalog no. EZGLP1T-36K; Millipore) and total GIP and PP were measured on the Luminex 200 IS (Luminex, Austin, TX) using a two-plex human gut hormone MILLIplex Kit (catalog no. HGT-68K-02; Millipore) (18). Intra- and inter-assay coefficients of variation were 5.4% and 8.2% for GLP-1 and 5.1% and 9.1% for GIP.
Calculations
Area-under-curve (AUC) was calculated with the use of the trapezoidal method. During the OGTT, early-phase responses were calculated as the AUC for the first 30 min and late-phase responses as the AUC for the last 90 min after the glucose challenge (18). Mathematical modeling of plasma glucose and C-peptide concentrations during the OGTT were employed to assess β-cell function parameters, according to a previously developed model by Mari et al. (25, 26). β-cell function parameters included β-cell glucose sensitivity (OGTT-βCGS) (in pmol.min−1.m−2.mM−1), rate sensitivity (in pmol.m−2.mM−1) and potentiation. OGTT-βCGS reflects the ability of the β-cell to respond to changes in prevailing plasma glucose concentration at any time point during the OGTT through a dose-response function relating insulin secretion and glucose concentration (26). Rate sensitivity is the magnitude of the β-cell response to the rate of change in plasma glucose concentrations. The potentiation factor modulates the relationship between glucose concentration and insulin secretion by increasing the sensitivity of the β-cells to subsequent plasma glucose concentration (26). The potentiation factor comprises several mechanisms including release of endogenous incretin hormones, neuronal inputs, and changes in incremental plasma glucose concentrations after ingestion of the glucose load. Total insulin output relates to the AUC of insulin secretion during the 2-hr OGTT (expressed in nmol.m−2). Basal and stimulated insulin clearance was calculated from the ratio of insulin secretion AUC/insulin AUC (27). A model-based index of insulin sensitivity (Oral Glucose Insulin Sensitivity, OGIS), validated against the hyperinsulinemic euglycemic clamp, was calculated using the plasma glucose and insulin concentrations during the 2-hr OGTT (28).
Statistical Analysis
Subject characteristics, β-cell function parameters and early and late phase incretin response were determined using a Student T-test for quantitative variables and χ2 test for categorical variables between groups. ANCOVA models were used to assess between group differences adjusting for Tanner stage. A repeated-measures ANCOVA (within-subjects factor of time; between-subjects factor of race, i.e., black vs. white; time × race interaction) was used to analyze glucose, hormone, and incretin response to oral glucose. Log transformations were used to normalize the distribution for insulin and GLP-1. All other variables were normally distributed. Data are presented as mean ± SEM. Statistical significance was set at p<0.05 and the statistical analyses were performed using PASW Statistics (version 20, SPSS Inc., Chicago, IL).
Results
Participant Characteristics
Black and white adolescents were similar in age, gender, BMI, FFM, and %BF. Compared with white youth, a greater proportion of blacks were Tanner stage IV–V, had lower VAT, lower VAT-to-SAT ratio, and higher HbA1c (Table 1). Oral glucose insulin sensitivity was similar between blacks and whites before and after adjusting for differences in VAT and Tanner stage.
Glucose, C-Peptide, Glucagon and Modeled β-cell Function Parameters During the OGTT
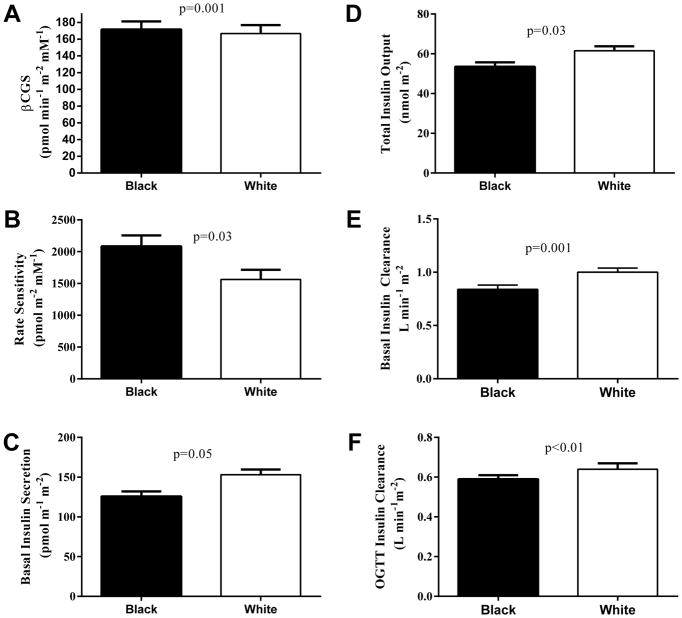
Fasting glucose and C-peptide were lower in blacks compared with whites (Figure 1). During the OGTT, glucose concentrations (Figure 1A) and AUC (795.6 ± 10.6 vs. 846.4 ± 10.6, p=0.001) were lower in blacks compared with whites (main effect of race, p=0.001). Early insulin secretion showed a rapid and pronounced increase followed by a rapid decline in black youth compared with whites (Figure 1C) (time x race interaction, p<0.001) consistent with the reduced late-phase rise in C-peptide (Figure 1B) (time x race interaction, p=0.03), which remained significant after controlling for differences in glucose response during the OGTT and Tanner stage. Coherent with the insulin secretion, early potentiation factor and AUC in the first 30 minutes of the OGTT were higher in blacks vs. whites (p<0.02 and p=0.05, respectively) (Figure 1D), together with higher βCGS and rate sensitivity in blacks (Figure 2A–B). C-peptide AUC was lower in blacks (328.1 ± 13.6 vs. 373.2 ± 14.1, p=0.01), but insulin AUC was similar between black and whites (110908.7±8806.3 vs. 114450.0 ± 8240.9, p=NS). Basal insulin secretion, total insulin output and basal and stimulated insulin clearance rates were lower in blacks compared with whites (Figure 2 C–F).
Figure 1.
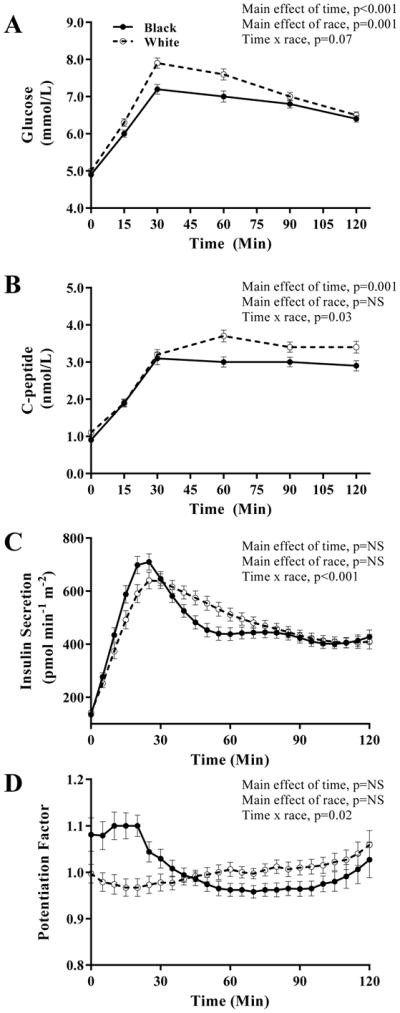
Glucose (A), C-peptide (B), insulin secretion (C) and potentiation factor (D) in obese black (N=85) and white (N=78) NGT adolescents during a 2-hr OGTT. Plots are mean ± SEM.
Figure 2.
βCGS (A), rate sensitivity (B), basal insulin secretion (C), total insulin output (D), basal insulin clearance (E), and OGTT insulin clearance (F) in obese black (N=85) and white (N=78) NGT adolescents during a 2-hr OGTT. Plots are mean ± SEM.
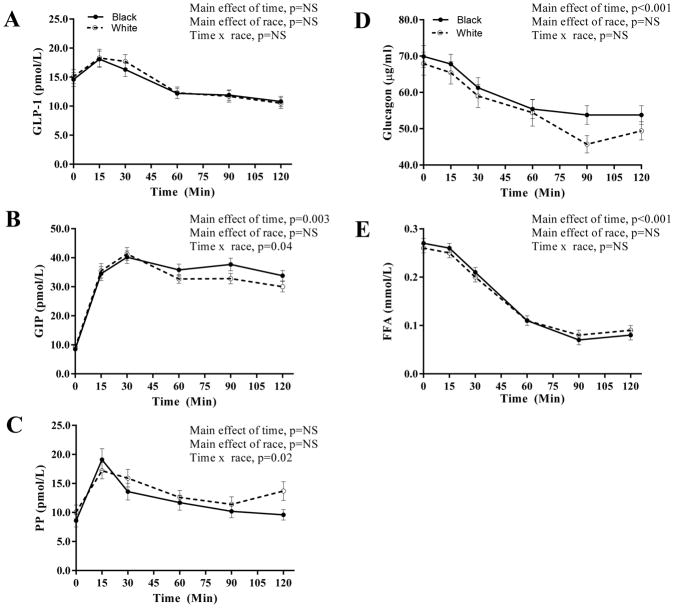
Incretin response, PP, glucagon and FFA concentrations during the OGTT are depicted in Figure 3. Fasting incretin and FFA concentrations did not differ between the two groups. During the OGTT, GLP-1 AUC was similar between blacks and whites (1644.9±117.4 vs. 1711.9±118.2; p=0.67) (Figure 3A). GIP and PP responses showed a significant time x race interaction with blacks having a more pronounced early PP response and a greater sustainability of GIP concentrations compared with whites (Figure 3B–C). Glucagon and FFA concentrations were similar between blacks and whites (Figure 3D–E).
Figure 3.
Incretin, glucagon and FFA concentrations during the OGTT: GLP-1 (A), GIP (B), PP (C), glucagon (D) and FFA (E) in obese black (N=85) and white (N=78) NGT adolescents during a 2-hr OGTT. Plots are mean ± SEM.
Determinants of glucose, C-peptide and Insulin Clearance during the OGTT
In an effort to examine the determinants of glycemia and β-cell function during the OGTT, multiple linear regression models were used (Table 2). Race was a significant independent contributor to the variance in C-peptide AUC of the OGTT and insulin clearance but not OGTT glucose AUC. For the latter, 73% of the variance was explained by insulin sensitivity, βCGS, rate sensitivity, insulin clearance, and Tanner stage (independent variables: age, sex, Tanner stage, race, VAT, insulin sensitivity, GLP-1 AUC, βCGS, potentiation ratio, rate sensitivity and insulin clearance). Since βCGS, rate sensitivity, and insulin clearance were significantly different between blacks and whites, a second model was constructed removing these parameters which yielded insulin sensitivity, race, VAT and sex explaining 34% of the variance in glucose AUC. For C-peptide AUC, 68% of the variance was explained by insulin sensitivity, rate sensitivity, race and sex, each independently and significantly contributing to the model. With respect to OGTT insulin clearance, insulin sensitivity, race, and VAT explained 46% of the variance.
Table 2.
Significant Independent Determinants of glucose, C-peptide and Insulin Clearance during the OGTT
| Dependent Variable: Glucose AUC | Partial r | Model r2 | Change in r2 | P |
|---|---|---|---|---|
|
|
||||
| Model 1 | ||||
| Insulin sensitivity | −0.79 | 0.24 | 0.24 | <0.001 |
| βCGS | −0.74 | 0.56 | 0.32 | <0.001 |
| Rate Sensitivity | −0.52 | 0.67 | 0.11 | <0.001 |
| OGTT insulin clearance | 0.38 | 0.71 | 0.04 | <0.001 |
| Tanner | −0.30 | 0.73 | 0.03 | <0.001 |
| Model 2 | ||||
| Insulin sensitivity | −0.51 | 0.24 | 0.24 | <0.001 |
| Race | 0.28 | 0.28 | 0.05 | <0.001 |
| VAT | −0.22 | 0.32 | 0.04 | 0.001 |
| Sex | −0.16 | 0.34 | 0.02 | 0.021 |
| Dependent Variable: C-peptide AUC | ||||
| Insulin sensitivity | −0.77 | 0.62 | 0.62 | <0.001 |
| Rate sensitivity | 0.33 | 0.65 | 0.04 | 0.001 |
| Race | 0.27 | 0.67 | 0.02 | 0.002 |
| Sex | 0.18 | 0.68 | 0.01 | 0.03 |
| Dependent Variable: Insulin clearance | ||||
| Insulin sensitivity | 0.54 | 0.38 | 0.38 | <0.001 |
| Race | 0.30 | 0.41 | 0.03 | 0.011 |
| VAT | −0.28 | 0.46 | 0.05 | 0.001 |
Discussion
In our continued efforts to examine the mechanism(s) behind the upregulated β-cell function and the hyperinsulinemia in black vs. white youth, the current investigation, using mathematical modeling of β-cell function during an OGTT revealed that: 1) black youth have a heightened early insulin secretion compared with whites coincident with 2) a higher βCGS, rate sensitivity and early potentiation factor but 3) no differences in mean incretin concentrations except for a race-dependent difference in the temporal pattern in GIP and PP, and 4) lower insulin clearance.
During an iv glucose challenge and for the same degree of insulin sensitivity, black youth have an exaggerated early insulin response compared with whites (6–8). However, during an OGTT racial disparities in insulin secretion are inconsistent (12–14). Compared with their white counterparts, black obese adults exhibited hyperinsulinemia and decreased insulin clearance (12). Conversely, black youth had reduced insulin concentrations compared with Latino youth (13), yet black females demonstrated a higher acute insulin response and lower insulin sensitivity compared with whites (14). The present study, employing mathematical modeling of β-cell function revealed consistent findings with iv glucose challenge in adults (29–31) and youth (21, 32–37). Black youth had approximately 15% higher early insulin secretion in harmony with the 12% higher βCGS and the 33% higher rate sensitivity, corresponding to a greater β-cell response to changes in glucose concentrations. However, unlike previous investigations (8, 10) where blacks had lower insulin sensitivity compared with whites, OGTT insulin sensitivity by OGIS, was similar between black and white youth. We previously demonstrated that the racial disparity in insulin sensitivity is only detected in normal weight youth and not in obese youth (6, 38) akin to the present investigation. This is because the obesity-associated insulin resistance overshadows the race-associated insulin resistance of much lesser magnitude (7). Furthermore, our past observations of lower insulin sensitivity in normal weight black vs. white youth were made using the hyperinsulinemic-euglycemic clamp, which is a much more sensitive method of assessing insulin sensitivity (where both insulin and glucose concentrations are clamped) than an OGTT- derived index of insulin sensitivity (where glucose and insulin concentrations vary among participants). However, the OGTT-derived OGIS is in agreement with others employing the OGTT-derived whole-body insulin sensitivity index (12) and the frequently-sampled iv glucose tolerance test using MINMOD (14). Irrespective, the present data and past observations, suggests that while interpreting insulinemia during an OGTT, race should be taken into consideration.
The literature with respect to racial differences in GLP-1 and GIP are controversial showing increased or decreased concentrations in blacks compared with whites. In severely obese black adults, fasting GLP-1 and GLP-1 AUC were significantly higher than in whites (12, 39). On the other hand, in overweight to obese adolescents, total GLP-1 AUC was significantly lower in blacks compared with whites (17). Using a mixed macronutrient meal, black children demonstrated lower early- and late phase GLP-1 response whereas GIP response did not differ compared with whites (16). In our study, early – and late – phase AUC for GLP-1, GIP, PP, and FFAs were similar. The inconsistencies in the literature may be due to differences in measuring active vs. total GLP-1 (40), differences in participant characteristics, and the different liquid meals used to assess incretin response. The observed lower GLP-1 in black vs white youth by Higgins et al. may be the result of measuring active GLP-1 and having used a mixed macronutrient meal (16) in contrast to total GLP-1 and the oral glucose load in our study. Furthermore, several investigations included a mixed-sample of participants with normal and impaired glucose metabolism (IGM) (12, 17, 39). Differences in incretin concentrations and effect have been described, with IGM youth having higher GLP-1 AUC than NGT youth (17), and IGT youth showing impaired incretin effect compared with NGT (18). Therefore, heterogeneity of glucose metabolism may be contributing to the different study findings with respect to incretin hormones.
We also observed significant differences in the temporal patterns in PP and GIP between races. Blacks demonstrated a greater early augmentation in PP followed by a rapid and consistent decline at minute 30 compared with whites. Whereas GIP increased similarly for the first 30 minutes, blacks sustained higher GIP concentrations over the remainder of the OGTT unlike their white peers. It remains to be determined if these subtle temporal differences in incretin concentrations translate to functional differences since the former only partially relates to the function of the incretin hormones. In this regard, we examined the potentiation factor, which comprises several mechanisms including release of endogenous incretin hormones, neuronal inputs, and changes in incremental plasma glucose concentrations after ingestion of the glucose load. Blacks demonstrated a higher early phase potentiation factor (Figure 1D) consistent with the higher early-phase insulin secretion compared with whites (Figure 1C). It remains to be investigated if race-related differences in neuronal input are operational in modulating insulin secretion. Lastly and consistent with past observations (6, 9, 41), insulin clearance was significantly lower in black vs. white youth, partially explaining the frequently reported hyperinsulinemia in blacks. In the present study, the determinants of insulin clearance were race, VAT and insulin sensitivity explaining 46% of its variance. Thus, to avoid the confounding influence of insulin clearance, C-peptide may be a better measure of insulin secretion especially when comparing and contrasting race-related differences in insulinemia and β-cell function. Finally, there were no race-related differences in FFA concentrations during the OGTT to explain differences in insulin secretion or clearance.
The strengths of this investigation include 1) the large number of carefully characterized and uniformly NGT obese black and white youth and 2) the mathematical modeling. The fact that insulin sensitivity was similar between groups may be viewed as a strength in the current investigation although this may be biased by the significant differences in pubertal stage between the two groups.
In conclusion, during an OGTT black obese youth with normal glucose tolerance demonstrate a pronounced early insulin secretion jointly with heightened β-cell glucose sensitivity, rate sensitivity and early potentiation factor which do not appear to be driven by differences in incretin hormones. The mechanism(s) behind these racial disparities remain to be investigated further in an effort to probe the metabolic basis for the increased risk of type 2 diabetes in obese black youth compared with their white peers.
Acknowledgments
SA is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors report any conflict of interest with respect to this work. We would like to thank all the children and their parents who participated in this study, without whom science would not advance. We are grateful to the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and meticulous attention to the research and to Resa Stauffer, Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, for her laboratory analytical contributions.
Funding
R01 HD-27503 (SA), K24 HD-01357 (SA), Richard L. Day Endowed Chair (SA), American Diabetes Association 7-08-JF-27 (SJL), Thrasher Research Fund (FB) and UL1 RR024153 and UL1 TR000005 CTSA.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Author Contribution
SFM first authored the manuscript, and contributed to the data analyses and interpretation; SJL, FB and HT contributed participants to the research project and contributed data and reviewed the manuscript; LF maintained the database and contributed data analysis; AM performed the mathematical modeling of the OGTT, the hyperglycemic clamp, and the incretin effect, and critically reviewed/edited the manuscript; EF critically reviewed/edited the manuscript; SA provided the study concept and design, acquired data, obtained funding, provided administrative, technical and material support, supervised the study and critically reviewed/edited the manuscript.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, et al. Incidence of diabetes in youth in the United States. JAMA : the journal of the American Medical Association. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatric diabetes. 2015 doi: 10.1111/pedi.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 4.Whitt-Glover MC, Crespo CJ, Joe J. Recommendations for advancing opportunities to increase physical activity in racial/ethnic minority communities. Preventive medicine. 2009;49:292–3. doi: 10.1016/j.ypmed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Wiecha JM, Fink AK, Wiecha J, Hebert J. Differences in dietary patterns of Vietnamese, white, African-American, and Hispanic adolescents in Worcester, Mass. Journal of the American Dietetic Association. 2001;101:248–51. doi: 10.1016/S0002-8223(01)00064-5. [DOI] [PubMed] [Google Scholar]
- 6.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: Decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–9. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 7.Haidet J, Demirci C, Arslanian S. Racial Differences in Childhood Obesity: Pathogenesis and Complications. In: Freemark M, editor. Pediatric Obesity: Etiology, Pathogenesis and Treatment. Springer; 2010. pp. 75–89. [Google Scholar]
- 8.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes care. 2002;25:2184–90. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Srinivasan SR, Radhakrishnamurthy B, Dalferes ER, Berenson GS. Racial (black-white) differences in insulin secretion and clearance in adolescents: the Bogalusa heart study. Pediatrics. 1996;97:357–60. [PubMed] [Google Scholar]
- 10.Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–8. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 11.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes care. 2008;31:1445–7. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, Lustig RH, Cashion AK, Burghen GA. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. International journal of obesity and related metabolic disorders : Journal of the International Association for the Study of Obesity. 2003;27:1359–64. doi: 10.1038/sj.ijo.0802415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. The Journal of clinical endocrinology and metabolism. 2010;95:4048–51. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goree LL, Darnell BE, Oster RA, Brown MA, Gower BA. Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity. 2010;18:247–53. doi: 10.1038/oby.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabe D, Seino Y. Two incretin hormones GLP-1 and GIP: comparison of their actions in insulin secretion and beta cell preservation. Progress in biophysics and molecular biology. 2011;107:248–56. doi: 10.1016/j.pbiomolbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Higgins PB, Fernandez JR, Garvey WT, Granger WM, Gower BA. Entero-insular axis and postprandial insulin differences in African American and European American children. The American journal of clinical nutrition. 2008;88:1277–83. doi: 10.3945/ajcn.2008.26357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velasquez-Mieyer PA, Cowan PA, Perez-Faustinelli S, Nieto-Martinez R, Villegas-Barreto C, Tolley EA, et al. Racial disparity in glucagon-like peptide 1 and inflammation markers among severely obese adolescents. Diabetes care. 2008;31:770–5. doi: 10.2337/dc07-1525. [DOI] [PubMed] [Google Scholar]
- 18.Michaliszyn SF, Mari A, Lee S, Bacha F, Tfayli H, Farchoukh L, et al. beta-Cell Function, Incretin Effect, and Incretin Hormones in Obese Youth Along the Span of Glucose Tolerance From Normal to Prediabetes to Type 2 Diabetes. Diabetes. 2014 doi: 10.2337/db13-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? The Journal of clinical endocrinology and metabolism. 2010;95:2426–32. doi: 10.1210/jc.2009-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns SF, Lee S, Arslanian SA. In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes care. 2009;32:2087–93. doi: 10.2337/dc09-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjaarda LA, Michaliszyn SF, Lee S, Tfayli H, Bacha F, Farchoukh L, et al. HbA(1c) diagnostic categories and beta-cell function relative to insulin sensitivity in overweight/obese adolescents. Diabetes care. 2012;35:2559–63. doi: 10.2337/dc12-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Kuk JL, Kim Y, Arslanian SA. Measurement site of visceral adipose tissue and prediction of metabolic syndrome in youth. Pediatr Diabetes. 2011;12:250–7. doi: 10.1111/j.1399-5448.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008;16:1066–71. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 24.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care. 2010;33:632–8. doi: 10.2337/dc09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta -cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283:E1159–66. doi: 10.1152/ajpendo.00093.2002. [DOI] [PubMed] [Google Scholar]
- 26.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51(Suppl 1):S221–6. doi: 10.2337/diabetes.51.2007.s221. [DOI] [PubMed] [Google Scholar]
- 27.Natali A, Ribeiro R, Baldi S, Tulipani A, Rossi M, Venturi E, et al. Systemic inhibition of nitric oxide synthesis in non-diabetic individuals produces a significant deterioration in glucose tolerance by increasing insulin clearance and inhibiting insulin secretion. Diabetologia. 2013;56:1183–91. doi: 10.1007/s00125-013-2836-x. [DOI] [PubMed] [Google Scholar]
- 28.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–48. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 29.Festa A, Williams K, D’Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–20. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 30.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Banerji MA, Bray GA, Buchanan TA, Clement S, Henry RR, et al. Determinants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW) study. Diabetologia. 2010;53:435–45. doi: 10.1007/s00125-009-1614-2. [DOI] [PubMed] [Google Scholar]
- 32.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32:100–5. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining beta-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care. 2011;34:2033–40. doi: 10.2337/dc11-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes. 2005;6:95–9. doi: 10.1111/j.1399-543X.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–7. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–12. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 37.Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61:606–14. doi: 10.2337/db11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. The Journal of pediatrics. 2006;148:188–94. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Velasquez-Mieyer PA, Umpierrez GE, Lustig RH, Cashion AK, Cowan PA, Christensen M, et al. Race affects insulin and GLP-1 secretion and response to a long-acting somatostatin analogue in obese adults. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:330–3. doi: 10.1038/sj.ijo.0802561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heijboer AC, Frans A, Lomecky M, Blankenstein MA. Analysis of glucagon-like peptide 1; what to measure? Clinica chimica acta; international journal of clinical chemistry. 2011;412:1191–4. doi: 10.1016/j.cca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism: clinical and experimental. 1997;46:53–8. doi: 10.1016/s0026-0495(97)90167-0. [DOI] [PubMed] [Google Scholar]