Abstract
Resolution of infection and inflammation is governed by innate immune cells. The resolvin family of n-3 mediators produced by resolving exudates stimulates clearance of neutrophils and attenuates pro-inflammatory signals. Using metabololipidomics, endogenous resolvin D3 (RvD3) was identified in self-resolving exudates during active E. coli infection. Through a new, independent synthetic route for RvD3, we matched endogenous and synthetic RvD3 and determined that RvD3 (ng doses) potently reduced the resolution interval (Ri) by ~4.5 h during E. coli peritonitis after administration at peak inflammation (Tmax = 12 h) and increased leukocyte phagocytosis of E. coli and neutrophils as well as reduced proinflammatory cytokines, chemokines, MMP-2 and MMP-9. At pM-nM concentrations, RvD3 also enhanced human macrophage efferocytosis and bacterial phagocytosis, increased neutrophil bacterial phagocytosis and intracellular ROS generation, and reduced human platelet-PMN aggregation. These results provide additional evidence for potent RvD3 immunoresolvent actions in host defense, host protection and antimicrobial defense.
Keywords: inflammation, infection, human leukocyte, specialized proresolving mediator
1. Introduction
Acute inflammatory responses are critical for host survival from infection and trauma [1][2]. In some disease states, the time-dependent signaling of cellular responses that promote clearance of pathogens and catabasis becomes altered so that chronic inflammation and infection persist. Anti-inflammatory therapies alone can further deregulate these responses by disrupting cytokine signaling and lipid mediator class switching [3][4].
In recent years, a number of bioactive lipid mediator families were elucidated from inflammatory exudates that possess pro-resolving and anti-inflammatory actions, named resolvins, protectins and maresins, reviewed in Ref. 1. Resolvin E1 enhances macrophage phagocytosis of E. coli [5], and resolvin D1 and resolvin D5 enhance in vivo clearance of bacteria while reducing antibiotic resistance [6]. These results prompted us to evaluate in detail the role that all resolvins play in microbial regulation in order to more fully understand their importance in acute inflammation and infection.
The complete stereochemical assignments for resolvin D3 (RvD3) was recently determined as: 4S, 11R, 17S-trihydroxydocosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid and its potent bioactions were confirmed by total organic synthesis [7][8][9]. Together, these results established RvD3 immunoresolvent actions that include inhibition of neutrophil transmigration and enhancement of macrophage phagocytosis and efferocytosis [7][8]. RvD3 exerts these actions, at least in part, due to activation of the human receptor GPR32 [8]. Here, we employed LC-MS/MS based lipid mediator (LM) metabololipidomics to identify RvD3 during infections in mice and report a new second total organic synthesis of RvD3. This total organic synthesis permits larger scale production. We also present new host protective actions for RvD3 during infection and inflammation.
2. Materials and Methods
2.1. NMR of synthetic RvD3
Data for RvD3 (7): 1H NMR (400 MHz, methanol-d4) δ 6.54 (dd, J=11.99, 13.63 Hz, 1H), 6.49 (dd, J=10.06, 15.19 Hz, 1H), 6.27 (dd, J=11.71, 13.36 Hz, 2H), 6.07 (dd, J=10.25, 11.07 Hz, 2H), 5.75 (dd, J=6.59, 14.36 Hz, 1H), 5.67 (dd,J=6.50, 15.19 Hz, 1H), 5.40–5.50 (m, 2H), 5.35 (dd, J=9.79, 10.98 Hz, 2H), 4.55–4.80 (m, 3H), 4.06–4.18 (m, 2H), 2.39–2.48 (m, 2H), 2.32–2.39 (m, 3H), 2.21–2.32 (m, 2H), 2.04 (q, J=13.70 Hz, 3H), 1.82 (td, J=7.00, 14.00 Hz, 1H), 1.72 (td, J=8.20, 13.50 Hz, 1H), 1.31–1.50 (m, 5H), 0.86–1.12 (m, 3H); UV (EtOH) λmax 238, 272, 282 nm; HPLC/UV: Gemini C18, 5 mm, 250 × 4.6mm, 272nm, MeOH/H2O (0.1% acetic acid) 70/30, 1ml/min, tR = 8.60 min; MS (m/z) 375.80 [M-H]−
Preparation of Zn (Cu/Ag) alloy: 5 gm of zinc dust was suspended in 25 ml of 2 M HCl (aq). The suspension was filtered after 2 minutes before washing with 25 ml of 2 M HCl (aq) and 50 ml of water. The zinc dust was resuspended in 25 ml of water. 500 mg of Cu (OAc)2-H2O was added followed 15 minutes later with 500 mg of AgNO3. The suspension stirred for another 15 minutes before filtering. The alloy was washed with water, methanol, acetone, and diethyl ether before drying in a desiccator.
Reduction of 6 to 7: To a suspension of Zn/Cu/Ag alloy (10 equivalents by mass) in water was added 88 mg of the alkyne 6 as a solution in methanol. The reaction was stirred at 40°C for 15 hr and was then diluted with methanol and filtered through Celite to remove the solids. Concentration to 5ml followed by overnight reaction with 0.5 M K2CO3 yielded crude RvD3 (7), which was purified by HPLC (Gemini C18, 5 µm, 250 × 21.2 mm, 270 nm, MeOH/H2O (0.1% acetic acid) 70/30, flow rate 21.2ml/min) to produce 11.4 mg. Yield over final two steps was 13%.
2.2. Acute inflammation
Male FVB mice (10–12 weeks old) purchased from Charles River Laboratories were fed ad libitum Laboratory Rodent Diet 20–5058 (Lab Diet, Purina Mills). All animal experimental procedures were approved by the Standing Committee on Animals of Harvard Medical School (protocol no. 02570) and complied with institutional and US National Institutes of Health (NIH) guidelines. Peritonitis: zymosan (1 mg/mL saline; Sigma-Aldrich) was injected intraperitoneally (i.p.) 15 min after intravenous (i.v.) administration of RvD3 (10 ng), or vehicle in 100 µL saline. Peritoneal lavages were collected 4 h after zymosan administration. Leukocyte numbers and differential counts were assessed using Turks solution and flow cytometry analysis as detailed below. Cytokine, chemokine, and enzyme levels were assessed in cell-free supernatants by multiplex membrane-based array analysis using the Proteome Profiler Mouse XL Cytokine Array Kit (R&D Systems) using manufacturer’s instructions; levels were determined by chemiluminescence and densitometry using ImageJ. Lavages were also placed in two volumes of methanol and subjected to lipid mediator metabololipidomics.
2.3. Microbial-induced mouse peritonitis
FVB mice, 6–8-week-old, purchased from Charles River Laboratories (Wilmington, MA, USA) were fed ad libitum Laboratory Rodent Diet 20-5058 (Lab Diet; Purina Mills, St. Louis, MO, USA). Mouse experimental procedures were approved by the Standing Committee on Animals of Harvard Medical School (Protocol 02570) and complied with institutional and U.S. National Institutes of Health (NIH) guidelines. E. coli (serotype O6:K2:H1) was cultured in Luria-Bertani broth and harvested at midlog phase (OD600 nm ≈ 0.5 absorbance units; 5 × 108 CFU/ml). Mice were given an intraperitoneal injection containing E. coli (1×105 CFU/mouse) and 12 h later (at peak of inflammation) mice were administered either RvD3 (50 ng/mouse) or vehicle (saline + 0.1% ethanol). Peritoneal exudates were collected at indicated time intervals. Cellular composition was determined by differential leukocyte count and flow cytometry. For flow cytometry, cells were labeled with fluorescently conjugated antibodies against mouse surface CD11b (clone M1/70; eBioscience, San Diego, CA, USA), F4/80 (clone BM8; eBioscience), Ly6G (clone RB6-8C; eBioscience), and intracellular stained with anti-E. coli antibody (clone GTX408556; GeneTex, Irvine, CA, USA). Resolution indices were calculated: Ψmax (maximal PMN counts in the exudates); Tmax (the time interval when PMN numbers reach maximum); T50 (the time interval when PMN numbers reach 50% of maximum, or Ψ50) and Ri (resolution interval, the interval between Tmaz and T50), as in [10].
2.4. Flow cytometry
Murine peritoneal exudate cells were suspended in fluorescence-activated cell sorting (FACS) buffer (5% BSA in DPBS) and incubated with FC block (15 min, 4°C; BD PharMingen) and then rat antimouse from eBioscience CD11b-PerCP/Cy5.5 (clone M1/70), F4/80 (clone BM8; eBioscience), and Ly6G-fluorescein isothiocyanate (FITC) (clone RB6-8C; eBioscience) (30 min, 4°C) or appropriate isotype controls. Staining was assessed using FACSDiva Canto II (BD Biosciences) and analyzed using FlowJo (TreeStar).
2.5. Macrophage and PMN phagocytosis, efferocytosis, and phagolysosomal acidification
Human PMN were isolated by density-gradient Ficoll-Histopaque from human peripheral blood. Blood was obtained from healthy human volunteers giving informed consent under protocol # 1999-P-001297 approved by the Partners Human Research Committee. Apoptotic neutrophils were prepared by plating 1 × 107 cells/mL in 5 mL DPBS+/+ for 24 hr in 100 mm × 20 mm petri dish. Macrophages were prepared from peripheral blood mononuclear cells (PBMCs) purchased from Children’s Hospital Blood bank. PBMCs collected from healthy donors were differentiated using GM-CSF (20 ng/mL) in RPMI culture media (Lonza) containing 10% FBS (Invitrogen), 5 mM L-Glutamine (Lonza), and 5% penicillin and streptomycin (Lonza) for 7 days. Cells were then plated in 96-well plate at 5 × 104 cells/well overnight before experiments.
Human macrophages or PMN were pre-incubated with vehicle (DPBS+/+) or RvD3 (1 pM–10 nM) for 15 minutes at 37°C before addition with 1:50 fluorescently labeled E. coli (5 × 108 c.f.u. /mL) (7 µg/mL, BacLight, Molecular Probes) or 1:3 fluorescently labeled apoptotic PMN (10 ng/mL, bisbenzimide H 33342, Sigma Aldrich) for 1 hour. Plates were gently washed, extracellular fluorescence quenched using 1:15 trypan blue, and phagocytosis determined measuring total fluorescence (E. coli, excitation 480 nm/emission 516 nm; apoptotic PMN, excitation 355 nm/emission 465 nm) using a fluorescent plate reader (Molecular Probes).
PMN phagolysosomal acidification was assessed by incubating macrophages with pHrodo dye (Invitrogen) following the manufacturer’s instructions. Subsequently, cells were incubated with 0.01–10 nM of test compounds (15 min at 37°C), E. coli (2.5 × 106 CFU/well) were added, and fluorescence was assessed after 60 min (37°C) using a BZ9000 microscope equipped with a 320 objective (Keyence, Itasca, IL, USA) and a fluorescence plate reader.
2.6. Human PMN-platelet interactions in whole blood
Fresh human blood was collected in acid citrate–dextrose (Sigma-Aldrich) from healthy volunteers giving informed consent under protocol # 1999-P-001297 approved by the Partners Human Research Committee. Immediately after collection, 500 µL of whole blood were incubated with RvD3 (1–100 nM) or vehicle (0.05% EtOH in PBS) for 15 min at 37°C, followed by addition of PAF (500 nM) or vehicle (0.05% EtOH in PBS) and incubations continued for 20 min. To assess PMN-platelet interactions samples were stained with mouse anti-human CD16-PE and mouse anti-human CD41-FITC (20 min, RT), red blood cells lysed (RBC lysis buffer (BD); 10 min) and cells washed. Staining was assessed immediately using FACS Canto II (BD Biosciences) and analyzed using FlowJo (TreeStar).
2.7. Human PMN-platelet incubations
Human PMN and platelets were isolated by density-gradient Ficoll-Histopaque from human peripheral blood collected in acid citrate-dextrose (Sigma Aldrich). Human PMN (1×106) were incubated with 10 nM RvD3 or vehicle (0.1% ethanol in DPBS, 15 min, 37°C) followed by addition of PAF (500 nM) and platelets (1:5 PMN to platelets, 20 min, 37°C). Incubations were stopped with ice-cold MeOH containing deuterium labeled internal standards and taken to LC-MS-MS-based LM metabololipidomics following solid phase extractions (see detailed below).
2.8. Lipid mediator metabololipidomics
All samples for LC-MS-MS-based lipidomics were subject to solid-phase extraction as described [11]. Prior to sample extraction, d4-LTB4 and d4-PGE2 internal standards (500 pg each) were added to facilitate quantification. Extracted samples were analyzed by a liquid chromatography-ultraviolet-tandem mass spectrometry system, QTrap 5500 (AB Sciex) equipped with a Shimadzu LC-20AD HPLC (Tokyo, Japan). A Poroshell 120 EC-18 column (100 mm × 4.6 mm × 2.7 µm; Agilent Technologies, Santa Clara, CA, USA) was kept in a column oven maintained at 50°C, and lipid mediators (LMs) were eluted with a gradient of methanol/water/acetic acid from 55:45:0.01 (v/v/v) to 100:0:0.01 at 0.5 mL/min flow rate. To monitor and quantify the levels of targeted LMs, multiple reaction monitoring (MRM) was used with MS/MS matching signature ion fragments for each molecule (six diagnostic ions and calibration curves) as in [11].
2.9. Statistics
All data are expressed as means ± SEM. Differences between groups were compared using Student’s t test (two groups) and one-way ANOVA (more than two groups). The criterion for statistical significance was p < 0.05.
3. Results and Discussion
3.1. A new total organic synthesis of RvD3
Biosynthesis of RvD3 results from sequential lipoxygenase reactions starting with insertion of molecular oxygen at the C-17 position of docosahexaenoic acid (DHA) [12] followed by peroxidation of C-4 leading to 4S, 5S-epoxidation and 11S hydroxylation (Figure 1A). Resolvin D3 was prepared as shown in (Figure 1B). Our strategy utilizes two Sonogashira reactions (Supplementary Figure 1), each one followed by a selective reduction to install the conjugated diene and then the conjugated triene. The configuration of the alcohols at C-11 and C-17 are derived from commercially available chiral pure starting materials ((S)-glycidol and (R)-glycidol, respectively) and the alcohol at C-4 from an enantioselective reduction of a propargyl ketone. Reaction of butynyl lithium with (R)-glycidol protected as its THP ether, followed by P-2 nickel semi-hydrogenation, silyl protection of the secondary alcohol, deprotection of the THP ether, Swern oxidation and then Takai olefination yielded iodide 1 [13][14][15][16].
Figure 1. Biosynthesis of RvD3 and retrosynthetic scheme.
Palladium (0) mediated cross-coupling of 1 with 2 (generated from (S)-glycidol, as shown in Supplementary Figure 2) [7] yielded the alkyne which was reduced to the conjugated Z,E diene using P-2 nickel semi-hydrogenation. Selective deprotection of the primary alcohol followed by Swern oxidation produced the aldehyde, which was transformed into the iodo diene 3 as shown in (Supplementary Figure 3).
For the final Sonogashira step, 3 was coupled to 4 (generated from the enantioselective reduction of the propargyl ketone as shown in (Supplementary Figure 4)) to produce the silyl protected 5,6-dehydro-RvD3 methyl ester 5 (Supplementary Figure 5). Deprotection using TBAF resulted in a mixture of the expected methyl ester product 6, the free acid, and the γ-lactone. The crude mixture was treated with TMS-diazomethane before flash chromatography. The γ-lactone and methyl ester were not separated.
Reduction of the mixture of 6 and the γ-lactone using Boland’s Zn/Cu/Ag alloy in methanol/water yielded the methyl ester of RvD3 in 13% yield. Hydrolysis of the ester produced the free acid of RvD3 7 (as shown in Supplementary Figure 6) which was purified by preparative HPLC to provide RvD3 in greater than 95% purity.
3.2. Matching of endogenous RvD3 with synthetic material
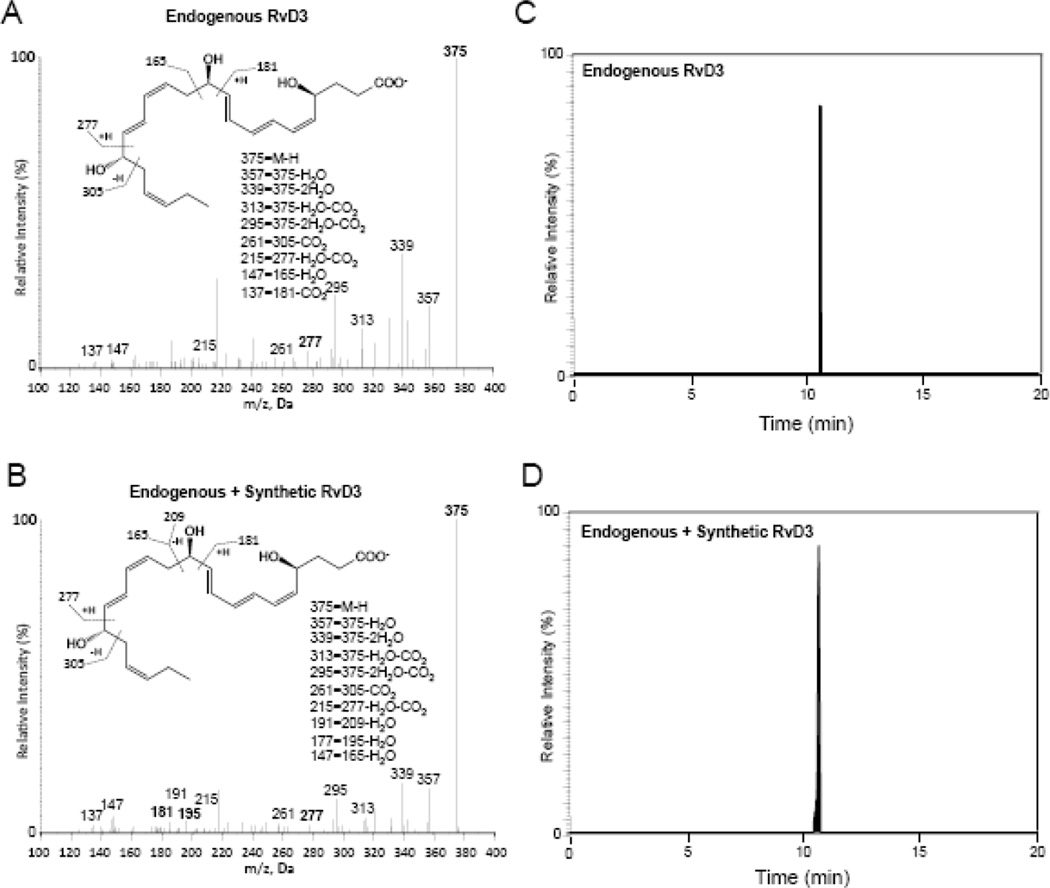
RvD3 is produced endogenously in sterile inflammatory exudates [8][17][12]. To extend these findings to infection, we first identified the presence of RvD3 in murine inflammatory peritoneal exudates after 24 h intraperitoneal administration of E. coli using LC-MS/MS metabololipidomics (Figure 2A). Material prepared by total organic synthesis (described above) contained the characteristic conjugated triene and diene chromophores corresponding to triplet λmaxMeOH ~260, 271, 281 nm and ~236 nm absorbance bands, respectively (Supplementary Figure 7 inset). Synthetic material and endogenous RvD3 from infectious exudates both gave a sharp peak in liquid chromatography with retention time (Tr) = 10.7 min (Figure 2C,D). Endogenous RvD3 and synthetic material both gave fragmentation patterns with m/z 375 = M-H, m/z 357 = M-H-H2O, m/z 339 = M-H-2H2O, m/z 313 = M-H-CO2-H2O, m/z 295 = M-H-CO2-2H2O, m/z 261 = 305-M-H-CO2 m/z 215 = 249-CO2, m/z 177 = 195-H2O, m/z 147 = 165-H2O, and m/z 137 = 181-CO2. These results confirmed the stereochemistry and structure of synthetic material produced by a new total synthetic route matched with authentic RvD3 and that RvD3 is produced endogenously during active bacterial infection in vivo.
Figure 2. RvD3 is produced in infectious exudates and matches synthetic compound.
Endogenous RvD3 was obtained from mice inoculated with E. coli (105 CFU; i.p.) and exudates collected at 24 hr. Exudates and synthetic material were subjected to lipid mediator metabololipodomics. (A and B) MS/MS spectrum for (A) endogenous RvD3; (B) coinjection of endogenous RvD3 from resolving exudate and synthetic RvD3. (C and D) Selected ion chromatograms (m/z 375-147) depict (C) murine-resolving exudate-derived RvD3 and (D) coinjection with synthetic material. Representative MRM chromatograms and MS-MS spectra (n=3).
3.3. RvD3 potently attenuates exudate neutrophil levels and pro-inflammatory proteins
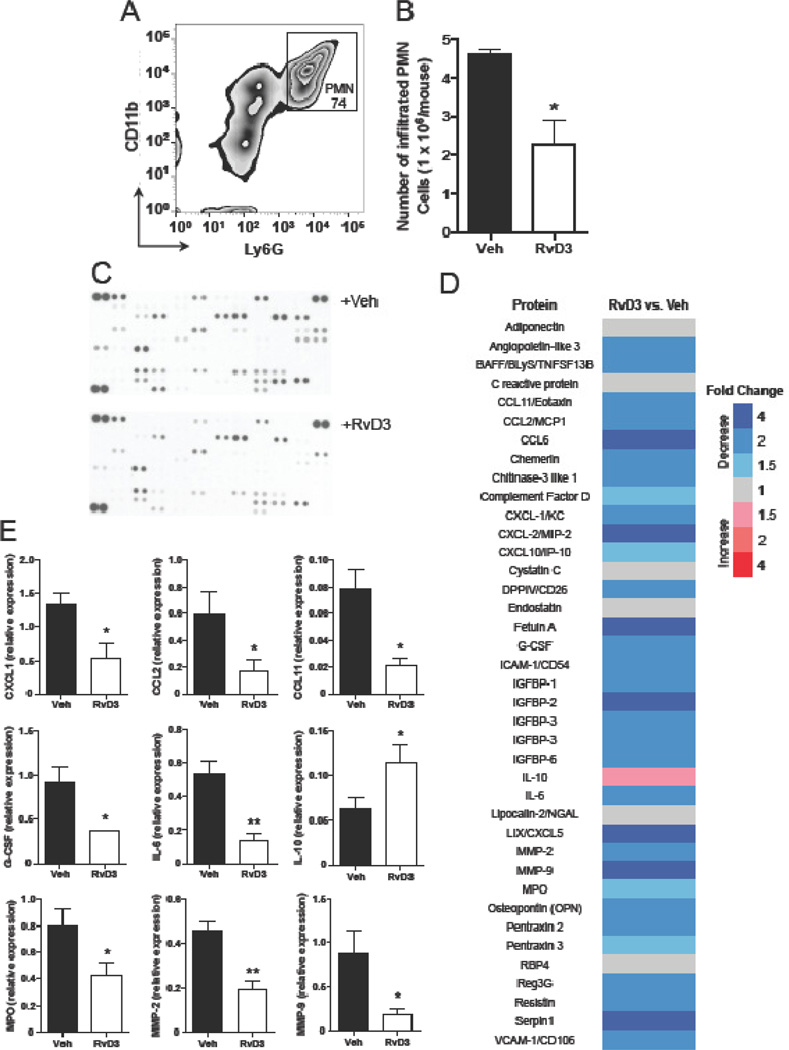
Earlier, RvD3 was found to limit neutrophil numbers and reduce pro-inflammatory cytokines, prostaglandins (PG), and leukotrienes (LT) in zymosan-induced peritonitis in mice [8][12]. In order to confirm that the newly synthesized RvD3 retains this bioactivity, we subjected mice to zymosan peritonitis (1 mg/mouse; i.p.) after 15 min pretreatment with new synthetic RvD3 (10 ng/mouse; i.v.) or saline control. After 4 h zymosan administration, neutrophil (PMN) levels were reduced by ~45% (Figure 3A–C), and the pro-inflammatory lipid mediators LTB4, PGD2, and thromboxane B2 (TxB2) were also significantly reduced (Supplementary Figure 8).
Figure 3. RvD3 potently stops neutrophil recruitment and reduces proinflammatory cytokines, chemokines, and enzymes.
RvD3 (10 ng/mouse) or vehicle (saline) was administered i.v. 15 min prior to i.p. injection of zymosan (1 mg/mouse). Exudates were collected 4 hr later. (A and B) Cells were enumerated and PMN were identified using flow cytometry. (C–E) Pro-inflammatory cytokines, chemokines and enzymes were measured using the Proteome Profiler Mouse XL Cytokine Array Kit (R&D Systems). Results are mean ± SEM, n=3–4 mice per group. *P < 0.05, **P<0.01 versus zymosan plus vehicle.
Proteomic analysis of zymosan exudates revealed a global reduction of pro-inflammatory proteins, including cytokines, chemokines, and enzymes with RvD3 treatment vs. vehicle (Figure 3D–E). Of interest, the neutrophil chemoattractant, CXCL1/KC, as well as G-CSF and the neutrophil marker, MPO, were significantly reduced (Figure 3E); consistent with the observed decrease in neutrophil numbers. Other leukocyte chemoattractants (CCL2/MCP-1 and CCL11/eotaxin) as well as matrix-degrading enzymes (MMP2 and MMP9) were also significantly reduced (Figure 3E). These results confirmed the bioactive profile of synthetic RvD3 matching that of RvD3 established earlier [8][12].
3.4. RvD3 enhances bacterial clearance and efferocytosis during infection
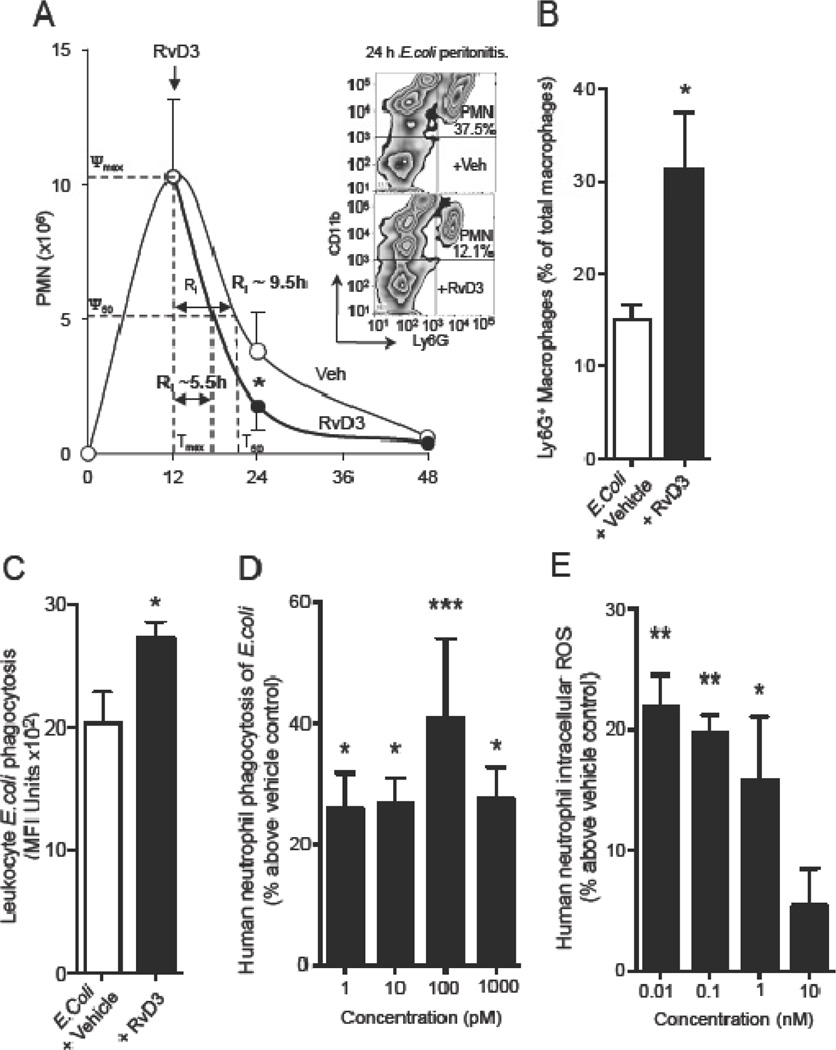
Based on the potent proresolving actions of RvD3 in sterile inflammation and its identification in resolving exudates from mice with active E. coli infection, it was important to determine if RvD3 also carries proresolving and antimicrobial actions during infection. Mice were inoculated with active E. coli (105 C.F.U.; i.p.) followed by administration of RvD3 (50 ng/mouse; i.p.) or saline control 12 h later (Figure 4A). Peritoneal exudates were removed at various times within 48 h and PMN were enumerated to assess previously established resolution indices [10]. RvD3 reduced PMN numbers at 24 h post inoculation and reduced the time to half maximal PMN recruitment (resolution interval; Ri) from ~9.5 h to ~5.5 h, which included a ~50% increase in the proportion of macrophages undergoing phagocytosis of PMN (Figure 4B) and increased the number of leukocytes undergoing phagocytosis of E. coli (Figure 4C). These results show that RvD3 is able to potently stimulate proresolving and antimicrobial actions after the onset of infection.
Figure 4. Resolvin D3 stimulates infectious-resolution by enhancing phagocyte microbial clearance.
(A–C) Mice were inoculated with E. coli (1×105 CFU per mouse, i.p.) followed by either RvD3 (50 ng/mouse, i.p.) or vehicle (saline containing 0.1% EtOH) 12 h later. (A) Exudate PMN numbers and resolution indices were determined (Material and Methods); Inset shows representative flow cytometry zebra plot; PMNs identified as CD11b+Ly6G+ events (n=4). In vivo (B) increased macrophage efferocytosis identified as Ly6G+ macrophages and (C) increase in leukocyte E. coli phagocytosis in 24 h peritoneal exudates. Results are mean ± SEM from n = 4 mice for 12 h and treatment with vehicle, n = 6 for treatment with RvD3. *P < 0.05 vs E. coli peritonitis mice. Human neutrophils (1 × 105 cells per well) were (D) incubated with RvD3 at indicated concentrations (10−12 to 10-9 M for 15 min at 37°C) or (E) pre-incubated with H2-CFFDA (5µM, 30 min, 37°C) prior to addition of RvD3 (10−11 to 10−8 M for 15 min at 37°C), followed by addition of fluorescently labeled E. coli (45 min, 37°C) and (D) phagocytosis or (e) intracellular ROS assessed with SpectraMax M3 plate reader. Results are mean ± SEM for (d) n= 4 or (e) n=3 neutrophil donors. *P < 0.05, ***P < 0.001 vs. vehicle group.
3.5. RvD3 stimulates proresolving responses in human tissues
Given the potent antimicrobial actions of RvD3 with murine leukocytes, we next determined if RvD3 enhanced these actions in human leukocytes. Fresh human neutrophils obtained from healthy donors were treated with 1–1000 pM RvD3 for 15 min before addition of fluorescently labeled E. coli for 45 min. A significant increase in PMN phagocytosis of E. coli (over 20% vs. control) was observed at each concentration of RvD3 administered across the pM range (Figure 4D). Additionally, intracellular reactive oxygen species (ROS) production was significantly increased with RvD3 (over 15% vs. control) at sub-pM to low pM concentrations of RvD3.
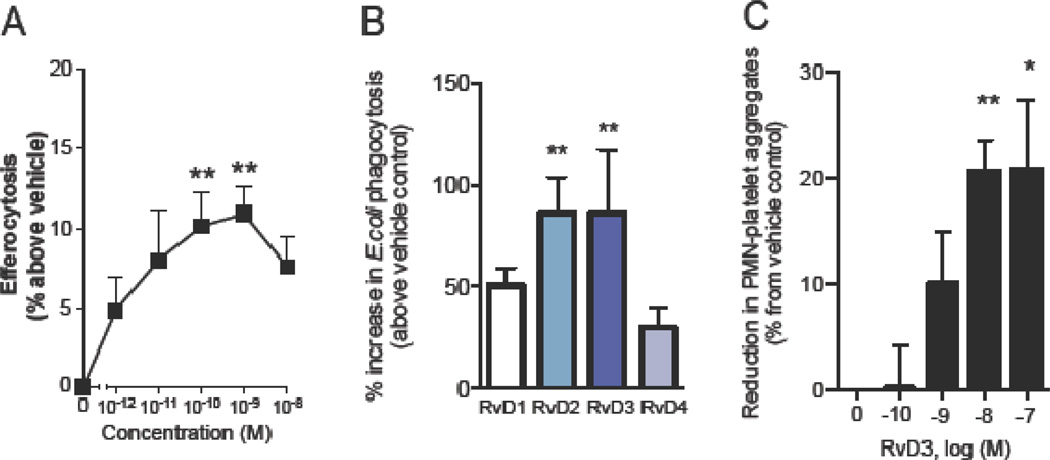
RvD3 dose-dependently enhanced phagocytosis of apoptotic PMN by human macrophages (from 7 day 20 ng/mL GM-CSF differentiated PBMCs) with significant increases in the high pM to low nM range (Figure 5A). To test the relative potency of RvD3 vs. other D-series resolvins on enhancement of phagocytosis, macrophages were treated with RvD1–4 each at 10 nM; 15 min before addition of fluorescently labeled E. coli. Highest increases in phagocytosis vs. control were observed with RvD2 and RvD3; both at ~80% over control (Figure 5B).
Figure 5. Proresolving actions of RvD3 in human cells.
Enhanced macrophage phagocytosis and efferocytosis. Human macrophages (derived from 7 d, 20 ng/mL GMCSF differentiated PBMCs) were incubated with RvD3 (0–10 nM) for 15 min at 37° C followed by addition of (A) fluorescently-labeled E. coli (1:50) or (B) fluorescently-labeled apoptotic PMN (1:50) for 1 hr. Results are mean ± SEM of n=4. **P < 0.01, ***P < 0.001 versus vehicle.
Based on the observation that RvD3 significantly reduced LTB4 and thromboxane levels during zymosan peritonitis (Supplementary Figure 8C), we tested whether RvD3 could reduce platelet-leukocyte interactions. Fresh human whole blood was obtained from healthy donors and was pretreated with RvD3 or vehicle prior to activation with 10 nM platelet aggregating factor (PAF). Using flow cytometry analysis, 10 and 100 nM RvD3 treated blood samples were found to have significantly reduced aggregates (~20% less than control) (Figure 5C). We then isolated fresh PMN and platelets from healthy donors to assess direct responses of RvD3 and activated platelets on PMN eicosanoid production. PMN were pretreated with 10 nM RvD3 or control before addition of platelets activated with 10 nM PAF (Figure 6). Significant decreases in LTB4 (~45%) and PGD2 (~20%) were found in incubations with RvD3 treatment vs. control. Together, these results demonstrate that the proresolving, anti-inflammatory, and antimicrobial actions of RvD3 in murine infection and inflammation are also potently effective on human leukocytes.
Figure 6. Resolvin D3 reduces eicosanoid production in PAF+platelet stimulated human PMN.
Peripheral blood PMN were isolated from healthy volunteers and incubated with RvD3 (10 min, 37°C) followed by addition of PAF (10 nM) and platelets (1:5 PMN:platelets). Incubations were stopped after 30 min with ice-cold MeOH containing deuterium labeled internal standards and LM assessed using LC-MS-MS based LM metabololipidomics following solid phase extraction. (A) Representative MRM chromatograms of selected ion pairs for arachidonic acid-derived eicosanoids. (B) Representative MS-MS spectra with diagnostic ions employed for the identification of LTB4 and PGF2α (see Material and Methods). (C) Reduction in PAF+platelet stimulated eicosanoids with RvD3 treatment is expressed as % reduction from vehicle plus PAF and platelets. Results are mean ± SEM from n = 4 healthy donors. ***P < 0.001, ****P < 0.0001 vs. vehicle plus PAF and platelets.
Here, we identified endogenous RvD3 from infectious-inflammatory exudates using lipid mediator metabololipidomics. RvD3 accelerated resolution of E. coli infections in part by increasing macrophage efferocytosis and phagocytosis of bacteria providing new evidence for the role of RvD3 in host protection. In human leukocytes, RvD3 potently enhanced bacterial phagocytosis as well as the intraphagolysosomal generation of reactive oxygen species necessary for efficient bacterial killing.
The ability of RvD1 and RvD2 to each increase mouse survival after E coli inoculation or sepsis has been documented [6][18], and suggests a crucial role for this family of lipid mediators in the containment and limitation of leukocyte mediated collateral tissue damage associated with pathogenic diseases [8][12]. We also report herein an independent second total organic synthesis of RvD3 that permits scale up of this resolvin. The RvD3 produced by this second route matched the bioactivity and physical properties of biologic RvD3. Taken together, these results underscore the mechanisms of resolvins and specifically RvD3 in host defense, pro-resolution and antiinflammation via the control of pro-inflammatory cytokines and other pro-inflammatory lipid mediators involved in regulating immunity during infection.
Supplementary Material
Acknowledgments
The authors thank Mary Halm Small for expert assistance in manuscript preparation.
Grant support: This work was supported in part by the U.S. National Institutes of Health (grant # P01GM095467).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
CNS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler JW, Uddin J, Serhan CN, Petasis NA. Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17 R-epimer. Org. Lett. 2013;15:1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haeggström JZ, Hamberg M. Resolving resolvins. Chem. Biol. 2013;20:138–140. doi: 10.1016/j.chembiol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 11.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolaou KC, Ramphal JY, Abe Y. Stereocontrolled total synthesis of (5Z,8Z,10E,12R,14Z)-12-hydroxy-5,8,10,14-eicosatetraenoic acid [(12R)-HETE] Synthesis (Stuttg) 1989:898–901. [Google Scholar]
- 14.Rodriguez A, Nomen M, Spur BW, Godfroid JJ, Lee TH. Total synthesis of 12(R)-HETE, 12(S)-HETE, 2H2-12(R)-HETE and LTB4 from racemic glycidol via hydrolytic kinetic resolution. Tetrahedron. 2001;57:25–37. [Google Scholar]
- 15.Takai K, Nitta K, Utimoto K. Simple and selective method for aldehydes (RCHO) → (E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system. J. Am. Chem. Soc. 1986;108:7408–7410. [Google Scholar]
- 16.Rodriguez AR, Spur BW. First total synthesis of the E type I phytoprostanes. Tetrahedron Lett. 2003;44:7411–7415. [Google Scholar]
- 17.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 18.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis Na, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.