Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, with about 30% of new cases presenting with extranodal disease. Lesions originating from soft tissues of the upper extremities are extremely rare and may mimic other malignancies like sarcoma. We present a case of an elderly patient with right upper extremity (RUE) mass which was proven to be DLBCL instead of sarcoma. We emphasize the increasing need for investigating new therapeutic options for patients of extreme age and/or with underlying heart disease.
Keywords: Diffuse large B-cell lymphoma (DLBCL), right upper extremity (RUE), targeted agents
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common amongst non-Hodgkin lymphomas, accounting for approximately 30% of all cases. Most of the times, disease primarily occurs in the lymph nodes, but extranodal involvement is not uncommon. About 30% of DLBCL cases present with disease outside the lymph nodes, which may incur different clinicopathologic features and prognosis for these patients (1-3). Cases involving soft tissues often mimic other entities like sarcoma. Treatment options remain the same for both nodal and extranodal DLBCL, with the first choice being chemoimmunotherapy including anthracyclines (4-10). Age and preexisting medical conditions are the main limiting factors in planning the treatment. Regimens including alternatives to the cardiotoxic therapies have been investigated. Phases I and II studies incorporating the liposomal form of doxorubicin showed promising results for the patients who would otherwise be deprived of the most effective therapies (11-13). We present a case of an elderly patient with right upper extremity (RUE) mass which was proven to be DLBCL instead of sarcoma. We emphasize the increasing need for investigating new therapeutic options for patients of extreme age and/or with underlying heart disease.
Case presentation
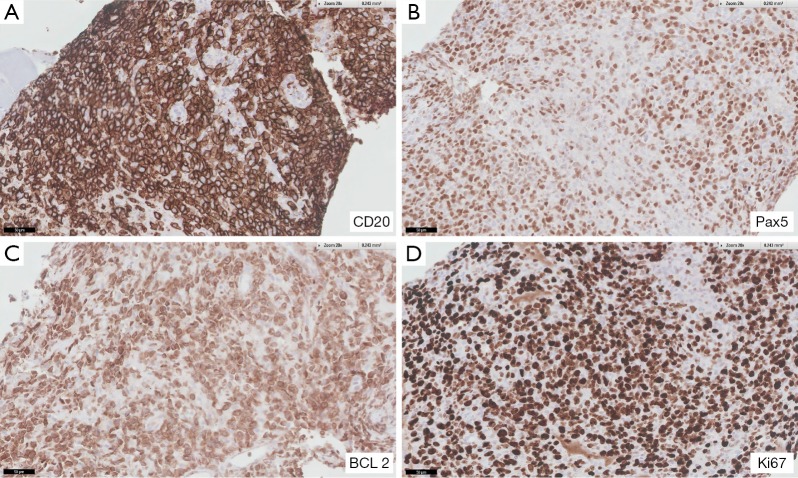
The patient was a 90-year-old male who was referred for evaluation of a RUE mass. The patient and family reported decreased appetite and fatigue, as well as weight loss of about 20 pounds in the preceding 6 months. Patient’s past medical history was significant for hypertension, cerebrovascular accident with residual expressive aphasia and benign prostate hyperplasia. Located in the anterior aspect of the right upper arm, just above the antecubital fossa, the mass was described as a large violaceous fleshy tumor of approximately 10 cm × 7 cm in size. Not tender, with intact skin, the lesion was associated with mild RUE edema. Three months earlier patient was evaluated by surgery for the same lesion. The ultrasound study of the lesion showed heterogeneous mass or collection of about 7.8 cm × 4.6 cm × 5.2 cm in size, MRI study showed a mass of 10.1 cm × 5.3 cm × 6.8 cm within the musculature of the right arm, as well as additional 1.4 cm × 1.9 cm × 2.5 cm focus surrounding the brachioradialis neurovascular bundle, which was in contiguity with the larger mass. The radiological picture was highly suggestive of soft tissue sarcoma or other malignant neoplasm. At this time, patient was reluctant to pursue further work up and was noncompliant with follow up. After presenting to oncology the patient was referred for biopsy of the mass, which again he did not pursue. Two months later the patient was admitted to the hospital; the mass was then fungating and ulcerated, with purulent discharge. Patient was managed for wound infection. MRI of the RUE showed interval growth and ulceration of the mass lesion measuring 15.4 cm × 4.9 cm × 7.7 cm, encasing the brachial vessels, as well as possible extension into the axilla with involvement of the brachial plexus. Biopsy was performed during the hospital stay and the H&E sections revealed multiple cores with diffuse proliferation of atypical lymphocytes with focal crush/degeneration artifacts and areas of tumor necrosis. Scattered entrapped skeletal muscle fibers were seen in focal areas. The atypical lymphocytes were large in size and had moderate cytoplasm, round to irregular nuclear contour, some indented, clumped to fine vesicular chromatin and some with one to multiple nucleoli. Mitosis/apoptosis were easily seen. Scattered small reactive lymphocytes were seen in the background. The atypical lymphocytes were diffusely positive for CD20, Pax5 and BCL2 (Figure 1), while they were negative for CD3, CD5, CD10, BCL6, BCL1, MUM-1, CD30, CD56, CD34, pancytokeratin (AE1/AE3), MyoD1, and HMB-45. CD3 and CD5 highlighted background reactive small T-cells. Ki-67 stain showed high nuclear proliferation index (85–90% of neoplastic cells were positive). The findings were consistent with DLBCL, activated B-cell like (ABC). Staging work up was performed. CT of the chest, abdomen and pelvis did not reveal any changes suspicious for disease spread, however bone marrow biopsy showed minimal involvement with B-cell lymphoma. Upon presentation patient had hemoglobin level 12.6 g/dL, white blood cell count (WBC) of 6,800/mm3 with predominance of neutrophils (88%) and a normal platelet count (207 K/mm3). LDH was 337 U/L. Echocardiography revealed left ventricular ejection fraction (LVEF) of 45%. His Eastern Cooperative Oncology Group (ECOG) performance status was 2. After extensive discussion with the patient and the family, chemotherapy with R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) was advised with modifications. Due to decreased LVEF and advanced age the regimen included the pegylated liposomal formulation of doxorubicin given at the dose of 30 mg/m2, based on regimens cited in the literature (11,12). The remaining medications were administered at standard doses. After the first cycle of chemotherapy there was some noticeable improvement in the extent of the RUE lesion. However, when blood counts decreased, the patient’s overall status started to deteriorate and his activity and oral intake significantly declined. The patient and the family desired for comfort and supportive care only. Therefore the patient was discharged to a hospice facility.
Figure 1.
Immunohistochemical studies of the specimen from core biopsies of the right upper extremity mass. The specimens were positive for CD20, Pax5, BCL2, and Ki67. The magnifications are 20× for the figures.
Discussion
The analysis of the Surveillance, Epidemiology End Results Database between 2004 and 2009 identified 31.6% of DLBCL cases presenting with primary extranodal involvement. Gastrointestinal tract was the most common site (10.7%), followed by head and neck (4.3%), with skin and soft tissue representing 3.3% of cases. In the same population study extranodal presentation is related with older age, early presentation and, depending on location, incurs different prognosis. Better outcomes were observed with head and neck involvement, whereas worse outcomes with GI tract, liver, pancreas and lung involvement. In a single institution report combined with meta-analysis of published cases and case series (2), authors included a total of 83 patients with primary extranodal lymphoma. The most common subtype was DLBCL. Authors observed a trend towards inferior outcomes in DLBCL compared to indolent lymphomas and increased chance of CNS relapse. Because of the limitations of the data (lacking information about prognostic factors) and small sample size, no conclusion about best treatment options could have been made. In another single institution study of 262 patients, authors compared outcomes of the nodal and extranodal DLBCL before and after introduction of the immunotherapy with rituximab to standard treatment (3). Authors point to the fact that the group with the greater benefit from the addition of rituximab was the primary nodal involvement DLBCL, emphasizing potential clinicobiological differences between the two groups, like IPI (international Prognostic Index) scores at presentation or single gene alterations (3,14). Cases involving soft tissues often mimic other entities like sarcomas, malignant melanoma, or metastatic carcinomas (14-22). In our case, the initial presentation, imaging studies, as well as fast growth of the lesion raised strong suspicion of a soft tissue sarcoma. Our patient presented with high risk disease. The first line therapy for this type of high risk DLBCL recommended by the National Comprehensive Cancer Network is R-CHOP or dose-adjusted R-EPOCH (4-8,23,24). However, about 50% of patients are above 60 years old at the time of diagnosis. Therefore, regimens including alternatives to the cardiotoxic therapies have been investigated. Phase I and II studies incorporating liposomal form of doxorubicin showed promising results (11-13,23,24). The pegylated form of doxorubicin compared to traditional doxorubicin has longer half-life, reduced volume of distribution, as well preferential distribution into neoplastic tissues, at the same time carrying the benefit of lower toxicity and improved therapeutic activity (12,13). Other measures which might be implemented in order to reduce cardiac toxicity are to treat the patient with conventional doxorubicin in continuous infusion over 72–96 h (25) and to use dexrazoxane (26). The use of the latter however, raised concerns of increased myelotoxicity and possible tumor protective effect. Its administration in the setting of chemotherapy induced cardiomyopathy is currently limited to women with breast cancer, who had received a cumulative dose of doxorubicine of 300 mg/m2. Regimen with decreased doses of cyclophosphomide, doxorubicin, vincristine and prednisone (R-miniCHOP) is another possibility for frail patients older than 80 years old (27). In the patient presented in our report, the regimen containing liposomal doxorubicin was a reasonable choice in attempt to control an advanced, high-risk disease in an elderly patient with deteriorating performance status and decreased cardiac function. However, all of the above therapies carry a burden of significant toxicities and there is an increasing need for novel targeted agents for the treatment of the population of elderly and frail patients (28-34).
Conclusions
This patient represents an extremely rare case of RUE lymphoma, mimicking soft tissue sarcoma. This elderly patient with poor performance status and decreased cardiac function had limited treatment options. Novel targeted agents should be explored for this population of patients
Acknowledgements
None.
Informed Consent: Written informed consent was obtained and available for review by the editor-in-chief.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: an analysis of the Surveillance, Epidemiology and End Results database. Am J Hematol 2014;89:310-4. 10.1002/ajh.23638 [DOI] [PubMed] [Google Scholar]
- 2.Derenzini E, Casadei B, Pellegrini C, et al. Non-hodgkin lymphomas presenting as soft tissue masses: a single center experience and meta-analysis of the published series. Clin Lymphoma Myeloma Leuk. 2013;13:258-65. 10.1016/j.clml.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-García G, Colomo L, Villamor N, et al. Clinico-biological characterization and outcome of primary nodal and extranodal diffuse large B-cell lymphoma in the rituximab era. Leuk Lymphoma 2010;51:1225-32. 10.3109/10428194.2010.483301 [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. 10.1182/blood-2010-03-276246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005;23:4117-26. 10.1200/JCO.2005.09.131 [DOI] [PubMed] [Google Scholar]
- 6.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22. 10.1016/S1470-2045(11)70235-2 [DOI] [PubMed] [Google Scholar]
- 7.Purroy N, Bergua J, Gallur L, et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. Br J Haematol 2015;169:188-98. 10.1111/bjh.13273 [DOI] [PubMed] [Google Scholar]
- 8.Salit RB, Fowler DH, Wilson WH, et al. Dose-adjusted EPOCH-rituximab combined with fludarabine provides an effective bridge to reduced-intensity allogeneic hematopoietic stem-cell transplantation in patients with lymphoid malignancies. J Clin Oncol 2012;30:830-6. 10.1200/JCO.2011.37.0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol 2008;26:2717-24. 10.1200/JCO.2007.13.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica 2012;97:758-65. 10.3324/haematol.2011.056531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martino R, Perea G, Caballero MD, et al. Cyclophosphamide, pegylated liposomal doxorubicin (Caelyx), vincristine and prednisone (CCOP) in elderly patients with diffuse large B-cell lymphoma: results from a prospective phase II study. Haematologica 2002;87:822-7. [PubMed] [Google Scholar]
- 12.Zaja F, Tomadini V, Zaccaria A, et al. CHOP-rituximab with pegylated liposomal doxorubicin for the treatment of elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma 2006;47:2174-80. 10.1080/10428190600799946 [DOI] [PubMed] [Google Scholar]
- 13.Rafiyath SM, Rasul M, Lee B, et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol 2012;1:10. 10.1186/2162-3619-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Zhang F, Fang H, et al. Clinicopathologic features of primary lymphoma in soft tissue. Leuk Lymphoma 2010;51:2039-46. 10.3109/10428194.2010.520049 [DOI] [PubMed] [Google Scholar]
- 15.Comez G, Goktepe MF, Oztuzcu S, et al. The isolated extranodal relapse of the isolated extranodal non- Hodgkin lymphoma: A case report. J Cancer Res Ther 2015;11:645. 10.4103/0973-1482.147706 [DOI] [PubMed] [Google Scholar]
- 16.Damron TA, Le MH, Rooney MT, et al. Lymphoma presenting as a soft tissue mass. A soft tissue sarcoma simulator. Clin Orthop Relat Res 1999;221-30. 10.1097/00003086-199903000-00026 [DOI] [PubMed] [Google Scholar]
- 17.Knowles B, Serpell JW. Extra-nodal lymphoma presenting as a mimic of soft-tissue sarcoma. ANZ J Surg 2003;73:26-30. 10.1046/j.1445-2197.2003.02613.x [DOI] [PubMed] [Google Scholar]
- 18.Lanham GR, Weiss SW, Enzinger FM. Malignant lymphoma. A study of 75 cases presenting in soft tissue. Am J Surg Pathol 1989;13:1-10. 10.1097/00000478-198901000-00001 [DOI] [PubMed] [Google Scholar]
- 19.O'Neill JK, Devaraj V, Silver DA, et al. Extranodal lymphomas presenting as soft tissue sarcomas to a sarcoma service over a two-year period. J Plast Reconstr Aesthet Surg 2007;60:646-54. 10.1016/j.bjps.2006.03.040 [DOI] [PubMed] [Google Scholar]
- 20.Salamao DR, Nascimento AG, Lloyd RV, et al. Lymphoma in soft tissue: a clinicopathologic study of 19 cases. Hum Pathol 1996;27:253-7. 10.1016/S0046-8177(96)90065-9 [DOI] [PubMed] [Google Scholar]
- 21.Suresh S, Saifuddin A, O'Donnell P. Lymphoma presenting as a musculoskeletal soft tissue mass: MRI findings in 24 cases. Eur Radiol 2008;18:2628-34. 10.1007/s00330-008-1014-x [DOI] [PubMed] [Google Scholar]
- 22.Travis WD, Banks PM, Reiman HM. Primary extranodal soft tissue lymphoma of the extremities. Am J Surg Pathol. 1987;11:359-66. 10.1097/00000478-198705000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Oki Y, Ewer MS, Lenihan DJ, et al. Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: an open label, single arm, phase II trial. Clin Lymphoma Myeloma Leuk 2015;15:152-8. 10.1016/j.clml.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohlfing S, Aurich M, Schöning T, et al. Nonpegylated liposomal doxorubicin as a component of R-CHOP is an effective and safe alternative to conventional doxorubicin in the treatment of patients with diffuse large b-cell lymphoma and preexisting cardiac diseases. Clin Lymphoma Myeloma Leuk 2015;15:458-63. 10.1016/j.clml.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 1982;96:133-9. 10.7326/0003-4819-96-2-133 [DOI] [PubMed] [Google Scholar]
- 26.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997;15:1318-32. [DOI] [PubMed] [Google Scholar]
- 27.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460-8. 10.1016/S1470-2045(11)70069-9 [DOI] [PubMed] [Google Scholar]
- 28.Akinleye A, Chen Y, Mukhi N, et al. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol 2013;6:59. 10.1186/1756-8722-6-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:323-32. 10.1056/NEJMoa1509981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cang S, Iragavarapu C, Savooji J, et al. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol 2015;8:129. 10.1186/s13045-015-0224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Fu J, Zhang M, et al. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol 2015;8:96. 10.1186/s13045-015-0188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Fu J, Zhang M, et al. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol 2015;8:104. 10.1186/s13045-015-0195-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novero A, Ravella PM, Chen Y, et al. Ibrutinib for B cell malignancies. Exp Hematol Oncol 2014;3:4. 10.1186/2162-3619-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das A, Wei G, Parikh K, et al. Selective inhibitors of nuclear export (SINE) in hematological malignancies. Exp Hematol Oncol 2015;4:7. 10.1186/s40164-015-0002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]