Abstract
With a photoreceptor mosaic containing ~85% cones, the ground squirrel is one of the richest known mammalian sources of these important retinal cells. It also has a visual ecology much like the human’s. While the ground squirrel retina is understandably prominent in the cone biochemistry, physiology, and circuitry literature, far less is known about the remodeling potential of its retinal pigment epithelium, neurons, macroglia, or microglia. This review aims to summarize the data from ground squirrel retina to this point in time, and to relate them to data from other brain areas where appropriate. We begin with a survey of the ground squirrel visual system, making comparisons with traditional rodent models and with human. Because this animal’s status as a hibernator often goes unnoticed in the vision literature, we then present a brief primer on hibernation biology. Next we review what is known about ground squirrel retinal remodeling concurrent with deep torpor and with rapid recovery upon re-warming. Notable here is rapidly-reversible, temperature-dependent structural plasticity of cone ribbon synapses, as well as pre- and post-synaptic plasticity throughout diverse brain regions. It is not yet clear if retinal cell types other than cones engage in torpor-associated synaptic remodeling. We end with the small but intriguing literature on the ground squirrel retina’s remodeling responses to insult by retinal detachment. Notable for widespread loss of (cone) photoreceptors, there is surprisingly little remodeling of the RPE or Müller cells. Microglial activation appears minimal, and remodeling of surviving second- and third-order neurons seems absent, but both require further study. In contrast, traumatic brain injury in the ground squirrel elicits typical macroglial and microglial responses. Overall, the data to date strongly suggest a heretofore unrecognized, natural checkpoint between retinal deafferentiation and RPE and Müller cell remodeling events. As we continue to discover them, the unique ways by which ground squirrel retina responds to hibernation or injury may be adaptable to therapeutic use.
Keywords: Cone photoreceptor, Microglia, Müller cell, Reactive gliosis, Retinal detachment, Retinal pigment epithelium, Synaptic plasticity
1. Introduction
The ground squirrel (GS) photoreceptor mosaic contains ~85% cones, including a large, nearly pure-cone region near the posterior pole (Kryger et al., 1998; Long & Fisher, 1983; Sakai et al., 2003). Even those GS species that are smaller than rats have eyes that are substantially larger than the rat’s. These features combine to make the GS a rich source of mammalian cones and the circuitry underlying their function. Hence, GS retina has been invaluable for landmark cone discoveries including outer segment morphogenesis (Steinberg et al., 1980), disc shedding (Anderson et al., 1978; Long et al., 1986), retinoid binding proteins (Anderson et al., 1986), glucose metabolism (Winkler et al., 2008), and visual transduction (Mata et al., 2002; von Schantz et al., 1994; Wang & Kefalov, 2011; Weiss et al., 1998).
Since cone damage is catastrophic for human vision, it is somewhat surprising how seldom the GS has been used to model injury responses, including retinal remodeling. Some of this is likely due to its status as a wild animal, though captive breeding of one species is possible (Merriman et al., 2012). As this review will describe, what limited information we have suggests that photoreceptor loss from the GS retina results in rather different downstream responses relative to what has been recorded in other animal models and indeed in humans.
We begin this review by briefly reviewing the GS visual system. We then overview GS hibernation and what is currently known about retinal remodeling as a seasonal phenomenon. We end by considering GS retinal remodeling after experimental insult. Given the relative underutilization of this model species, more questions are raised than answers provided. Where relevant, studies of other parts of the GS central nervous system are referenced.
2. Ground squirrel visual system
2.1. Visual ecology, life history, and genome
Ground squirrels are strictly diurnal, omnivorous rodents that routinely engage in visually-guided predation on fast-moving prey including insects, other rodents, snakes, and birds. Ground squirrels also serve as prey for agile, fast-moving predators. Favoring open short-grass habitats and bright sunny days, GSs commonly adopt an erect vigilance posture. As such, GSs share much of the human’s visual ecology and thus make useful complements to traditional rat and mouse models of visual function and disease (van Hooser & Nelson, 2006).
Unlike rats or mice, GSs do not become reproductive until nearly one year of age, after their first winter hibernation (section 3.1). Wild GSs experience substantial mortality as juveniles but adults may survive 3–4 years (Michener, 1989). In our captive colony of 13LGSs, lifespan typically extends to 5–6 years. The GS’s longer natural “childhood” and lifespan eases some of the scalability problems presented by traditional rodent models used in CNS damage research (Agoston, 2015).
Other assets of the GS model include the 13-lined GS genome (13LGS, Ictidomys tridecemlineatus, formerly Spermophilus, formerly Citellus), which was chosen for sequencing by the National Human Genome Research Institute in 2005. Currently, over 187,000 13LGS nucleotide sequences are found in GenBank. The 13LGS mitochondrial genome has also recently been sequenced (Zhang et al., 2015). The markedly slow rate of evolution of GSs makes them useful for comparative genomics vis-à-vis rats and mice (reviewed by Rodriguez-Ramos Fernandez & Dubielzig, 2013). A retina-specific RNAseq database with 20,000 genes has also been acquired from the 13LGS (Wei Li, unpublished).
2.2. General ocular anatomy
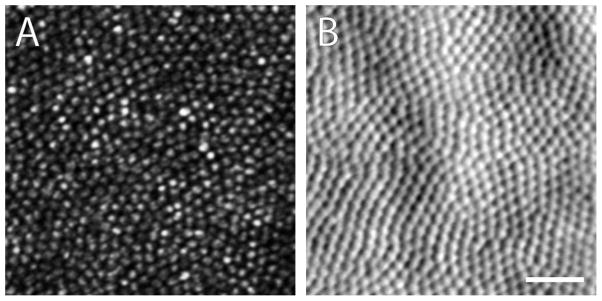
Ocular parameters for several species of GS are shown in Table I. Ground squirrel eyes are set deeply and laterally within the skull, making enucleation and in situ access to the optic nerve more challenging than in rats or mice. The entire sclera is darkly pigmented. Their emmetropic eyes are thought to have low spherical and chromatic aberration (Sussman et al., 2011; Gur & Sivak, 1979). The GS lens is spherical, yellow, and small relative to the size of the globe (Vaidya 1965), which facilitates intraocular manipulations of the retina. Its UV filters resemble those of human lens (Hains et al., 2006). These features may contribute to what makes the GS retina remarkably amenable to non-invasive retinal imaging by adaptive optics scanning light ophthalmoscopy (AOSLO) (Figure 1; Sajdak et al., in press).
Table I.
Ocular measurements of ground squirrel eyes. From aGur & Sivak, 1979 (Mexican G S, 13LGS); bChou & Cullen, 1984 (13LGS); cMcCourt & Jacobs, 1984b (California GS); dHughes, 1977 (European GS); eour data (13LGS).
| Eye diameter | 8.23 mma; 10.5 mmd |
| Axial length | 9.00 mmd; 9.45 mme |
| Lens thickness | 2.91 mma |
| Anterior chamber depth | 1.23 mma |
| Posterior chamber depth | 3.90 mma |
| Corneal thickness | 0.28 mmb |
| Anterior cornea radius* | 3.27 – 3.48 mm1 |
| Vitreous humor | 9.90 mmb |
| Focal length | 5.00 mmc |
| Depth of focus | ± 10.7 Dc |
| Accommodation range | 2–6 Dc |
| Retinal magnification factor | 0.10 mm/degreed |
| Intraocular pressure** | 10.7 mm Hge |
| Dilated pupil diameter*** | 4.40 mme |
By ex vivo cross section and in vivo photokeratoscopy, respectively.
Light isoflurane anesthesia.
After 1% tropicamide + 2.5% phenylephrine.
Figure 1.
In vivo 13LGS photoreceptor mosaic images captured with a custom adaptive optics scanning light ophthalmoscope. Left: confocal image of cone outer segments. Right: non-confocal image of cone inner segments. Scale bar = 20 μm.
The GS optic nerve head is located well above the posterior pole and extends horizontally for several millimeters (Vaidya 1965); less extensive examples are also seen in rabbit and some cervids (R. Dubielzig, personal communication). Hence, instead of a blind “spot” in its visual field, the GS will have a blind “band”, but its dorsal position does not interfere with images of the world above the ground (Rodriguez-Ramos Fernandez & Dubielzig, 2013). Dorsal from the horizontal nerve head, the outer nuclear layer (ONL) is 1–2 nuclei thick and the photoreceptor:ganglion cell ratio is 4:2, whereas the ventral ONL is 2–3 nuclei thick and the photoreceptor:ganglion cell ratio is only 3:2 (Rodriguez-Ramos Fernandez & Dubielzig, 2013; Vaidya, 1965). This retinal asymmetry has long been ascribed to the “danger above” posed by predators.
As in human, the GS’s retinal vasculature is holangiotic (Rodriguez-Ramos Fernandez & Dubielzig, 2013). Also in common with human (Besharse & Bok, 2011), the GS outer plexiform and photoreceptor layers lack blood capillaries. The GS retinal pigment epithelium (RPE) forms a “particularly robust association” with photoreceptor outer segments (Linberg et al., 2002) that makes stripping the neuroretina from the RPE challenging in vitro, but does not impede experimental retinal detachment.
2.3. Photoreceptors
Ground squirrels were once thought to have a pure-cone retina (Hollenberg & Bernstein, 1966), but subsequent microscopic analysis clearly demonstrated rods (West & Dowling, 1975). Thereafter, electrophysiological and immunocytochemical studies differentiated M-cones (λmax 518 nm), S-cones (λmax 436 nm), and rods (λmax 500 nm; reviewed in Jacobs, 1990). Squirrel Scones are not UV-sensitive (Peichl, 2005). In California GS, M-cone and rod responses are mature about two weeks after eyelid opening (P40-45), whereas S-cone responses mature later (P70-80; Jacobs & Neitz, 1984).
Ground squirrel S-cones are distinguished from M-cones by their larger ellipsoids (Ahnelt, 1985) and by differential visual pigment immunocytochemistry (Szél & Röhlich, 1988), although certain immunolabeling confounds must be taken into account (Kryger et al., 1998). This is in contrast to bona fide mouse cones (Applebury et al., 2000; Ortin-Martinez et al., 2014), which may co-express two opsins, and to the cone-like photoreceptors of Nrl−/− mice which may express only S-opsin (Daniele et al., 2005). In a recent paper (Cao et al., 2014), 13LGS cones gave a monophasic hyperpolarizing flash response that those authors propose is the norm for primate, mammalian, and lower vertebrate cones. Notably, compared to primate cones, GS cones appear to have lower light sensitivity but faster light responses (Cao et al., 2014; Kraft, 1988).
Squirrel rod and cone synapses appear to have similar vesicle release kinetics (Li et al., 2010) but scotopic function is notoriously difficult to record from GS eyes (Jacobs, 1990). Interestingly, GS rods exhibit a sort of hybrid physiology, suggesting special post-receptoral signal processing (Jacobs, 1990). Corbo & Cepko (2005) have attributed this to relaxed selective pressure on scotopic vision in this exclusively diurnal animal. Additionally, the GS’s b2 ERG component (ascribed to rod function) dark adapts just as rapidly as its b1 component (ascribed to cone function) (Jacobs, 1990), possibly advantageous in a fossorial animal that frequently transitions between surface bright light and subterranean darkness in the course of a day.
The only GS species to date in which the photoreceptor mosaic has been mapped is the California GS. First studied by microscopy (Long & Fisher, 1983) and then by immunocytochemistry (Kryger et al., 1998; Sakai et al., 2003), the California GS retina has ~8.75 million photoreceptors comprising ~80% M-cones, ~6% S-cones, and ~14% rods. The highest photoreceptor density (ca. 45,000/mm2) is in an elliptical region of central retina that contains only ~5% rods. Photoreceptor density falls by about half at the retinal periphery. A recent study using AOSLO to image the living 13LGS retina obtained similar results (Sajdak et al., in press).
Long & Fisher (1983) pointed out that the isodensity lines of the California GS’s photoreceptor mosaic are intermediate between the very narrow ones of rabbit and the concentric ones of cat. Kryger and colleagues (1998) confirmed this asymmetric distribution of rods and also reported a novel S-cone-rich dorsonasal “rim” region. This rim’s far peripheral placement obviates any role in image formation, so its functional significance is yet to be discovered.
The highest proportion of rods within the GS mosaic (~32%) is found in ventral retina as defined by the unusual placement of the optic nerve head (Kryger et al., 1998; Long & Fisher, 1983). Notably, GS rods display diurnal nuclear architecture, just as human rods do (Solovei et al., 2009; Solovei et al., 2013). In contrast, rod nuclei of nocturnal mammals (including mouse and rat) have an inverted chromatin pattern that maximizes photon acquisition (Solovei et al., 2009). The relevance of this diurnal-nocturnal rod dimorphism was recently shown to go beyond photon capture in a study comparing the ability of adult mouse retinal cells to repair double-strand nuclear DNA breaks induced by X-irradiation (Frohns et al., 2014). Of the cell types examined, mouse rods were only half as efficient at repairing DNA as the other cell types – including mouse cones. One implication is that rods of diurnal mammals, which exhibit the normal chromatin pattern, will not suffer the same handicap at repairing DNA, but to our knowledge this hypothesis has not yet been directly tested. Should this be the case, despite their disparate proportions of rods amongst the total photoreceptor population, at least GS and human retinas would share rods that are equally efficient at DNA repair.
2.4. Retinal circuitry
The cone-rich GS retina is especially suitable for studying cone synapses and cone pathways in general. Thus, both the outer plexiform layer (OPL) and inner plexiform layer (IPL) of the GS retina have been productive areas of study. Anatomical studies using either 13LGS or California GS have progressively documented various bipolar, amacrine, interplexiform, and ganglion cell types (Abreu et al., 1993; Cuenca et al., 2002; Cuenca et al., 2003; Li & DeVries, 2006; Linberg et al., 1996; Lugo-Garcia & Blanco, 1993; Lugo-Garcia & Kicliter, 1988; Puller et al., 2011; West, 1976).
Notable findings have included a comparison of amacrine cell populations in cone-dominant versus rod-dominant species (Cuenca et al., 2002). Using multiple labeling methods, Light and colleagues (2012) documented a near complete inventory of 13LGS bipolar cell types and identified several organizational motifs, which provided a valuable addition to the bipolar classifications of the mammalian retina.
Physiologically, functional gap junctions between mammalian cones were first characterized in the GS retina (DeVries et al., 2002). Glutamate spillover between neighboring cone pedicles has been shown to contribute to cone signaling (Szmajda & DeVries, 2011). Temporal filtering of cone signals by postsynaptic glutamate receptors (DeVries & Schwartz, 1999; DeVries 2000), as well as glutamate microenvironments sampled by bipolar cells (DeVries et al., 2006), have been demonstrated in the 13LGS retina, which established a foundation for understanding parallel processing of the temporal features of the cone signals. In addition, a bipolar cell type that generates both graded and all-or-none action potential has been thoroughly characterized in the 13LGS retina (Saszik & DeVries, 2012). Different ganglion cell types, including direction-selective ganglion cells, have been characterized by optic nerve fiber recordings (McCourt and Jacobs, 1984a,c).
The cone-rich GS retina, in conjunction with non-overlapping opsin expression in M- and S-cones, render the GS retina particularly suitable for color vision study. The developmental course of spectral mechanisms has been delineated in the California GS retina (Jacobs and Neitz, 1984). It has been shown that, due to the lack of functional gap junctions between S- and M-cones, S-cones are separated from the M-cone network (Li & DeVries, 2004). This provided a foundation for separate S- and M-cone signals that can then be contrasted in subsequent processing to generate color opponency. S- and M-cone synaptic connections with different types of bipolar cells have been examined anatomically and functionally to outline multiple pathways carrying luminance and color information (Li & DeVries, 2006). An S-cone amacrine cell has been identified to provide S-OFF signals to the downstream color opponent ganglion cells (Chen & Li, 2012), which have been recorded using the multi-electrode array technique (Sher & DeVries, 2012). Together, these two studies identified the pathway that propagates Scone OFF signals to the color opponent ganglion cells in the GS retina (for reviews, see Marshak & Mills, 2014; Miyagishima et al., 2014). Spectral responses of California GS ganglion cells have also been recorded at optic nerve fibers (Jacobs & Tootell, 1981, Jacobs et al., 1981).
2.5. Optic nerve and central projections
The GS optic nerve carries an estimated 1.2 million axons (Johnson et al., 1998), roughly 25x that of the mouse (Templeton et al., 2014) and 15x that of the rat (Marina et al., 2010), and yet in line with the rhesus macaque (Cull et al., 2003) and the human (Jonas et al., 1990). The optic nerve is somewhat flattened and 5–6 mm long (Vaidya, 1965). This is a further indicator of the pertinence of diurnal rodent species to model-based research on human visual pathophysiology.
Retinal projections to the lateral geniculate nucleus and elsewhere exhibit contralateral dominance (Vaidya, 1963) and are consistent with those of other mammals (Agarwala et al., 1989; Major et al., 2003; Rivera & Lugo, 1998). Compared to the rat or mouse, a greater proportion of dorsolateral cerebral cortex subserves vision in the GS brain. Of that visual cortex, a greater proportion is devoted to area 17 and 18 than is seen in rats or mice (Campi & Krubitzer, 2010). In common with the visual cortex of diurnal primates, GS visual cortex is activated by diffuse illumination (Cooper, 2002). Superior colliculus pathways have also been described (Fredes et al., 2012; Lugo-Garcia & Kicliter, 1987; Major et al., 2003; Rivera & Lugo, 1998).
3. Ground squirrel hibernation
3.1. Torpor physiology
Ground squirrels meet the many life-threatening challenges of winter by fattening in fall and then hibernating winter into spring sealed in dark underground chambers. Transcription and translation mostly halt; lipid fuels become preferred over carbohydrates; homeothermy is replaced by heterothermy; and metabolic rate falls dramatically (van Breukelen & Martin, 2015). There is substantial relocation of immune cells and platelets out of the bloodstream (Bouma et al., 2011; de Vrij et al., 2014). Urinary function halts (Jani et al., 2013). Entering hibernation with an empty gut and caching little or no food, torpid squirrels survive on tissue stores for up to 8 months in the case of the Arctic GS.
Cortical electrical activity eventually ceases in deep torpor, although hypothalamic functions continue (Bratincsak et al., 2007; Schwartz et al., 2013) and animals respond to even gentle handling by artificial arousal from torpor (Christian et al., 2014). Spatial memory is somewhat impaired by hibernation (Millesi et al., 2001) whereas social memory, memory of operant conditioning tasks, and ability to acquire mechanosensory habituation are not (Christian et al., 2014; Clemons et al., 2009; Millesi et al., 2001).
During the (summer) active portion of its circannual cycle, GS Tbody is ~38°C. As befits the GS’s classification as a “deep” hibernator, during (winter) torpor, an active physiological mechanism loosens control of Tbody, which comes to match Tambient. In captive GS facilities, winter Tambient is typically ~4°C. In nature, the Arctic GS burrow’s Tambient routinely falls well below 0°C, and has been experimentally manipulated to an astonishing −26°C. Under these conditions, elevated metabolic rate and thermogenesis defend a minimal Tbody of about −3°C (Richter et al., 2015). The fall in Tbody is accompanied by a heart rate only ~5% of the (summer) euthermic rate, and episodic breathing every 30 min or so. Despite this, the extremities of torpid squirrels remain a healthy pink.
Torpor bouts last 1–2 weeks in winter, interrupted by “interbout arousals” (IBAs) to euthermia lasting 12–24 hours. These IBAs occur at remarkably regular intervals (e.g. Figure 1 of Hindle & Martin, 2013), suggesting that the hibernation season is under a high degree of control. Re-warming begins in the head and spreads posteriorly over the body. Recent evidence suggests that 13LGS brain neurons themselves are thermogenic (Laursen et al., 2014). Most body functions resume, though the animals do not eat or drink. During IBAs, GSs may reposition themselves, but have been shown to mostly sleep (Larkin et al., 2002; Walker et al., 1977). The staggeringly high metabolic costs of IBAs raise the question of their purpose, if any. Evidence that extracellular mouse brain metabolites are far more efficiently flushed by glymphatic flow during sleep than waking (Xie et al., 2013) suggests an attractive hypothesis for IBAs that has yet to be tested.
3.2. Transition season physiology
Spring and fall represent transition phases of the circannual cycle, known as “emergence” and “immergence” respectively. The exact timing of spring emergence varies depending on sex, soil temperature, snow cover, and the circannual clock. The process of re-establishing homeothermy, full organ function, and consciousness is termed “arousal”.
Female GSs are fertile within 1–2 days of arousal because they enter winter hibernation in a sort of paused estrus. In contrast, male GS gonads regress in late spring after a mere 4 weeks or so of spermatogenesis, and so require a few days of early spring euthermia to regain reproductive capacity. Curiously, male Arctic GSs seem to maintain a 12 day head start on female emergence regardless of female timing, which is in turn closely tied to the variable of snow cover (Sheriff et al., 2013). How this staggered timing is accurately signaled is unknown.
In our captive colony, synchronous spring emergence is induced on a calendar that obeys the “males first” rule seen in the wild. After removal to a warm, lit room, torpid 13LGs are euthermic and mobile within an hour. Handling triggers and even accelerates arousal from torpor (Christian et al., 2014), evidence of a rapid, extreme physiological transition. Melatonin signaling has recently been shown to play a role in brain neuroprotection during arousal, by inhibiting caspase activity and optimizing mitochondrial function (Schwartz et al., 2015).
In late summer and early fall, GSs increase body mass (captive adult 13LGSs to ~300 g) as they acquire extra white adipose tissue. This visible fattening is but one example of the alterations in physiology that precede, i.e. anticipate, entry into actual torpor. It has been estimated that hibernation cuts metabolic costs to 6% of euthermic values (Ruf & Geiser, 2014). The observed savings cannot be ascribed to lower Tbody alone. This underscores the importance of ascertaining a GS’s physiological state, particularly in late summer and fall.
Landmark events of the immergence transition are spontaneous, brief test bouts of torpor (Russell et al., 2010). Test bouts are recognized by an animal that is cool to the touch, is difficult to “waken”, and staggers if it moves. There is also anorexia accompanied by lack of urination and defecation. Those individuals who tend to immerge earliest are male and/or older. In captive adult 13LGSs, we have observed test bouts as early as late June, though August is more typical. We have been unable to prevent test bouts in captivity despite the continued imposition of summertime zeitgebers (e.g. 16:8 LD photoperiod), more evidence that the immergence transition is strongly tied to a circannual clock that has long been of interest to hibernation physiologists.
3.3 Circannual rhythm generation
Circannual rhythms have evolved in many organisms, even simple ones like protists (for an excellent review, see Helms et al., 2013). Notably, the GS’s months-long hibernation in constant darkness deprives its circannual clock of an important zeitgeber for an extended period. Another challenge is the GS’s profound Tbody cycling from 0°C – 38°. Whereas an extensive literature exists describing transition season physiological adjustments, less is known for certain about the light- and temperature-independent clock mechanism that controls them. Reflecting the uncertainty at present, Drew and colleagues (2007) have explored routes of GS CNS regulation that reveal candidate central locations of the circannual clock. More recently, Hut et al. (2014) have proposed a new model placing the circannual clock in the pars tuberalis of the pituitary.
The circannual rhythm does not appear to be coupled to the suprachiasmatic nucleus (SCN) circadian clock system. Studies using the European hamster, a deep hibernator with a winter cycle similar to that of the GS, have demonstrated that SCN clock genes remain transcriptionally active throughout winter, but lose their circadian rhythm of output signals (Revel et al., 2007). This has been interpreted as the cessation of circadian function for about half of every year of the animal’s life. A study of European GSs revealed disrupted circadian patterns of activity and Tbody that lasted up to 2 weeks after the animals had resumed euthermia (Hut et al., 2002), suggesting that GS circadian clock function is re-entrained by zeitgebers upon emergence from hibernation. With respect to the retina, one wonders if photoreceptor disc shedding might also be dysregulated in the first days aboveground in spring, an idea which to our knowledge has not been examined.
3.4. Retinal remodeling associated with hibernation
3.4.1. Early studies
Kuwabara (1975) was the first to examine the GS retina during hibernation. His primary interest was the retinal pigment epithelium-cone outer segment (RPE-COS) interface. His transmission electron microscopy (TEM) study included the 13LGS, frog, and bat. Squirrels were experimentally induced to hibernate in a cold room during two successive falls. Hibernation was ended after two months by removing the 13LGSs to a warm, lit room and reinstating food and water. Three time points were examined using light and electron microscopy: before hibernation (presumably, a euthermic animal in what we understand today to have been in the pre-immergence transition state); during hibernation (presumably, a hypothermic animal in full torpor; interbout arousals are not mentioned); and after hibernation (a euthermic animal 1–2 weeks after removal from the cold room).
Kuwabara (1975) reported the progressive loss of synaptic vesicles and ribbons from the cone pedicles of torpid animals, alongside a marked shortening of COSs but without any increase in RPE phagosomes. Time of day of collection was not mentioned, and the dark period timing of squirrel COS shedding would not be published for several years (Long et al., 1986). Indeed, by the end of two months’ hibernation, COSs were “almost absent in several animals” (Kuwabara, 1975). No changes were reported in rod OS length nor in the inner segment mitochondria of rods or cones. Partial recovery to normal COS length and synaptic morphology was observed one week after arousal, and was complete another week after that.
Overall, Kuwabara’s study appeared to demonstrate progressive, seasonal, reversible, cone-specific degeneration. Complicating this interpretation is that one eye from each GS was enucleated before, during, or after hibernation. The now-monocular GS was allowed to survive until the next time point, at which time its remaining eye was collected. Time post-enucleation was not provided for any data shown. This protocol thus added variables of surgical stress, wound healing, and chronology to the underlying hibernation physiology.
A second TEM study of the hibernating 13LGS retina appeared two years later (Remé & Young, 1977). These authors monitored their GSs daily, keeping track of interbout arousals. Retinas collected in July, August, and September served as controls for the torpid condition but, based on reported body masses, some control animals were well into the immergence transition stage. Hibernation was initiated by placing all GSs in a cold, dark room on the same fall day. Lights were on occasionally for “a few hours”, a condition that would never occur in nature. Retinas from both eyes of torpid animals were collected after 3–10 weeks in the cold room. To assess the retina’s recovery from hibernation, one eye was enucleated from a small number of torpid GSs that aroused and survived for 3–9 days in the warm room with photoperiodic lighting. Hence, as in the Kuwabara (1975) study, recovery from torpor was also on a background of surgical stress and wound healing.
Remé & Young (1977) found little evidence for COS shedding even in (summer) controls, possibly because euthanasia occurred in daytime. As did Kuwabara (1975), they recorded the loss of synaptic vesicles and most ribbons from cone pedicle active sites. However, while shortened COSs were observed in torpid animals, it was a far subtler effect than Kuwabara (1975) reported. Also in contrast to the earlier study, the size and number of ellipsoid mitochondria were markedly reduced in torpid GS cones. Upon arousal, mitochondria and synaptic ribbons recovered more quickly than did COS length (~3 days versus ~7 days). Overall, the second study found little evidence that cone degeneration was progressive, suggesting instead a new steady state during torpor that rapidly recovered upon arousal.
3.4.2. Contemporary studies
About ten years ago, we began to re-examine retinal remodeling during hibernation using TEM plus a variety of methods not available to the hibernation pioneers. These methods include immunoconfocal microscopy and computational molecular phenotyping (CMP), which is further explained below. Some of our data have been published only in abstract form.
Using standard immunocytochemistry, we observed limited COS shortening but significantly reduced immunostaining for cytochrome oxidase in cone ellipsoids of 13LGS during torpor (Gruber et al., 2006), in agreement with Remé and Young (1977). Our subsequent study of ellipsoid mitochondria showed evidence of either increased fission or decreased fusion during torpor, and underscored the notion that mitochondrial activity is regulated by the amount of mitochondrial protein (Kaden et al., 2013). We have also used optical coherence tomography (OCT) imaging to examine the 13LGS retina during torpor, in comparison with arousal (Li et al., 2014). Signal amplitude and contrast are quite reduced during torpor, but alterations in cone ellipsoids are most evident.
Our TEM data also confirmed shorter ribbons at the cone pedicle active sites and aggregates of ribbon material some distance away in retinas collected from torpid 13LGS (Figure 2AC). We showed this to be a homogeneous feature across the torpid GS retina using anti-CtBP2/RIBEYE to immunostain synaptic ribbons (Mehta et al., 2013; Qiao et al., 2013; Vaughan et al., 2007). In hibernating GS, RIBEYE aggregate formation is strongly temperature-dependent, since it can be induced by cold treatment of 13LGS retinal tissues collected from euthermic (summer) animals (Wei Li, unpublished). Most RIBEYE aggregates disappeared within 8 hours of rewarming to euthermia, apparently re-deployed to the cone pedicle active sites.
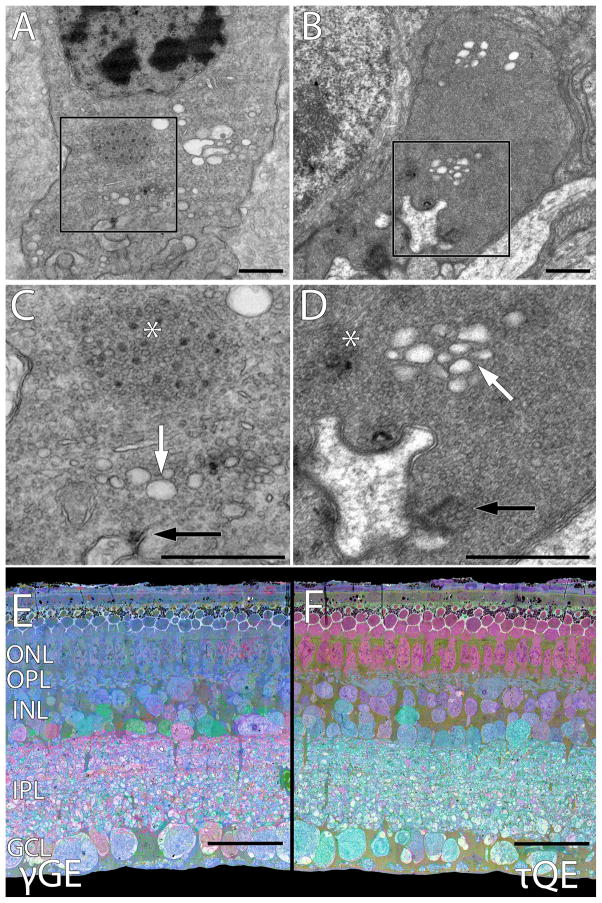
Figure 2. A–D: Transmission electron microscope (TEM) images showing similar cone synapse ultrastructure of the torpid 13LGS and the Tg P347L rabbit. The latter is a model of retinitis pigmentosa. E–F: Computational molecular phenotyping (CMP) of retina from a 13LGS recently emerged from torpor. Small molecule signals have been superimposed onto TEM structural data, quantitatively informing as to cell type and metabolic state.
A. Torpid 13LGS cone terminal with an aggregate of degenerate ribbon structures and synaptic vesicles that has moved ~500 nm away from the remnant active zone ribbon. Scale bar, 500 nm.
B: Tg P347L rabbit cone terminal showing degenerate ribbon structures resembling hibernating squirrel and human RP. Scale bar, 500 nm.
C: Higher magnification view of torpid 13LGS cone terminal (inset box from A) demonstrating an ectopic synaptic cloud (asterisk) and clear vesicles from fragmented Golgi apparatus (white arrow) along with the remnant synaptic ribbon (black arrow). Scale bar, 500 nm.
D: Higher magnification view Tg P347L rabbit cone terminal (inset box from B), demonstrating an ectopic synaptic cloud (asterisk) as well as truncated or remnant synaptic ribbon (black arrow) and the clear fragmented Golgi vesicles (white arrow). Scale bar, 500 nm.
E: CMP overlay mapping of GABA (red), glycine (green), and glutamate (blue) signals. Both excitatory (photoreceptor, bipolar and ganglion cells) and inhibitory retinal neurons (amacrine cells) can be visualized. Scale bar, 30 μm.
F: CMP overlay mapping of taurine (red), glutamine (green), and glutamate (blue) signals. Alternate mapping of the same specimen in E demonstrates Müller cells alongside excitatory and inhibitory cell classes. Scale bar, 30 μm.
Interestingly, TEM images of cone synapses from torpid 13LGS are strikingly similar to those of degenerative cone synapses from the Tg 347L rabbit model of retinitis pigmentosa (Figure 2BD; Jones et al., 2011). Cone degeneration in the rabbit model is, however, progressive and irreversible, in contrast to what seems to be the alternative steady state in hibernation.
Subsequently, our anti-BASSOON immunolabeling showed that BASSOON largely remains at the cone active site during torpor, even while RIBEYE aggregates detach from it (Qiao et al., 2013). In view of the fact that torpor occurs in continuous darkness, we note that (in mice) light exposure contributes to BASSOON’s ability to anchor ribbons at the rod spherule’s active zone (Spiwoks-Becker et al., 2013). Curiously, whereas 13LGS brain transcriptomics demonstrate upregulation of BASSOON transcripts during fall pre-immergence, winter torpor, and winter IBAs (Schwartz et al., 2013), this is not seen in 13LGS retinal transcriptomes collected at similar hibernation stages (Wei Li, unpublished).
A follow-up tested the hypothesis that cone ribbon disruption during torpor would disrupt postsynaptic signaling (Mehta et al., 2013). Cone resting membrane potential, and the placement and amount of glutamate receptor immunopositivity, were unchanged during torpor, and cone calcium currents were not significantly different. However, there was a significant reduction in size and number of the miniature-like excitatory post-synaptic currents (mlEPSC) reflecting reduced vesicle release, particularly multi-vesicular release. With cone depolarization, mlEPSC frequency and amplitude did increase in retinas of torpid GSs, but never to the level seen in retinas of aroused GSs. The detailed molecular mechanisms and physiological consequences associated with GS ribbon synapse plasticity during hibernation remain to be illustrated.
We have only just begun to use CMP to explore the GS retina’s histology, ultrastructure, metabolism and plasticity over the hibernation cycle. In CMP, small molecule signals are superimposed onto TEM structural data, quantitatively informing as to cell type and metabolic state (Jones & Marc, 2007). This approach has been very useful in addressing phenotype reprogramming and synaptology shifts that occur during retinal degenerations (Jones et al., 2011; Jones et al., 2012; Marc et al., 2007), i.e. during changes in physiological state. Here we present only a sampling of our preliminary data, which does not yet encompass all of the GS’s circannual cycle.
As shown in Figure 2E, CMP mapping of GABA, glycine, and glutamate signals readily reveals excitatory and inhibitory neurons of the 13LGS retina, which will allow us to appropriately identify any alterations in synaptic relationships going forward. Moreover, alternate mapping of taurine, glutamine, and glutamate signals in the same retinal sample provides valuable metabolic status information about Müller cells (Figure 2F) and how this might change over time.
We have been particularly interested in applying CMP to GS bipolar cells because, by standard TEM, their synaptic ribbons appear unaffected by hibernation. In a typical (rod-dominated) mammalian retina, total bipolar cells comprise ON, OFF, and rod types in roughly equal proportions (e.g. mouse, Wässle et al., 2009). It is to be expected that, in the cone-dominant GS retina (only 15% rods), the proportion of rod bipolar cells would be smaller. Using CMP, ON cone bipolar cells are readily demonstrated by their glycine signals, which they acquire through gap junction coupling with AII amacrine cells. Indeed, in 13LGS retina just after emergence from torpor, glycinergic signatures clearly predominate (Figure 3A). One interpretation is that, as 13LGS arouse from torpor, there are alterations in bipolar cell coupling patterns with amacrine cells. If confirmed in our ongoing work, this is the first evidence of hibernation-associated synaptic remodeling outside of cone pedicles.
Figure 3. CMP mapping of retina from a recently-emerged 13LGS.
A: GABA (γ), glycine (G), and glutamate (B) signals are shown with γGE → RGB mapping. ON cone bipolar cells are revealed by glycine content derived from coupling with AII amacrine cells. A surprisingly large number of total bipolar cells (>90%) exhibit a glycinergic signature. B: Taurine (T), glutamine (Q), and glutamate (E) signals are shown with TQE→RGB. Müller cells appear uniformly yellow/olive across the retina.
In the healthy retina of every species examined up till now, CMP mapping has revealed homogeneous small molecular signals within all Müller cells, representing tightly controlled metabolic regulation (Fig. 3B). Only in retinas undergoing retinal degeneration have Müller cells within the same region exhibited dissimilar CMP signals (reviewed in Jones et al., 2012). We therefore note that, during torpor, individual 13LGS Müller cells do in fact exhibit dissimilar metabolic signals, particularly in glutamine and glutathione (Figure 4). This variation is once again reminiscent of retinal degeneration, but in GS represents a readily-reversible physiological state in a healthy animal. Overall, CMP has great potential to reveal the part that retinal macroglia play in modulating retinal metabolism over the hibernation cycle.
Figure 4.
CMP overlay mapping of retina collected from a torpid 13LGS. Taurine (T), glutamine (Q), and glutamate (E) signals are shown with TQE→RGB demonstrating Müller cell metabolic signatures. Note the variation in the glutamine signal of individual Müller cell endfeet (arrows).
3.5. Brain remodeling associated with hibernation
A growing literature on hibernating GS brain increasingly documents physiological adaptations that may well extend to retina. Recent reviews address the GS’s intrinsic tolerance of brain hypoxia (Garbarino et al., 2015; Larson et al., 2014) and brain ischemia (Lee & Hallenbeck, 2013), supporting the notion that hibernation is a neuroprotected state (Dave et al., 2012). Moreover, presynaptic ribbon dynamics similar to that seen in cones have also been reported in GS pinealocytes (McNulty et al., 1990). Other studies report dendrite withdrawal in hibernating GS frontal cortex (Ruediger et al., 2007), cerebellum (Popov & Bocharova, 1992), thalamus (von der Ohe et al., 2007), and hippocampus (Popov et al., 2007; Sallmen et al., 2003; von der Ohe et al., 2007). All findings point to reversible, temperature-driven changes in the location of, and association between, pre- and postsynaptic proteins. Thus, synaptic recovery on rewarming relies upon a reservoir of nearby proteins, not on new protein synthesis (von der Ohe et al., 2007).
An elegant study has compared the proteomes of 13LGS forebrains collected during multiple physiological states across the hibernation year (Hindle & Martin, 2013). Of the more than 3,000 forebrain proteins surveyed, fewer than 3% showed significant differences, and there was surprisingly little change in metabolic enzyme content. Most of the altered proteins were related to the cytoskeleton, cytoskeletal regulation, and Ca+2 regulation, with the majority differing based on Tbody not time of year. The relevance of these findings to neuroplasticity is obvious.
While hibernating brain neuron remodeling studies vastly outnumber retinal neuron remodeling studies at this time, the knowledge base for GS retinal circuitry may recommend it as a useful tissue to explore this phenomenon in detail.
4. Ground squirrel retina after insult
4.1. Early studies
Relatively few studies have examined GS retinal responses to physical, nutritional, and pharmacotoxic insults. Vaidya (1965) used retinal cautery solely to elucidate 13LGS central visual pathways. Berson (1973) fed 13LGSs a Vitamin A-deficient diet and maintained them in either dim or moderate cyclic light, preliminarily reporting pathology only in those animals maintained in moderate light. Farber and colleagues (1983, 1981) induced photoreceptor degeneration in 13LGS retinas using intracardiac injections of iodoacetate, which inhibits glycolysis. Anderson and colleagues (1988) induced (cone) photoreceptor degeneration in California GS retinas using intraocular injections of tunicamycin, which inhibits protein glycosylation.
While ground-breaking in their own ways, these early GS papers did not directly address neuronal or glial remodeling responses. That changed about 15 years ago with a group of experimental retinal detachment studies using the California GS.
4.2. Remodeling after retinal detachment
In retinal detachment (RD), physical distancing of the neuroretina from the choriocapillaris separates photoreceptors from their lifeline as well as from their companion RPE. The result in the detachment zone is the deconstruction of all photoreceptor outer and inner segments and the retraction of rod spherules (Fisher et al., 2006). Over time, many photoreceptors die by apoptosis (Fisher et al., 2005; Fisher et al., 2007; Mervin et al., 1999). Impact on human vision is worst with macular detachment. Examination of rare human RD specimens long after initial injury demonstrates not only photoreceptor loss, but also widespread remodeling of the RPE, surviving photoreceptors, second and third order neurons, Müller cells, and nerve fiber layer astrocytes (Sethi et al., 2005).
It bears mention that, alone among retinal cell types and in all mammalian species examined to date, cones within detachment zones rapidly downregulate their expression of cone-specific proteins, making it impossible to detect them using immunocytochemistry. This is true of cones that survive RD and remain deconstructed for months or years (Fisher et al., 2005). Upon successful reattachment to the RPE, deconstructed cones quickly resume cone-specific protein expression, becoming identifiable by immunolabeling once more. This unlucky phenomenon, plus the numeric rod dominance of most mammals (including humans), explains why rhodopsin immunolabeling is routinely used to assess photoreceptor responses to detachment, even in overwhelmingly cone-dominant mammals like the GS. The assessment of cone responses to RD thus depends on low-throughput methods like TEM that do not depend on protein expression.
Rescue of photoreceptor structure and function by reattachment to the RPE is possible so long as subretinal scars due to Müller cell gliosis have not formed. Reattachment does not reverse all abnormal changes (Fig. 5A) and indeed seems to trigger additional remodeling (Lewis et al., 2003), including the expansion of Müller cell endfoot processes into the vitreous where they contribute to fibrocontractile complications such as proliferative vitreoretinopathy (Fisher et al., 2005).
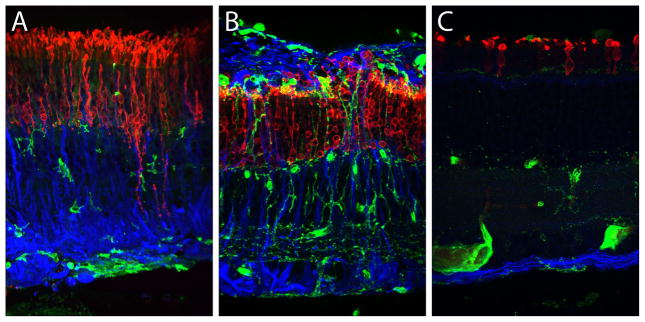
Figure 5. Immunoconfocal microscopy of detached human, cat, and California GS retina co-labeled with antibodies to rhodopsin and GFAP, plus a lectin that identifies microglia, blood vessels, and epiretinal membranes. Cones are not shown because they cease to express cone markers after RD. In all three images, the ILM is at the bottom and the SRS is at top. This work was originally published in Lewis GP, Sethi CS, Carter KM, Charteris DG, Fisher SK, 2005. Microglial cell activation following retinal detachment: a comparison between species. Mol Vis 11, 491–500. Reprinted courtesy of the Authors and Molecular Vision. Copyright by the Authors (2005).
A, Human, >30 days post-RD, had undergone reattachment prior to its excision. Note surviving but deconstructed rods (red), upregulated GFAP (blue), scattered microglia (green), and epiretinal membrane at ILM (green, bottom).
B, Cat, 28 days post-RD (no reattachment). Note surviving but deconstructed rods (red), upregulated GFAP including a glial scar in the SRS (blue, top), and scattered microglia (green).
C, California GS, 7 days post-RD (no reattachment). Note surviving but deconstructed rods (red), normal expression of GFAP (blue), and normal position of microglia (green; large green objects near ILM are blood vessels).
Human RD sequelae are recapitulated in animal models, with the distinct advantage of monitoring events chronologically both post-RD and post-reattachment as a way to assess intervention opportunities (Cuenca et al., 2014; Jones et al., 2012). The question of how well any animal RD model stacks up to human RD cannot truly be answered at this time, because human data are sporadic with many patient variables. Particularly lacking are ultrastructural and histochemical studies of human foveal detachment that could be compared to existing animal data. The animal data themselves have employed different times post-RD and different types of analysis have been applied to these samples. Studies on non-human primates, which arguably have the most relevance, have small sample sizes due to the expense and logistics of primate use in research.
Outside of non-human primates, the cat has proven the best match overall (Fig. 5B; Fisher et al., 2007; ibid., 2005), clearly demonstrating not only reactive gliosis but also extensive, aberrant neuritogenesis by primary, secondary, and tertiary retinal neurons (Coblentz et al., 2003; Sakai et al., 2014; Lewis et al., 1998; Linberg et al., 2006; Linberg et al., 2009). Post-RD remodeling of remnant neuropil likely explains why physical reattachment fails to coincide with functional recovery (Jones et al., 2012), especially with macular reattachment (Ross, 2002). Aberrant neuritogenesis is also a feature of human AMD (Sullivan et al., 2007) and of epiretinal membranes from multiple causes (Lesnik-Oberstein et al., 2011).
Experimental RD was originally applied to the California GS because of its numeric cone dominance and its central retina’s unique potential to model foveal RD in a sub-primate (Jacobs et al., 2002). As in cat, sterile injections of sodium hyaluronate are used to create detachments of controlled height and diameter as desired, aided by the GS’s small lens. The following summary derives from a quartet of GS studies (Lewis et al., 2005; Linberg et al., 2002; Sakai et al., 2001; Sakai et al., 2003) and an authoritative review of experimental RD (Fisher et al., 2005). Time points post-RD ranged from 10 hours to 28 days; a reattachment study followed GSs out to 96 days of recovery. All data derive from euthermic, aroused California GSs whose hibernation stage was otherwise undetermined. It is debated whether California GSs hibernate in the southern part of their range where these studies were conducted, but they do so in the northern part.
4.2.1. Photoreceptors and RPE
After detachment, GS rods and M-cones deconstructed as described in other mammalian species. Outer segments were disorganized and lost, cones stopped expressing cone-specific proteins, and rod visual pigment redistributed over most of the cell (Figure 5C). As GS photoreceptor OSs degenerated, the RPE continued to phagocytose them, at least in the early stages. Ultimately, GS photoreceptors died by apoptosis and were cleared from the subretinal space by cells identified as macrophages, with possible contributions from microglia and Müller cells.
The detachment zone in GS retina was notable for how many of its photoreceptors died and how quickly (Figure 5C), something it has in common with rabbit, but not with cat or human where up to 50% of photoreceptors may survive for over a year in their deconstructed state even when reattachment is not attempted (Figure 5AB). Despite enhanced photoreceptor death, the squirrel OPL appeared unaffected, other than the obvious loss of cone pedicles and rod spherules. The outer limiting membrane (OLM) appeared intact, and a flat outer retinal surface in the detachment zone (formed by branched Müller cell processes) was the result. Remarkably, despite near-complete photoreceptor loss, squirrel RPE did not proliferate or dedifferentiate to any appreciable degree.
4.2.2. Interneurons
Studied only with structural means to date, there were no apparent changes in the GS inner nuclear or ganglion cell layers, and the plexiform layers appeared unaffected other than the loss of photoreceptor terminals from the OPL. This finding probably should be considered preliminary until it is confirmed using immunocytochemical probes that identify inner retinal neurons.
4.2.3. Macroglia
Outside of the GS visual system papers reviewed here, we have found only one paper that has examined reactive gliosis in any GS: Zhou and colleagues (2001) documented local astrocytosis in euthermic Arctic GS brain three days after damage commenced. Thus, we know that the euthermic GS central nervous system is capable of reactive gliosis.
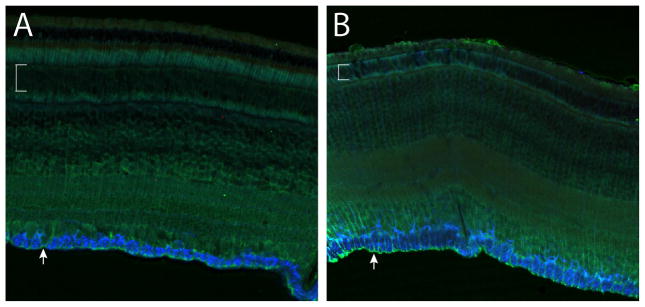
With this positive control in mind, it is remarkable that no evidence of Müller cell reactivity was found in detached (euthermic) California GS retina. Instead, Müller cell processes neatly filled only the empty space left by dead photoreceptors, preserving the general structure of what were once the OLM and ONL, save for an occasional presumed horizontal cell. There was no evidence of Müller cell proliferation, migration, or remodeling associated with scarring (Figure 5C). We have confirmed this finding in detached retina of a second GS species, the 13LGS (Figure 6).
Figure 6. Immunoconfocal images of control and detached 13LGS retina co-labeled with antibodies to nestin (red), GFAP (blue), and vimentin (green). Brackets show the ONL, arrowheads the ILM.
A, Normal control retina.
B, Retina 4 days after detachment. As in California GS, detached 13LGS retina remains nestin-immunonegative, and vimentin and GFAP labeling remain unchanged, indications of quiescent macroglia despite widespread cone death.
In fact, after detachment, California GS Müller cells actually increased expression of normal function proteins, including CRALBP, glutamine synthetase, and EAAT1, responses proposed to improve the metabolic health of surviving (cone) photoreceptors. Occasional nerve fiber layer (NFL) astrocytes extended persistent GFAP-immunopositive processes into the outer retina, more often in reattached GS retina. This evidence suggests a natural inhibition, in at least two GS species, of what we think of as normal macroglial responses to photoreceptor degeneration.
4.2.4. Microglia
Outside of the GS visual system papers reviewed here, we have found only one paper that has examined microglial activation in any GS: Zhou and colleagues (2001) documented local microglial activation in euthermic Arctic GS brain three days after damage commenced. Thus, we know that the euthermic GS central nervous system is capable of responding to insult in this way.
With this positive control in mind, California GS microglia did not mount the response to cone death after RD that we have come to expect from other mammalian species (Figure 5C). A caveat here, fully acknowledged by the study authors, is that the probes used at the time (Griffonia and Ricinis lectins and antibody to CD11b) cannot always distinguish between microglia and macrophages. Microglial response to detachment should be re-examined in GS with additional probes that differentiate activated from resting states (reviewed in Karlstetter et al., 2015).
4.2.5. Responses to intervention after detachment
As in cat, GS cone apoptosis was reduced when squirrels were continuously exposed to 70% O2 immediately after experimental RD up to 3 days later. Hyperoxia also lessened (but did not eliminate) other signs of cone deconstruction. Additionally, reattachment of GS retina 24 hours after detachment dramatically boosted the number of viable (cone) photoreceptors, with concomitant recovery of the cone ERG contrast gain. The overall picture from California GS after reattachment is a slow recovery over ~3 months, similar to what little is known about cone recovery in human patients after reattachment. Thus, GS cones perish quickly after detachment, and recover slowly after reattachment.
5. Conclusions and future directions
5.1. Hibernation repeatedly alters the metabolic background on which GS retina functions
Most GS species, including all of those employed in vision research to date, are either facultative or obligate hibernators, so it is fair to generalize that hibernation physiology is part of the GS model. Hibernation (along with estivation) is evolution’s solution to seasonal problems of physiological stress, but it is a solution that brought its own set of challenges. Hibernation’s challenges have been met not only by GSs but by a group of lower primates, the nocturnal dwarf lemurs of Madagascar, including one who burrows underground (Blanco et al., 2013).
Ground squirrels survive several years in the wild (Michener, 1989), experiencing multiple hibernation cycles in a lifetime. Then, within each winter, a torpid GS will experience perhaps a dozen IBAs. Its visual system has therefore evolved not only to make the most of bright light conditions during its ~5 months of euthermic activity, but to tolerate ~7 months of repeated, rapid, extreme fluctuations in blood flow and temperature, all on the background of continuous darkness.
Visual transduction ceases for the several months of winter sealed underground, which poses an interesting situation not only for the GS’s cones and rods, but also for its intrinsically photosensitive ganglion cells (Schmidt et al., 2014). Since cone membrane potential is quite stable when measured in room-temperature retinal slices collected from torpid 13LGSs (Mehta et al., 2013), we can infer that their dark current machinery remains intact during torpor. We thus expect a substantial increase in the GS retina’s energy demand during all the warm phases in constant darkness (Wong-Riley, 2010), including immergence, IBAs, and emergence. Yet electron microscopy has demonstrated a decrease in GS cone inner segment mitochondria during torpor (Remé & Young, 1977); immunopositivity for cytochrome C oxidase falls there as well (Kaden et al., 2013; Gruber et al., 2006). Irrespective of their activity in darkness, GS cones seem to share the body’s general metabolic downregulation during torpor.
In humans, moving from light to darkness increases retinal and choroidal blood flow (Kur et al., 2012). If this also occurs in the GS, presumably once torpor in the dark commences, it is counteracted by lower global perfusion when heart rate falls to ~5% of euthermic levels. Noninvasive imaging of retinal blood flow is now possible and should be able to clarify the interplay between GS blood supply and retinal demand in conditions of light and dark, torpor and arousal.
Extant studies of the hibernator’s retina tend to contrast the two most different states – deep torpor (“winter”) versus euthermic arousal (“summer”) – but it is increasingly important to acknowledge the possible impacts of transition stages, including IBAs. Practically speaking, outside of a ~5 month period each year, an adult GS with a euthermic Tbody could well be about to enter, or could have recently exited from, a torpor bout. Complicating matters, recent genomic analyses suggest that some GS tissues undergo no fewer than eight distinct physiological states (Grabek et al., 2011).
A priori the GS visual system should not dramatically alter its function unless and until the animal has committed itself to the long winter ahead by sealing itself inside its dark burrow. However, this assumption requires direct examination, especially in view of our experience that imposing summertime zeitgebers in a captive 13LGS colony do not prevent brief test bouts of torpor… bouts that might go unnoticed and yet influence retinal physiology. Until we are certain of any seasonality, vision researchers using GSs (even those which are merely facultative hibernators, e.g. California GS) should consider and report the timing of their experiments to avoid introducing variables. Unfortunately, this has not been done for most vision-related studies employing GSs.
5.2. Hibernation remodels cones, and perhaps more
Cone outer segments, ellipsoid mitochondria, and ribbon synapses of the 13LGS undergo deconstruction during torpor (Figure 2C; Gruber et al., 2006; Kaden et al., 2014; Kuwabara, 1975; Mehta et al., 2013; Qiao et al., 2014; Remé & Young, 1977), but this example of structural plasticity in the adult mammalian retina has a surprisingly small effect on cone synaptic function (Mehta et al., 2013). More details of the functional consequences of the synaptic ribbon plasticity have yet to be explored. Ribbon dynamics seem to be Tbody-dependent because they quickly recover as animals re-warm and they can be artificially induced by chilling retinal slices. Moreover, black bear photoreceptor ribbons do not exhibit these wintertime aggregations (Wei Li, unpublished), but then bear Tbody does not fall nearly so far during the bear’s mild version of torpor.
Despite the fact that hibernation is a normal part of life for the GS, there are intriguing parallels between torpor-associated cone remodeling and retinal disease in other species. Figure 2D illustrates the similar appearance of progressive cone ribbon degeneration in the Tg P347L rabbit, an animal model of retinitis pigmentosa (Jones et al., 2011). Moreover, preliminary data from RNA sequencing of whole retinas collected from torpid and aroused 13LGSs show changes in some genes associated with retinal diseases (Wei Li, unpublished).
To date, there has been only cursory examination of bipolar cell ribbon synapses during torpor, but they do not appear to undergo remodeling. This bears specific study because synaptic remodeling is so widespread in torpid GS brain. Additionally, Müller cells are normally very tightly constrained in their metabolism. Their metabolic signals revealed by CMP do not vary until a pathological insult or retinal degeneration occurs. Such variation has been robustly demonstrated in retinitis pigmentosa (RP) and in animal models of RP (reviewed in Jones et al., 2012). Notably, our preliminary CMP studies reveal variation in Müller cell metabolic profiles during torpor (Figure 4). All things considered, the GS retina during hibernation appears to bridge phase 1 and 2 remodeling following degeneration (Jones et al., 2012), but it recovers fully. There is considerable opportunity to explore this natural recovery further.
5.3. Retinal detachment is followed by atypical downstream events
Experimental retinal detachment has been applied to euthermic GSs in an effort to track the degenerative trajectory of cone-rich regions that may model the human fovea. After RD, more GS photoreceptors die, and die faster, than is the case in cat or human RD. That creates a narrower window for experimental intervention such as hyperoxia (Sakai et al., 2001) or reattachment (Sakai et al., 2003), but this is only the first of several ways in which RD sequelae differ in GS.
Notably, remodeling of RPE and Müller cells is negligible to absent. We cannot yet say for certain what downstream effects on microglial activation or interneuron synaptology might be, but early indications are that these responses are also less than what we’ve come to expect from the cat model of RD or indeed from human patients. As with the GS retina’s response to hibernation, the GS retina’s response to RD appears to bridge phase 1 and 2 remodeling (Jones et al., 2012), but with no progression at least up to the latest survival point studied to date (96 days). Despite photoreceptor loss, the GS retina’s detachment zone appears to stabilize in the least-corrupted form identified to date: the RPE appears normal, the subretinal space appears to be in no danger of acquiring a glial scar, Müller cells don’t proliferate or hypertrophy, and the downstream neurons and microglia seem to stay put. This could be the best possible scenario for photoreceptor transplantation, which has yet to be attempted using this model.
It’s not clear why, of all mammals examined to date, the GS has such a different response to RD. On the one hand, the GS’s relatively positive outcome cannot be attributed to some general CNS neuroprotection conferred by its status as a deep hibernator, because reactive gliosis and microglial activation quickly manifest after traumatic brain injury (Zhou et al., 2001). On the other hand, un-reactive retinal Müller cells are proposed to serve as deformable substrates that facilitate neurite outgrowth, perhaps even synaptic remodeling (Lu et al., 2006; Park & Lee, 2013). Perhaps protecting a deformable Müller cell phenotype expedites retina synaptic remodeling over the hibernation cycle, and the injured GS retina is simply the “unintended” beneficiary.
Another contributing factor could be the GS retina’s pronounced cone dominance (85%), a feature that cannot be separated from its hibernator status. Indeed, Lewis & Fisher (2000) have suggested that gliotic Müller cell outgrowth through the rod-dominant cat retina’s OLM during subretinal scar formation is especially associated with cones. If that holds true generally in mammals, OLM disruption might be greatly exaggerated in damaged cone-dominant retinas, potentially enacting a powerful selective pressure against it. In this regard, it would be useful to assess RD sequelae in non-hibernating, diurnal, cone-dominant rodent species (e.g. the Nile rat, 33% cones; the degu, 33% cones; the sand rat, 44% cones). Interestingly, the Nile rat requires unprecedented dosages of intense light and the DNA-disruptor MNU to attain (cone) photoreceptor degeneration, and even then there appears to be a muted Müller cell response (Boudard et al., 2011).
Taking this a step further, it would be instructive to examine RD in one of the diurnal species of tree shrew because they have even more cones than do GSs (95%; Müller & Peichl, 1993), a tropical animal that does not hibernate. While a favorite model of experimental myopia, the tree shrew doesn’t seem to have ever been used in experimental RD. Should the tree shrew exhibit muted Müller cell reactivity much as does the GS, we would conclude that hibernation physiology has little relationship with the attenuation. Instead, some aspect of the cone-rich retinal microenvironment might play a role, a finding that could possibly have relevance for the human fovea, albeit on a much smaller scale.
Strikingly, Müller cells of GFAP−/−Vimentin−/− mice also fail to mount a gliotic response after experimental RD. However, lack of these intermediate filaments renders the retinas of these knockout mice unusually vulnerable to shear stress (Verardo et al., 2008), and their inner retinal cells are unusually vulnerable to ischemia-reperfusion injury (Wunderlich et al., 2015). Both consequences would be terrible disadvantages in a wild-living hibernator.
Cebulla and colleagues (2012) have referred to the GS’s lack of post-RD reactive gliosis as a “research liability”. In truth, all animal models carry some sort of liability when it comes to modeling human vision. The mouse has certainly received its fair share of criticism (Baker, 2013). We take the alternate position that each model species is a vital natural experiment, in the spirit of August Krogh’s famous dictum, with something to contribute. It might be appropriate to view the GS at the far end of a gliosis continuum, with a checkpoint thanks to natural selection (Weil et al., 2008). After all, just within the mouse, Müller cell reactivity varies considerably depending on the inherited degeneration (Hippert et al., 2015). Moreover, different strains of mice subjected to experimental RD react differently to RD (Matsumoto et al., 2014). For all we know, this could be true in humans, too.
Debate continues whether typical retinal macro- and microglial responses to deafferentiation are helpful or harmful to surviving neurons. Strong arguments have been made in favor of limiting glial remodeling because it impedes clinical interventions (Jones et al., 2012; Marc et al., 2007; Pearson, 2014) and may exacerbate cone loss. The evidence to date in GS is that its limited glial response to RD does not attenuate photoreceptor loss, but does coincide with apparent stability of inner retinal neurons. Perhaps the two are co-regulated somehow, in a way that photoreceptor death and glial reactivity are not (Fisher et al., 2005). The knockout mouse literature continues to produce candidate control mechanisms that could be assessed in GS retina (Cuenca et al., 2014; Karlstetter et al., 2015). Some intriguing findings regarding Müller cell glucocorticoid receptor activation are being produced with a chick model of RD (Gallina et al., 2015). Post-injury, these receptors upregulate briefly and, when an agonist is applied, Müller cell proliferation and microglial reactivity are both reduced. If further examination of GS retina confirms minimal microglial reaction post-RD, it would be possible to determine if co-inhibition of negative remodeling (Wang & Wong, 2014) in the natural world occurs by a similar mechanism.
In addition, Müller cell reprogramming for the purposes of retinal regeneration has been proposed, with experiments done in mice (Goldman, 2014). To borrow a term from that author, with their uniquely stable properties in the face of severe retinal insult, how easily might GS Müller cells be “persuaded” in this regard?
Lastly, the GS’s lack of gliofibrotic scarring after RD is curiously echoed in its skeletal muscle. When muscle’s damaged state is prolonged, its eventual repair includes fibrosis, another version of scarring (Mann et al., 2011). Andres-Mateos and colleagues (2012) modeled focal muscle damage in 13LGS using a toxin injection. When damage occurred during torpor, muscle repair was inhibited, prolonging the damage state for weeks. When those animals were allowed to emerge naturally in spring, complete muscle repair resulted but, unexpectedly, without any fibrosis (Andres-Mateos et al., 2012).
5.4. Summary
Ground squirrel retinas contain a disproportionate number of cones, making them a worthy model of cone-based visual function. They share with human a diurnal habit that includes specific architecture of rod nuclei and attendant DNA repair ability. It is important to remain cognizant of seasonal hibernation physiology when using this animal model. Concurrent with low Tbody during hibernation torpor, GS cones exhibit deconstructive remodeling of their OS, ellipsoid mitochondria, and ribbon synapses. These features of the torpid GS retina resemble the early stages of retinal degeneration in non-hibernators, but do not progress and are rapidly reversed upon arousal from torpor. Additional study may well reveal more features of hibernation physiology in additional cell types, along with mechanisms underlying natural recovery.
Widespread cone death by experimental RD in the euthermic (“summer”) California GS and 13LGS has revealed the strongest disconnect yet between deafferentiation and remodeling of Müller cells. Interneuron remodeling and microglial reactivity also appear reduced or absent, but more investigation of th is needed. Ground squirrel evolution seems to have imposed a checkpoint in the retinal degenerative trajectory that may create a favorable environment for therapeutic approaches to photoreceptor loss.
Understanding the GS’s evolved solutions -- to many of the same problems faced by patients -- may provide insights for novel therapies (Buffenstein et al., 2014; van Breukelen & Martin, 2015; Weil et al., 2008). Hibernation’s relevance to neuroprotection afforded by ischemic preconditioning has not gone unnoticed (Dave et al., 2012). Moreover, the 13LGS’s suitability for noninvasive imaging technologies such as OCT and AOSLO, and its growing use in comparative genomics, epigenomics, transcriptomics, and proteomics, position this animal model well to facilitate translational hypotheses in the retina and more generally in the central nervous system (Alvarado et al., 2015; Biggar & Storey, 2014; Grabek et al., 2015; Schwartz et al., 2013; Xu et al., 2013).
Highlights.
Ground squirrels are highly visual hibernators and have a retina with 85% cones
Cone and brain synapses reversibly remodel during winter torpor
After retinal detachment and widespread cone death, reactive gliosis is attenuated
This natural attenuation may be relevant for candidate therapies
Acknowledgments
We thank T.B. Connor MD (Medical College of Wisconsin) for carrying out the experimental retinal detachments on 13LGS, and G.P. Lewis and G. Luna (University of California Santa Barbara) for providing the corresponding microscopy (Figure 6).
We thank Elsevier and G.P. Lewis and colleagues (University of California Santa Barbara) for permitting the reprint of the images shown in Figure 5.
We thank S.K. Fisher (University of California Santa Barbara) for helpful discussion during the preparation of this manuscript.
This work was funded by the University of Wisconsin Oshkosh Faculty Development and Student Collaborative Grant Programs and the AxleTech International Endowed Professorship (D.K.M.), NEI Core Grant P30EY001931 (B.S.S.), the NEI Intramural Research Program (W.L.), and NIH EY015128, EY02576, EY014800 Vision Core, and an unrestricted grant from Research to Prevent Blindness to the Moran Eye Center (B.W.J.).
Abbreviations footnote
The following abbreviations are used in this paper:
- AMD
age-related macular degeneration
- AOSLO
adaptive optics scanning light ophthalmoscopy
- CMP
computational molecular phenotyping
- CNS
central nervous system
- CRALBP
cellular retinaldehyde binding protein
- CRMP-2
collapsin response mediator protein 2
- CtBP2
C-terminal binding protein 2
- DPYSL2
dihydropyrimidinase-like 2
- EAAT1
excitatory amino acid transporter 1
- ERG
electroretinogram
- GFAP
glial fibrillary acidic protein
- GS
ground squirrel
- IBA
interbout arousal
- IPL
inner plexiform layer
- OCT
optical coherence tomography
- ONL
outer nuclear layer
- OLM
outer limiting membrane
- OPL
outer plexiform layer
- RBM3
RNA binding motif protein 3
- RD
retinal detachment
- RP
retinitis pigmentosa
- RPE
retinal pigment epithelium
- SCN
suprachiasmatic nucleus
- SRS
subretinal space
- TEM
transmission electron microscopy
- 13LGS
thirteen-lined ground squirrel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dana K. Merriman, Email: merrimad@uwosh.edu.
Benjamin S. Sajdak, Email: besajdak@mcw.edu.
Wei Li, Email: liwei2@nei.nih.gov.
Bryan W. Jones, Email: bryan.jones@m.cc.utah.edu.
Literature Cited
- Abreu M, Kicliter E, Lugo-Garcia N. Displaced amacrine cells in the ganglion cell layer of the ground squirrel retina. PR Health Sci J. 1993;12:137–141. [PubMed] [Google Scholar]
- Agarwala S, Petry HM, May JG., 3rd Retinal projections in the ground squirrel (Citellus tridecemlineatus) Vis Neurosci. 1989;3:537–549. doi: 10.1017/s0952523800009871. [DOI] [PubMed] [Google Scholar]
- Agoston D. Bench-to-bedside and bedside back to the bench; seeking a better understanding of the acute pathophysiological process in severe traumatic brain injury. Front Neurol. 2015;6:47. doi: 10.3389/fneur.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnelt PK. Characterization of the color related receptor mosaic in the ground squirrel retina. Vision Res. 1985;25:1557–1567. doi: 10.1016/0042-6989(85)90126-9. [DOI] [PubMed] [Google Scholar]
- Alvarado S, Mak T, Liu S, Storey KB, Szyf M. Dynamic changes in global and gene-specific DNA methylation during hibernation in adult thirteen-lined ground squirrels, Ictidomys tridecemlineatus. J Exp Biol. 2015;218:1787–1795. doi: 10.1242/jeb.116046. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- Anderson DH, Neitz J, Saari JC, Kaska DD, Fenwick J, Jacobs GH, Fisher SK. Retinoid-binding proteins in cone-dominant retinas. Invest Ophthalmol Vis Sci. 1986;27:1015–1026. [PubMed] [Google Scholar]
- Anderson DH, Williams DS, Neitz J, Fariss RN, Fliesler SJ. Tunicamycin-induced degeneration in cone photoreceptors. Vis Neurosci. 1988;1:153–158. doi: 10.1017/s0952523800001425. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Mejias R, Soleimani A, Lin BM, Burks TN, Marx R, Lin B, Zellars RC, Zhang Y, Huso DL, Marr TG, Leinwand LA, Merriman DK, Cohn RD. Impaired skeletal muscle regeneration in the absence of fibrosis during hibernation in 13-lined ground squirrels. PLoS ONE. 2012;7:e48884. doi: 10.1371/journal.pone.0048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Baker M. Through the eyes of a mouse. Nature. 2013;502:156–8. doi: 10.1038/502156a. [DOI] [PubMed] [Google Scholar]
- Berson EL. Experimental and therapeutic aspects of photic damage to the retina. Invest Ophthalmol. 1973;12:35–44. [PubMed] [Google Scholar]
- Besharse JC, Bok D. The retina and its disorders. Academic Press; 2011. [Google Scholar]
- Biggar KK, Storey KB. New approaches to comparative and animal stress biology research in the post-genomic era: a contextual overview. Comput Struct Biotechnol J. 2014;11:138–146. doi: 10.1016/j.csbj.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MB, Dausmann KH, Ranaivoarisoa JF, Yoder AD. Underground hibernation in a primate. Sci Rep. 2013;3:1768. doi: 10.1038/srep01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudard DL, Acar N, Bretillon L, Hicks D. Retinas of the diurnal rodent Arvicanthis ansorgei are highly resistant to experimentally induced stress and degeneration. Invest Ophthalmol Vis Sci. 2011;52:8686–8700. doi: 10.1167/iovs.11-8162. [DOI] [PubMed] [Google Scholar]
- Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc Natl Acad Sci USA. 2011;108:2052–2057. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratincsak A, McMuller D, Miyake S, Toth ZE, Hallenbeck JM, Palovits M. Spatial and temporal activation of brain regions in hibernation: c-fos expression during the hibernation bout in thirteen-lined ground squirrel. J Comp Neurol. 2007;505:443–58. doi: 10.1002/cne.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Nelson OL, Corbit KC. Questioning the preclinical paradigm: natural, extreme biology as an alternative discovery platform. Aging. 2014;6:913–920. doi: 10.18632/aging.100704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Krubitzer L. Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J Comp Neurol. 2010;518:4491–4512. doi: 10.1002/cne.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LH, Luo DG, Yau KW. Light responses of primate and other mammalian cones. Proc Natl Acad Sci USA. 2014;111:2752–2757. doi: 10.1073/pnas.1400268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nature Neurosci. 2012;15:954–956. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Rasley BT, Roe T, Moore JT, Harris MB, Drew KL. Habituation of Arctic ground squirrels (Urocitellus parryii) to handling and movement during torpor to prevent artificial arousal. Front Physiol. 2014;5:174. doi: 10.3389/fphys.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens LE, Heldmaier G, Exner C. Keep cool: memory is retained during hibernation in Alpine marmots. Physiol Behav. 2009;98:78–84. doi: 10.1016/j.physbeh.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Coblentz FE, Radeke MJ, Lewis GP, Fisher SK. Evidence that ganglion cells react to retinal detachment. Exp Eye Res. 2003;76:333–342. doi: 10.1016/s0014-4835(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Cooper RM. Diffuse light increases metabolic activity in the lateral geniculate nucleus, visual cortex, and superior colliculus of the cone-dominated ground squirrel visual system. Vision Res. 2002;42:2899–2907. doi: 10.1016/s0042-6989(02)00361-9. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genetics. 2005;1:e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Fisher SK, Kolb H. Choline acetyltransferase is expressed by non-starburst amacrine cells in the ground squirrel retina. Brain Res. 2003;964:21–30. doi: 10.1016/s0006-8993(02)04049-0. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Lewis GP, Fisher SK, Kolb H. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J Neurocytol. 2002;31:649–666. doi: 10.1023/a:1025791512555. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Ret Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Cull G, Cioffi GA, Dong J, Homer L, Wang L. Estimating normal optic nerve axon numbers in non-human primate eyes. J Glaucoma. 2003;12:301–306. doi: 10.1097/00061198-200308000-00003. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Christian SL, Perez-Pinzon MA, Drew KL. Neuroprotection: lessons from hibernators. Compar Biol Physiol. 2012;162:1–9. doi: 10.1016/j.cbpb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij EL, Vogelaar PC, Goris M, Houwertjes MC, Herwig A, Dugbartey GJ, Boerema AS, Strijkstra AM, Bouma HR, Henning RH. Platelet dynamics during natural and pharmacologically induced torpor and forced hypothermia. PLoS ONE. 2014;9:e93218. doi: 10.1371/journal.pone.0093218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Qi X, Smith R, Makous W, Sterling P. Electrical coupling between mammalian cones. Curr Biol. 2002;12:1900–1907. doi: 10.1016/s0960-9822(02)01261-7. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–26. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DB, Souza DW, Chase DG. Cone visual cell degeneration in ground squirrel retina: disruption of morphology and cyclic nucleotide metabolism by iodoacetic acid. Invest Ophthalmol Vis Sci. 1983;24:1236–1249. [PubMed] [Google Scholar]
- Farber DB, Souza DW, Chase DG, Lolley RN. Cyclic nucleotides of cone-dominant retinas. Reduction of cyclic AMP levels by light and by cone degeneration. Invest Ophthalmol Vis Sci. 1981;20:24–31. [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Ret Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Barawid E, Verardo MR. Cellular remodeling in mammalian retina induced by retinal detachment. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center; Salt Lake City, UT: 2007. [PubMed] [Google Scholar]
- Fredes F, Vega-Zuniga T, Karten H, Mpodozis J. Bilateral and ipsilateral ascending tectopulvinar pathways in mammals: a study in the squirrel (Spermophilus beecheyi) The J Comp Neurol. 2012;520:1800–1818. doi: 10.1002/cne.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohns A, Frohns F, Naumann SC, Layer PG, Lobrich M. Inefficient double-strand break repair in murine rod photoreceptors with inverted heterochromatin organization. Curr Biol. 2014;24:1080–1090. doi: 10.1016/j.cub.2014.03.061. [DOI] [PubMed] [Google Scholar]
- Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015;273:114–25. doi: 10.1016/j.expneurol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino VR, Orr ME, Rodriguez KA, Buffenstein R. Mechanisms of oxidative stress resistance in the brain: Lessons learned from hypoxia tolerant extremophilic vertebrates. Arch Biochem Biophys. 2015;576:8–16. doi: 10.1016/j.abb.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek KR, Karimpour-Fard A, Epperson LE, Hindle A, Hunter LE, Martin SL. Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics. 2011;43:1263–1275. doi: 10.1152/physiolgenomics.00125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek KR, Martin SL, Hindle AG. Proteomics approaches shed new light on hibernation physiology. J Comp Physiol B. 2015;185:607–627. doi: 10.1007/s00360-015-0905-9. [DOI] [PubMed] [Google Scholar]
- Gruber AR, Betts KE, Lewis GP, Fisher SK, Vaughan DK. Photoreceptor antigens are altered during season hibernation of a cone-dominant rodent. Invest Ophthalmol Vis Sci. 2006;47:a2845. [Google Scholar]
- Gur M, Sivak JG. Refractive state of the eye of a small diurnal mammal: the ground squirrel. Am J Optom Physiol Optics. 1979;56:689–695. doi: 10.1097/00006324-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Hains PG, Simpanya MF, Giblin F, Truscott RJ. UV filters in the lens of the thirteen lined ground squirrel (Spermophilus tridecemlineatus) Exp Eye Res. 2006;82:730–737. doi: 10.1016/j.exer.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc Biol Sci. 2013;280:20130016. doi: 10.1098/rspb.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle AG, Martin SL. Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PLoS ONE. 2013;8:e71627. doi: 10.1371/journal.pone.0071627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippert C, Graca AB, Barber AC, West EL, Smith AJ, Ali RR, Pearson RA. Muller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS ONE. 2015;10:e0120415. doi: 10.1371/journal.pone.0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MJ, Bernstein MH. Fine structure of the photoreceptor cells of the ground squirrel (Citellus tridecemlineatus tridecemlineatus) Am J Anat. 1966;118:359–373. doi: 10.1002/aja.1001180204. [DOI] [PubMed] [Google Scholar]
- Hut RA, Dardente H, Riede SJ. Seasonal timing: how does a hibernator know when to stop hibernating? Curr Biol. 2014;24:R602–5. doi: 10.1016/j.cub.2014.05.061. [DOI] [PubMed] [Google Scholar]
- Hut R, Van der Zee E, Jansen K, Gerkema M, Daan S. Gradual reappearance of post-hibernation circadian rhythmicity correlates with numbers of vasopressin-containing neurons in the suprachiasmatic nuclei of European ground squirrels. J Comp Physiol B. 2002;172:59–70. doi: 10.1007/s003600100227. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Duplicity theory and ground squirrels: linkages between photoreceptors and visual function. Vis Neurosci. 1990;5:311–318. doi: 10.1017/s0952523800000377. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Blakeslee B, Tootell RB. Color-discrimination tests on fibers in ground squirrel optic nerve. J Neurophys. 1981;45:903–914. doi: 10.1152/jn.1981.45.5.903. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Calderone JB, Sakai T, Lewis GP, Fisher SK. An animal model for studying cone function in retinal detachment. Doc Ophthalmol. 2002;104:119–132. doi: 10.1023/a:1014431701523. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J. Development of spectral mechanisms in the ground squirrel retina following lid opening. Exp Brain Res. 1984;55:507–514. doi: 10.1007/BF00235281. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Tootell RB. Spectral-response properties of optic-nerve fibers in the ground squirrel. J Neurophys. 1981;45:891–902. doi: 10.1152/jn.1981.45.5.891. [DOI] [PubMed] [Google Scholar]
- Jani A, Martin SL, Jain S, Keys D, Edelstein CL. Renal adaptation during hibernation. Am J Physiol Renal Physiol. 2013;305:F1521–1532. doi: 10.1152/ajprenal.00675.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Geller SF, Reese BE. Distribution, size and number of axons in the optic pathway of ground squirrels. Exp Brain Res. 1998;118:93–104. doi: 10.1007/s002210050258. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31:736–744. [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE. Retinal remodeling. Jap J Ophthalmol. 2012;56:289–306. doi: 10.1007/s10384-012-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol. 2011;519:2713–2733. doi: 10.1002/cne.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Visualizing the metabolome. Microsc Microanal. 2007;13(suppl 2) doi: 10.1017/S1431927607072601. [DOI] [Google Scholar]
- Kaden T, Du J, Goel A, Li W. Structural and functional properties of photoreceptor mitochondria in awake and hibernation ground squirrels. Invest Ophthalmol Vis Sci. 2013;54:a6082. [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Ret Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Kraft TW. Photocurrents of cone photoreceptors of the golden-mantled ground squirrel. J Physiol. 1988;404:199–213. doi: 10.1113/jphysiol.1988.sp017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger Z, Galli-Resta L, Jacobs GH, Reese BE. The topography of rod and cone photoreceptors in the retina of the ground squirrel. Vis Neurosci. 1998;15:685–691. doi: 10.1017/s0952523898154081. [DOI] [PubMed] [Google Scholar]
- Kuwabara T. Cytologic changes of the retina and pigment epithelium during hibernation. Invest Ophthalmol. 1975;14:457–467. [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JE, Franken P, Heller HC. Loss of circadian organization of sleep and wakefulness during hibernation. Am J Physiol Reg I. 2002;282:R1086–1095. doi: 10.1152/ajpregu.00771.2000. [DOI] [PubMed] [Google Scholar]
- Larson J, Drew KL, Folkow LP, Milton SL, Park TJ. No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J Exp Biol. 2014;217:1024–1039. doi: 10.1242/jeb.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen WJ, Funk O, Goodman J, Merriman DK, Ingolia NT, Bagriantsev SN, Gracheva EO. Molecular adaptations to extreme thermogenesis in mammalian hibernators. Biophys J. 2014;106:337a. [Google Scholar]
- Lee YJ, Hallenbeck JM. SUMO and ischemic tolerance. Neuromolec Med. 2013;15:771–781. doi: 10.1007/s12017-013-8239-9. [DOI] [PubMed] [Google Scholar]
- Lesnik Oberstein SY, Lewis GP, Dutra T, Fisher SK. Evidence that neurites in human epiretinal membranes express melanopsin, calretinin, rod opsin and neurofilament protein. British J Ophthalmol. 2011;95:266–272. doi: 10.1136/bjo.2010.180679. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK. Muller cell outgrowth after retinal detachment: association with cone photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:1542–1545. [PubMed] [Google Scholar]
- Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434. [PubMed] [Google Scholar]
- Lewis GP, Sethi CS, Linberg KA, Charteris DG, Fisher SK. Experimental retinal reattachment: a new perspective. Mol Neurobiol. 2003;28:159–75. doi: 10.1385/MN:28:2:159. [DOI] [PubMed] [Google Scholar]
- Li W, Chen S, DeVries SH. A fast rod photoreceptor signaling pathway in the mammalian retina. Nature Neurosci. 2010;13:414–416. doi: 10.1038/nn.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nature Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nature Neurosci. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Li W, Li Y, Qiao F, Hollyfield JG, Quian H, Bell BA. Retinal changes associated with hibernation revealed by OCT. Invest Ophthalmol Vis Sci. 2014;55:a2174. [Google Scholar]
- Light AC, Zhu Y, Shi J, Saszik S, Lindstrom S, Davidson L, Li X, Chiodo VA, Hauswirth WW, Li W, DeVries SH. Organizational motifs for ground squirrel cone bipolar cells. J Comp Neurol. 2012;520:2864–2887. doi: 10.1002/cne.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Lewis GP, Fisher SK. Retraction and remodeling of rod spherules are early events following experimental retinal detachment: an ultrastructural study using serial sections. Mol Vis. 2009;15:10–25. [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Lewis GP, Matsumoto B, Fisher SK. Immunocytochemical evidence that rod-connected horizontal cell axon terminals remodel in response to experimental retinal detachment in the cat. Mol Vis. 2006;12:1674–1686. [PubMed] [Google Scholar]
- Linberg KA, Sakai T, Lewis GP, Fisher SK. Experimental retinal detachment in the cone-dominant ground squirrel retina: morphology and basic immunocytochemistry. Vis Neurosci. 2002;19:603–619. doi: 10.1017/s095252380219506x. [DOI] [PubMed] [Google Scholar]
- Linberg KA, Suemune S, Fisher SK. Retinal neurons of the California ground squirrel, Spermophilus beecheyi: a Golgi study. J Comp Neurol. 1996;365:173–216. doi: 10.1002/(SICI)1096-9861(19960205)365:2<173::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Long KO, Fisher SK. The distributions of photoreceptors and ganglion cells in the California ground squirrel, Spermophilus beecheyi. J Comp Neurol. 1983;221:329–340. doi: 10.1002/cne.902210308. [DOI] [PubMed] [Google Scholar]
- Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp Eye Res. 1986;43:193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- Lu YB, Franze K, Seifert G, Steinhauser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei EQ, Kas J, Reichenbach A. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci USA. 2006;103:17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Garcia N, Blanco RE. Morphology and distribution of dopaminergic neurons in the ground squirrel retina. PR Health Sci J. 1993;12:143–146. [PubMed] [Google Scholar]
- Lugo-Garcia N, Kicliter E. Superior colliculus efferents to five subcortical visual system structures in the ground squirrel. Brain Res. 1987;426:131–141. doi: 10.1016/0006-8993(87)90432-x. [DOI] [PubMed] [Google Scholar]
- Lugo-Garcia N, Kicliter E. Morphology of ganglion cells which project to the dorsal lateral geniculate and superior colliculus in the ground squirrel. Brain Res. 1988;454:67–77. doi: 10.1016/0006-8993(88)90804-9. [DOI] [PubMed] [Google Scholar]
- Major DE, Rodman HR, Libedinsky C, Karten HJ. Pattern of retinal projections in the California ground squirrel (Spermophilus beecheyi): anterograde tracing study using cholera toxin. J Comp Neurol. 2003;463:317–340. doi: 10.1002/cne.10764. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skel Musc. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Bull ND, Martin KR. A semiautomated targeted sampling method to assess optic nerve axonal loss in a rat model of glaucoma. Nature Protocols. 2010;5:1642–1651. doi: 10.1038/nprot.2010.128. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Mills SL. Short-wavelength cone-opponent retinal ganglion cells in mammals. Vis Neurosci. 2014;31:165–175. doi: 10.1017/s095252381300031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kataoka K, Tsoka P, Connor KM, Miller JW, Vavvas DG. Strain difference in photoreceptor cell death after retinal detachment in mice. Invest Ophthalmol Vis Sci. 2014;55:4165–4174. doi: 10.1167/iovs.14-14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt ME, Jacobs GH. Directional filter characteristics of optic nerve fibers in California ground squirrel (Spermophilus beecheyi) J Neurophys. 1984a;52:1200–1212. doi: 10.1152/jn.1984.52.6.1200. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jacobs GH. Refractive state, depth of focus and accommodation of the eye of the California ground squirrel (Spermophilus beecheyi) Vision Res. 1984b;24:1261–1266. doi: 10.1016/0042-6989(84)90180-9. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jacobs GH. Spatial filter characteristics of optic nerve fibers in California ground squirrel (Spermophilus beecheyi) J Neurophys. 1984c;52:1181–1199. doi: 10.1152/jn.1984.52.6.1181. [DOI] [PubMed] [Google Scholar]
- McNulty JA, Fox LM, Spurrier WA. A circannual cycle in pinealocyte synaptic ribbons in the hibernating and seasonally reproductive 13-lined ground squirrel (Spermophilus tridecemlineatus) Neurosci Lett. 1990;119:237–240. doi: 10.1016/0304-3940(90)90842-w. [DOI] [PubMed] [Google Scholar]
- Mehta B, Snellman J, Chen S, Li W, Zenisek D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron. 2013;77:516–527. doi: 10.1016/j.neuron.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman DK, Lahvis G, Jooss M, Gesicki JA, Schill K. Current practices in a captive breeding colony of 13-lined ground squirrels (Ictidomys tridecemlineatus) Lab Anim NY. 2012;41:315–325. doi: 10.1038/laban.150. [DOI] [PubMed] [Google Scholar]
- Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164. doi: 10.1016/s0002-9394(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Michener GR. Sexual differences in interyear survival and life-span of Richardson’s ground squirrels. Can J Zool. 1989;67:1827–1831. doi: 10.1139/z89-260. [DOI] [Google Scholar]
- Millesi E, Prossinger H, Dittami JP, Fieder M. Hibernation effects on memory in European ground squirrels (Spermophilus citellus) J Biol Rhythms. 2001;16:264–271. doi: 10.1177/074873040101600309. [DOI] [PubMed] [Google Scholar]
- Miyagishima KJ, Grünert U, Li W. Processing of S-cone signals in the inner plexiform layer of the mammalian retina. Vis Neurosci. 2014;31:153–63. doi: 10.1017/S0952523813000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Peichl L. Horizontal cells in the cone-dominated tree shrew retina: morphology, photoreceptor contacts, and topographical distribution. J Neurosci. 1993;13:3628–46. doi: 10.1523/JNEUROSCI.13-08-03628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A, Nadal-Nicolas FM, Jimenez-Lopez M, Alburquerque-Bejar JJ, Nieto-Lopez L, Garcia-Ayuso D, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M. Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS ONE. 2014;9:e102392. doi: 10.1371/journal.pone.0102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee YJ. Nano-mechanical compliance of Müller cells investigated by atomic force microscopy. Int J Biol Sci. 2013;9:702–706. doi: 10.7150/ijbs.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA. Advances in repairing the degenerate retina by rod photoreceptor transplantation. Biotechnol Adv. 2014;32:485–91. doi: 10.1016/j.biotechadv.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat Rec A. 2005;287:1001–1012. doi: 10.1002/ar.a.20262. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Popov VI, Medvedev NI, Patrushev IV, Ignat’ev DA, Morenkov ED, Stewart MG. Reversible reduction in dendritic spines in CA1 of rat and ground squirrel subjected to hypothermia-normothermia in vivo: A three-dimensional electron microscope study. Neuroscience. 2007;149:549–560. doi: 10.1016/j.neuroscience.2007.07.059. [DOI] [PubMed] [Google Scholar]
- Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. The J Comp Neurol. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- Qiao F, Shen C, Merriman DK, Li W. Mechanisms of photoreceptor synaptic ribbon plasticity in the hibernating ground squirrel retina. Invest Ophthalmol Vis Sci. 2013;54:a2488. [Google Scholar]
- Remé CE, Young RW. The effects of hibernation on cone visual cells in the ground squirrel. Invest Ophthalmol Vis Sci. 1977;16:815–840. [PubMed] [Google Scholar]
- Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pévet M, Simonneaux V, Saboureau M, Pévet P. The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci USA. 2007;104:13816–20. doi: 10.1073/pnas.0704699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter MM, Williams CT, Lee TN, Toien O, Florant GL, Barnes BM, Buck CL. Thermogenic capacity at subzero temperatures: how low can a hibernator go? Physiol Biochem Zool PBZ. 2015;88:81–89. doi: 10.1086/679591. [DOI] [PubMed] [Google Scholar]
- Rivera N, Lugo N. Four retinal ganglion cell types that project to the superior colliculus in the thirteen-lined ground squirrel (Spermophilus tridecemlineatus) J Comp Neurol. 1998;396:105–120. doi: 10.1002/(sici)1096-9861(19980622)396:1<105::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ramos Fernandez J, Dubielzig RR. Ocular comparative anatomy of the family Rodentia. Vet Ophthalmol. 2013;16(Suppl 1):94–99. doi: 10.1111/vop.12070. [DOI] [PubMed] [Google Scholar]
- Ross WH. Visual recovery after macula-off retinal detachment. Eye. 2002;16:440–446. doi: 10.1038/sj.eye.6700192. [DOI] [PubMed] [Google Scholar]
- Ruediger J, Van der Zee EA, Strijkstra AM, Aschoff A, Daan S, Hut RA. Dynamics in the ultrastructure of asymmetric axospinous synapses in the frontal cortex of hibernating European ground squirrels (Spermophilus citellus) Synapse. 2007;61:343–352. doi: 10.1002/syn.20380. [DOI] [PubMed] [Google Scholar]
- Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2014 doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RL, O’Neill PH, Epperson LE, Martin SL. Extensive use of torpor in 13-lined ground squirrels in the fall prior to cold exposure. J Comp Physiol B. 2010;180:1165–1172. doi: 10.1007/s00360-010-0484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdak BS, Sulai YN, Langlo CL, Luna G, Fisher SK, Merriman DK, Dubra A. Noninvasive imaging of the thirteen-lined ground squirrel photoreceptor mosaic. Vis Neurosci. 2015 doi: 10.1017/S0952523815000346. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Calderone JB, Lewis GP, Linberg KA, Fisher SK, Jacobs GH. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci. 2003;44:416–425. doi: 10.1167/iovs.02-0633. [DOI] [PubMed] [Google Scholar]
- Sakai T, Lewis GP, Linberg KA, Fisher SK. The ability of hyperoxia to limit the effects of experimental detachment in cone-dominated retina. Invest Ophthalmol Vis Sci. 2001;42:3264–3273. [PubMed] [Google Scholar]
- Sakai T, Tsuneoka H, Lewis GP, Fisher SK. Remodelling of retinal on- and off-bipolar cells following experimental retinal detachment. Clin Exp Ophthalmol. 2014;42:480–485. doi: 10.1111/ceo.12246. [DOI] [PubMed] [Google Scholar]
- Sallmen T, Lozada AF, Anichtchik OV, Beckman AL, Panula P. Increased brain histamine H3 receptor expression during hibernation in golden-mantled ground squirrels. BMC Neurosci. 2003;4:24. doi: 10.1186/1471-2202-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saszik S, DeVries SH. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci. 2012;32:297–307. doi: 10.1523/jneurosci.2739-08.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Alam NM, Chen S, Kofuji P, Li W, Prusky GT, Hattar S. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82:181–8. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Ballinger MA, Andrews MT. Melatonin receptor signaling contributes to neuroprotection upon arousal from torpor in thirteen-lined ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00292.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Hampton M, Andrews MT. Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS ONE. 2013;8:e58427. doi: 10.1371/journal.pone.0058427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nature Neurosci. 2012;15:952–953. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Richter MM, Buck CL, Barnes BM. Changing seasonality and phenological responses of free-living male arctic ground squirrels: the importance of sex. Phil Trans Roy Soc Lond B. 2013;368:20120480. doi: 10.1098/rstb.2012.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Lamberti R, Tom Dieck S, Spessert R. Evidence for synergistic and complementary roles of Bassoon and darkness in organizing the ribbon synapse. Neuroscience. 2013;236:149–159. doi: 10.1016/j.neuroscience.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Sullivan RKP, WoldeMussie E, Pow DV. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Invest Ophthalmol Vis Sci. 2007;48:2782–91. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- Sussman D, Chou BR, Lakshminarayanan V. Eye model for the ground squirrel. J Mod Optic. 2011;58:1889–1896. doi: 10.1080/09500340.2010.548608. [DOI] [Google Scholar]
- Szel A, Rohlich P. Four photoreceptor types in the ground squirrel retina as evidenced by immunocytochemistry. Vision Res. 1988;28:1297–1302. doi: 10.1016/0042-6989(88)90060-0. [DOI] [PubMed] [Google Scholar]
- Szmajda BA, Devries SH. Glutamate spillover between mammalian cone photoreceptors. J Neurosci. 2011;31:13431–13441. doi: 10.1523/jneurosci.2105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton JP, Struebing FL, Lemmon A, Geisert EE. ImagePAD, a novel counting application for the Apple iPad, used to quantify axons in the mouse optic nerve. Exp Eye Res. 2014;128:102–108. doi: 10.1016/j.exer.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya PG. A study of the lateral geniculate body in the diurnal ground squirrel, Citellus tridecemlineatus and in the nocturnal guinea pig. Anat Rec. 1963;147:499–505. doi: 10.1002/ar.1091470405. [DOI] [PubMed] [Google Scholar]
- Vaidya PG. A comparative study of the visual system in the diurnal ground squirrel, Citellus tridecemlineatus tridecemlineatus and in the nocturnal guinea pig, Cavia cobaya. Z Anat Entwicklungs. 1965;124:505–521. doi: 10.1007/BF00520843. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. The hibernation continuum: physiological and molecular aspects of metabolic plasticity in mammals. Physiology. 2015;30:273–281. doi: 10.1152/physiol.00010.2015. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Nelson SB. The squirrel as a rodent model of the human visual system. Vis Neurosci. 2006;23:765–778. doi: 10.1017/s0952523806230098. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Linberg KA, Li W, Traub M, Lewis GP, Fisher SK. Seasonal dynamics of cone ribbon synapses in ground squirrel retina. Invest Ophthalmol Vis Sci. 2007;49:a1262. [Google Scholar]
- Verardo MR, Lewis GP, Takeda M, Linberg KA, Byun J, Luna G, Wilhelmsson U, Pekny M, Chen DF, Fisher SK. Abnormal reactivity of Müller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest Ophthalmol Vis Sci. 2008;49:3659–65. doi: 10.1167/iovs.07-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ohe CG, Garner CC, Darian-Smith C, Heller HC. Synaptic protein dynamics in hibernation. J Neurosci. 2007;27:84–92. doi: 10.1523/jneurosci.4385-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schantz M, Szel A, van Veen T, Farber DB. Expression of phototransduction cascade genes in the ground squirrel retina. Invest Ophthalmol Vis Sci. 1994;35:2558–2566. [PubMed] [Google Scholar]
- Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol. 1977;233:R213–221. doi: 10.1152/ajpregu.1977.233.5.R213. [DOI] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Ret Eye Res. 2011;30:115–128. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wong WT. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol. 2014;801:333–338. doi: 10.1007/978-1-4614-3209-8_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Puller C, Müller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–17. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Norman GJ, DeVries AC, Nelson RJ. The injured nervous system: a Darwinian perspective. Prog Neurobiol. 2008;86:48–59. doi: 10.1016/j.pneurobio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ER, Raman D, Shirakawa S, Ducceschi MH, Bertram PT, Wong F, Kraft TW, Osawa S. The cloning of GRK7, a candidate cone opsin kinase, from cone- and rod-dominant mammalian retinas. Mol Vis. 1998;4:27. [PubMed] [Google Scholar]
- West RW. Light and electron microscopy of the ground squirrel retina: functional considerations. J Comp Neurol. 1976;168:355–377. doi: 10.1002/cne.901680304. [DOI] [PubMed] [Google Scholar]
- West RW, Dowling JE. Anatomical evidence for cone and rod-like receptors in the gray squirrel, ground squirrel, and prairie dog retinas. J Comp Neurol. 1975;159:439–460. doi: 10.1002/cne.901590402. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Starnes CA, Twardy BS, Brault D, Taylor RC. Nuclear magnetic resonance and biochemical measurements of glucose utilization in the cone-dominant ground squirrel retina. Invest Ophthalmol Vis Sci. 2008;49:4613–9. doi: 10.1167/iovs.08-2004. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich KA, Tanimoto N, Grosche A, Zrenner E, Pekny M, Reichenbach A, Seeliger MW, Pannicke T, Perez MT. Retinal functional alterations in mice lacking intermediate filament proteins glial fibrillary acidic protein and vimentin. FASEB J. 2015;29:4815–28. doi: 10.1096/fj.15-272963. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shao C, Fedorov VB, Goropashnaya AV, Barnes BM, Yan J. Molecular signatures of mammalian hibernation: comparisons with alternative phenotypes. BMC Genomics. 2013;14:567. doi: 10.1186/1471-2164-14-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Storey KB, Yu DN, Hu Y, Zhang JY. The complete mitochondrial genome of Ictidomys tridecemlineatus (Rodentia: Sciuridae) Mito DNA. 2015:1–2. doi: 10.3109/19401736.2015.1041117. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158:2145–2151. doi: 10.1016/s0002-9440(10)64686-x. Merriman, Sajdak, Li, and Jones submission for: Experimental Eye Research Special Issue: Retinal Remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]