Abstract
Background
Primary vaginal carcinoma is rare, accounting for 1–2 % of all gynecological malignancies. Being rare, most observations are based on retrospective and comparative analyses. This study was aimed to retrospectively analyze the prognostic factors and its relevance in the outcomes of primary vaginal cancers.
Materials
Medical records of all cases of primary vaginal cancers, presented to Department of Oncology, from 2004 to 2012, at a tertiary care center in southern India, were retrieved from electronic medical records, and were analyzed.
Results
The total number of cases was 32. Median age at presentation was 64.28 years. Squamous histology accounted for 84.4 %, with the rest being adenocarcinoma. Surgery was offered for five (15.6 %), and concurrent chemotherapy for 14 (43.8 %) patients. Three patients had only surgery. All others received radiotherapy. Twenty received external beam radiation (EBRT) and vaginal brachytherapy (VBT); seven only EBRT and two, adjuvant radiation. Five patients had residual disease; two, stage III, and three stage IV. Median follow-up was 55.83 months. Twelve patients were alive at last follow-up (37.5 %), while 14 were dead (43.8 %—8 of disease and 6 of other causes). Six patients were lost to follow-up (18.8 %). Twenty patients were disease free. Seven had recurrence, three loco-regional and four distant. Median overall survival (OS) was 86.1 months, disease-free survival (DFS) 90.17 months, and disease-specific survival (DSS) 97.13 months. When well and moderately differentiated tumors were taken together, the 5-year OS, DFS, and DSS rates were, 56.6, 64.3, and 82.3 %. For poorly differentiated tumors, median OS, DFS, and DSS were, 20.9, 14.6, and 20.9 months, with statistically significant advantage for better grade tumors, for DSS (p 0.050). Better 5-year OS, DFS, and DSS rates were observed for stage I + II group, with 54.9, 79.8, and 78.9 %, compared with advanced stage where the same were 54.8, 38.2, and 68.6 % (DFS—p 0.003, DSS—p 0.009). Grade and stage of tumor had statistically significant predictive value over the outcomes, while tumor size showed a significant trend. Patients treated with combination of EBRT and VBT fared well.
Conclusion
Our study could conclude that grade of differentiation was a significant predictor of poor survival as was stage of disease. Combination of VBT and external beam radiotherapy provides good DFS.
Keywords: Primary vaginal cancer, Prognostic factors, Tumour grade, External beam radiation, Vaginal brachytherapy
Introduction
Primary vaginal carcinoma is rare, accounting for about 1–2 % [1] of all gynecological malignancies. Being rare, there are no prospective randomized studies. All studies are retrospective, either comparative or meta-analyses. Diagnosis is always confirmed after excluding primary malignancies of cervix and vulva. This, a retrospective analysis of patients with primary vaginal cancers who had presented to our institute, was done to analyze the prognostic factors and its relevance to clinical outcome.
Materials
Medical records of all cases of primary vaginal cancers, presented to Department of Oncology, from 2004 to 2012, were retrieved from electronic medical records, and were analyzed. Patients were called for review or contacted over telephone for follow-up. Statistical analysis was done using SPSS 11.0, for Windows. Log rank method was used for assessing significance. Survival analysis was done using Kaplan–Meier curves.
Results
During the 9-year period from 2004 to 2012, 32 primary vaginal cancers were treated. Median age at presentation was 64.28 years (range 42–85 years). Eighteen patients (56.3 %) had undergone hysterectomy for non-malignant causes earlier. Mean interval between hysterectomy and development of vaginal malignancy was 9.31 years. Rest (43.8 %-14/32) had intact uterus. All patients’ characteristics are tabulated in Table 1.
Table 1.
Patients’ characteristics
| No. | Character | Sub-group | Number | Percentage |
|---|---|---|---|---|
| n | 32 | 100 | ||
| 1 | Age | <65 | 17 | 53.1 |
| ≥65 | 15 | 46.9 | ||
| 2 | Hysterectomy | Done | 18 | 56.3 |
| Not done | 14 | 43.8 | ||
| 3 | Site of lesion | Upper | 21 | 65.6 |
| Middle | 1 | 3.1 | ||
| Lower | 3 | 9.4 | ||
| Entire | 4 | 12.5 | ||
| Not known | 3 | 9.4 | ||
| 4 | Size | <4 cm | 13 | 40.6 |
| >4 cm | 16 | 50 | ||
| Not known | 3 | 9.4 | ||
| 5 | Histology | Squamous | 27 | 84.4 |
| Adenocarcinoma | 5 | 15.6 | ||
| 6 | Differentiation | Well | 12 | 37.5 |
| Moderate | 15 | 46.9 | ||
| Poor | 5 | 15.6 | ||
| 7 | Stage | I | 6 | 18.8 |
| II | 11 | 34.4 | ||
| III | 9 | 28.1 | ||
| IVA + IVB | 6 (4 + 2) | 18.8 | ||
| 8 | Surgery | No | 27 | 84.4 |
| WLE | 3 | 9.4 | ||
| Hysterectomy + WLE | 1 | 3.1 | ||
| Pelvic Exenteration | 1 | 3.1 | ||
| 9 | Radiation | No RT | 3 | 9.4 |
| EBRT | 7 | 21.9 | ||
| EBRT + VBT | 20 | 62.5 | ||
| Adjuvant RT | 2 | 6.3 | ||
| 10 | Chemotherapy | No | 18 | 56.3 |
| Yes | 14 | 43.8 | ||
| 11 | Out come | No disease | 20 | 62.5 |
| Residual | 5 | 15.6 | ||
| Recurrence | 7 | 21.9 | ||
| 12 | Site of recurrence | Local | 2 | – |
| Pelvic | 1 | – | ||
| Distant | 4 | – | ||
| 13 | Status | Alive | 12 | 31.3 |
| Dead | 14 | 40.6 | ||
| Lost for FU | 6 | 28.1 | ||
| 14 | Status—disease specific | Alive | 12 | 37.5 |
| Died—due to disease | 8 | 25 | ||
| Died—due to other cause | 6 | 18.8 | ||
| Lost for FU | 6 | 18.8 | ||
| 15 | Toxicity | No | 28 | 87.5 |
| Local | 1 | 3.1 | ||
| Bladder | 1 | 3.1 | ||
| Rectal | 2 | 6.3 |
Except for three treated with only surgery, all others received radiotherapy. Seven patients received external beam radiation (EBRT) alone, while 20 had both EBRT and vaginal brachytherapy (VBT). Three-dimensional conformal radiotherapy techniques using multiple fields, ensuring that 95 % of target volume receives the prescription dose, was the protocol used in all EBRT. A dose of 45–50 Gy in 180–200 cGy fractions, at 5 fractions a week was delivered. Patients were treated with high dose rate (HDR) brachytherapy using intracavitary applicators. Most common fractionation schedule was three sittings of 700 cGy each. Two patients had adjuvant radiation after surgery.
Five patients had residual disease at treatment completion, two of stage III, and three of stage IV. Surgical options being unacceptable for patients and relatives, considering their advanced stage and co-existing morbidities, they were offered best supportive care. Recurrence was seen in seven patients, three being loco-regional and four, distant (Table 2). These patients were offered palliative radiation to metastatic sites and supportive symptomatic care. Twenty patients (62.5 %) were disease free at last follow-up. Median follow-up time was 55.83 months. Twelve patients were alive at last follow-up (37.5 %), while 14 had died (43.8 %): 8 due to disease, and 6 of other causes. Six patients were lost to follow-up (18.8 %).
Table 2.
Results of treatment: based on stage and treatment modality
| Stage | No. of patients | EBRT + BT | EBRT | Surgery ± RT |
|---|---|---|---|---|
| I | 6 | 3 | 0 | 3 |
| II | 11 | 8–3b | 2 | 1 |
| III | 9 | 6–1b, 1a | 3–1b, 1a | 0 |
| IV | 6 | 3–1b, 1a | 2–1b, 1a | 1–1a |
a Residual 5/32
b Recurrence 7/32—2 local, 1 pelvic, 4 distant
Disease-free patients 20/32—62.5 %
Median follow-up time 55.83 months
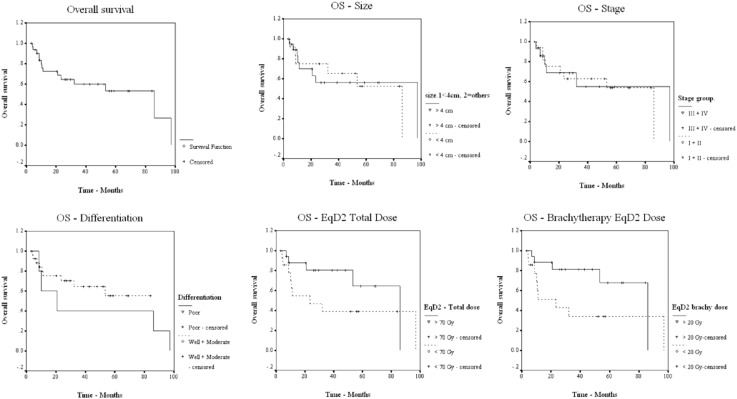
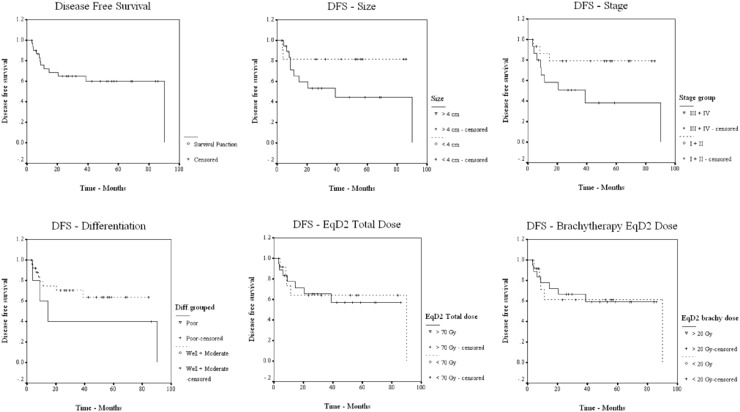
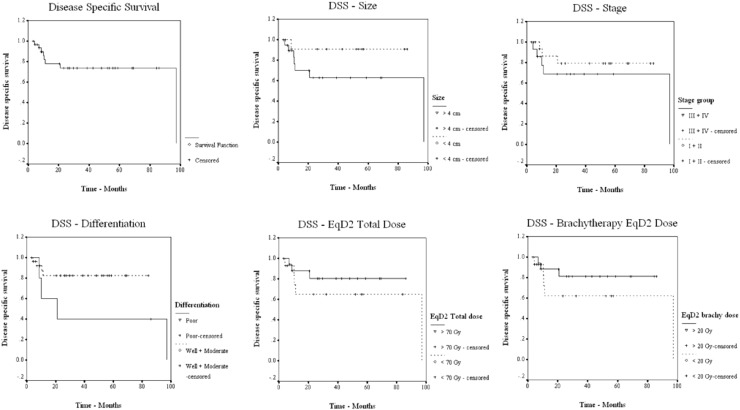
Median overall survival (OS) was 86.10 months, and the median disease-free (DFS) and disease-specific survival (DSS) rates were 90.17 and 97.13 months, respectively. Five-year OS, DFS, and DSS rates were 54.14, 60.43, and 73.92 %, respectively (Figs. 1, 2, 3). Patients were grouped according to their age as <65 and ≥65 years. The number of patients in age groups of <65 and ≥65 were 17 and 15. Median OS in <65 group was 97.1 and in ≥65 group, it was 86.1 months. Statistical significance was not observed. Median OS value in hysterectomy group was (18/32) 97.1 months and in intact-uterus group was (14/32) 53.3 months. (p 0.237; not statistically significant). Both groups had 5-year DFS of 61.4 months, with median reaching 90.2 in hysterectomy group. OS, DFS, and DSS for each of the prognostic factors are tabulated in Table 3.
Fig. 1.
OS in relation to prognostic factors
Fig. 2.
DFS in relation to prognostic factors
Fig. 3.
DSS in relation to prognostic factors
Table 3.
OS, DFS, and DSS for each prognostic sub-group & FIGO stage
| OS, DFS, and DSS for each prognostic sub-group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Characteristic | Sub group | Overall survival (event = 14) | Disease-free survival (event = 12) | Disease-specific survival (event = 8) | ||||||
| 5 year% | Median months |
p value | 5 year% | Median months | p value | 5 year% | Median months | p value | |||
| n = 32 | 44.1 | 86.1 | 60.4 | 90.2 | 73.9 | 97.1 | |||||
| 1 | Age group | <65 | 59.0 | 97.1 | 0.886 | 49.7 | 38.7 | 0.293 | 62.7 | 97.1 | 0.215 |
| ≥65 | 50.1 | 86.1 | 72.2 | – | 84.4 | – | |||||
| 2 | Hysterectomy status | Not done | 37.5 | 53.3 | 0.237 | 61.4 | – | 0.639 | 72.9 | – | 0.777 |
| Done | 63.6 | 97.1 | 61.4 | 90.2 | 75.0 | 97.1 | |||||
| 3 | Histology | Squamous | 52.9 | 86.1 | 0.789 | 64.1 | 90.2 | 0.214 | 76.7 | 97.1 | 0.261 |
| Adeno ca | 60.0 | – | 40.0 | 20.5 | 60.0 | – | |||||
| 4 | Grade | Well + mod | 56.6 | – | 0.396 | 64.3 | – | 0.244 | 82.3 | – | 0.050 |
| Poor | 40.0 | 20.9 | 40.0 | 14.6 | 40.0 | 20.9 | |||||
| 5 | Site | Upper 1/3 | 43.9 | 53.3 | 0.217 | 56.0 | – | 0.672 | 72.5 | – | 0.847 |
| Others | 76.2 | 86.2 | 63.2 | 90.2 | 76.2 | 97.1 | |||||
| 6 | Size | <4 cm | 54.7 | 86.1 | 0.945 | 83.3 | – | 0.112 | 90.9 | – | 0.143 |
| >4 cm | 56.1 | 97.1 | 44.6 | 44.6 | 63.1 | 97.1 | |||||
| 7 | Stage | I + II | 54.9 | 86.2 | 0.865 | 79.8 | – | 0.525 | 78.9 | – | 0.434 |
| III + IV | 54.8 | 97.1 | 38.2 | 38.7 | 68.6 | 97.1 | |||||
| 8 | Treatment modality | EBRT + BT | 63.6 | 86.1 | 0.128 | 60.2 | – | 76.3 | – | 0.879 | |
| EBRT | 42.9 | 32.1 | 57.1 | 90.2 | 0.899 | 57.1 | 97.1 | ||||
| Sx ± RT | 30.0 | 23.4 | 66.7 | – | – | – | |||||
| 9 | Brachytherapy EqD2 dose | <20 Gy | 34.3 | 23.4 | 0.080 | 63.3 | 90.2 | 0.971 | 61.9 | 97.1 | 0.284 |
| >20 Gy | 69.8 | 86.1 | 59.3 | – | 81.5 | – | |||||
| 10 | Radiotherapy EqD2 dose | <70 Gy | 38.9 | 23.4 | 0.151 | 60.1 | 90.2 | 0.799 | 65.0 | 97.1 | 0.380 |
| >70 Gy | 67.1 | 86.1 | 57.3 | – | 80.5 | – | |||||
| 11 | Chemotherapy | Yes | 66.7 | 97.1 | 0.418 | 54.6 | 90.2 | 0.365 | 66.7 | 97.1 | 0.424 |
| No | 47.3 | 53.3 | 65.0 | – | 79.9 | – | |||||
| 12 | Chemotherapy sequence | Concurrent | 63.7 | 97.1 | 0.547 | 58.3 | 90.2 | 0.534 | 91.7 | – | 0.421 |
| Others | 48.4 | 53.3 | 61.1 | – | 71.2 | – | |||||
| OS, DFS, and DSS based on stage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | No. | Overall survival | Disease-free survival | Disease-specific survival | ||||||
| 5 year% | Median | p value | 5 year% | Median | p value | 5 year% | Median | p value | ||
| I | 6 | 66.7 | – | 0.668 | 100 | – | 0.003 | 100 | – | 0.009 |
| II | 11 | 48.0 | 53.3 | 70.7 | – | 90.9 | – | |||
| III | 9 | 56.3 | 97.1 | 66.7 | 90.2 | 88.9 | – | |||
| IVa | 4 | 75.0 | – | 37.5 | 20.5 | 50.0 | 20.5 | |||
| IVb | 2 | 0 | 10.1 | 0 | 8.73 | 0 | 8.91 | |||
Well and moderately differentiated tumors fared better. For well and moderately differentiated tumors taken together, the 5-year OS, DFS, and DSS rates were 56.6, 64.3, and 82.3 %, respectively. For poorly differentiated tumors, median OS, DFS, and DSS were, 20.9, 14.6, and 20.9 months, respectively. There was statistically significant advantage for better grade tumors, i.e., for DSS (p 0.050).
Patients were staged according to FIGO (International Federation of Gynecology and Obstetrics) and were grouped as early (Stage I + II-17) and advanced (Stage III + IV-15). Better 5-year OS, DFS, and DSS rates were observed for stage I + II group, with 54.9, 79.8, and 78.9 %, compared with the advanced stage where the same were 54.8, 38.2, and 68.6 %, respectively. Statistical significance was not observed.
Significant treatment-related acute toxicities were observed only in 4 patients (12.5 %). Two patients had rectal complications, while one each had bladder and local reactions. These were managed symptomatically.
Discussion
Vaginal cancer treatment has evolved from the early twentieth century when a 5-year overall survival of 10 % was observed in 1935 by Taussig et al. [2] to around 37–42 % as documented by Stryker et al. [3]. Kosary et al., analyzing SEER data from 1988 to 2001, published in 2007, quoted a 5-year stage-wise OS rates of 68, 54, 35, and 20 % for stages I, II, III, and IV, respectively [4]. The improved survival outcome has been attributed to better radiotherapy facilities and combinations of conformal three-dimensional external beam treatments and optimized brachytherapy.
Various retrospective studies from across the world have analyzed outcomes in primary vaginal cancers and have predicted probable prognostic factors [5–8]. Major predictors of clinical outcome could be grouped as host or patients’ factors like age at presentation, and status of uterus; tumor factors like grade, FIGO stage, location, and size of lesion; and treatment parameters like modality of treatment, total radiation dose, total brachytherapy dose [5, 8], and chemotherapy status [9]. The results of our outcomes of primary vaginal cancer were analyzed based on these probable prognostic factors and were compared with the literature.
Host Factors
Younger age at presentation has been suggested by Creasman et al. [10] and Helman et al. [11] to be a better prognostic factor. In our study, median OS for <65 group was 97.1 months, while that of ≥65 was 86.1. The 5-year OS in <65 age group was 59.1 % and in ≥65, it was 50.1 %. Although no statistical significance was observed, a probable advantage was observed for OS among younger age. Same was not seen in either DFS or DSS. Platta et al. [8] had also failed to document any impact for age on treatment results.
Chyle et al. [6] had suggested a better prognosis for previous hysterectomy status, and this was also observed in Hiniker et al.’s [12] study. Our group also showed an advantage for earlier hysterectomy, in OS, DFS, and DSS, corresponding medians being 97.1, 90.2, and 97.1 months, respectively (Figs. 1, 2, 3), as observed by Chyle et al. from MD Anderson. Lian et al., in 2008, from the University of Alberta [5], on the other hand, had failed to observe any advantage for hysterectomy status.
Tumor Factors
Squamous tumors have an advantage in terms of OS and time to recurrence as shown by various authors [6, 13], and adenocarcinoma has twice the incidence of recurrence compared with squamous histology. In our group, 5-year OS and DFS rates for squamous cell carcinoma were 52.9 and 64.1 %, comparable to 68 % reported by Fleming et al. [13]. Median DFS for adenocarcinoma was only 20.5 months in our group, as evidenced by Chyle et al. However, Kirkebride et al. [14] reporting from Princess Margaret Hospital and Platta et al. [8] failed to support an advantage for squamous cell histology.
Grade of tumor, an inherent character throwing light on biology and behavior of tumor, was found to be a predictor of survival and recurrence in our group of patients. While well and moderately differentiated tumors together had 5-year OS, DFS, and DSS of 56.6, 64.3, and 82.3 %, medians of OS, DFS, and DSS for poorly differentiated tumors were, 20.9 (p 0.396), 14.6 (p 0.244), and 20.9 months (p 0.050). Statistically significant advantage was observed for DSS; OS and DFS showed definite advantages for well and moderately graded tumors (Figs. 1, 2, 3). Although comparable literature is lacking, grade needs to be considered as predictor of outcome in primary vaginal tumors.
Earlier authors like Kucera et al. and Ali et al. [15, 16] have suggested that tumors of upper one-third or upper half of vagina behave better compared with lower-located tumors. Lian et al. [5] also observed better DFS for lesions in upper third of vagina. Site and extent of vaginal involvement have been predicted as significant factors by Platta et al. also [8]. However, Perez et al. [17] and Stryker et al. [3], assessing response in relation to location of tumors, failed to document any significant advantage based on site. We compared survival in upper one third tumors with other sites, but failed to observe any significant relation. It has been suggested that with modern radiotherapy higher tumoricidal doses are being delivered to upper third of vagina, tolerance dose of which lies in the range of 140 Gy, compared with lower third, and this could be the reason for better behavior of these lesions, and may not be due to site per se.
Bigger the lesion, poorer the prognosis, has been the rule in malignancy. Lesion above 4 cm has been predicted to be poor prognostic factor by authors like Hiniker et al. [12] who documented a significant p value of 0.0266 in their series. Similar results were also obtained by Lian et al. [5] (p 0.020), although the cut-off they used was 5 cm. Frank et al. also found size >4 cm to be a statistically significant predictor of poor prognosis (p < 0.001) [7]. Jang et al. in 2012 [18] stressed that primary tumor size was a significant prognostic factor (p-0.039) for DFS. In our group, 5-year OS, DFS, and DSS for tumors <4 cm was, 54.7, 83.3, and 90.9 % while the same for those >4 cm was 56.1 % (p 0.945), 44.6 % (p 0.118), and 63.1 % (p 0.143) (Figs. 1, 2, 3). Increase in primary tumor size was associated with higher incidence of local failure, there by negatively affecting survival. A predictable trend was observed, although not statistically significant, probably due to small numbers.
FIGO stage was another factor with statistical significance in our series. Five-year OS, DFS, and DSS for each stage showed statistically significant correlation between advancing stage and DFS—p 0.003, and DSS—p 0.009 (Table 3). Stages I and II combined had a better 5-year OS, DFS, and DSS, compared with stages III and IV taken together (Figs. 1, 2, 3). Perez et al. and Lian et al. [5, 17] had also observed statistical significance for FIGO stage, and suggested this to be the most important predictor of OS and DFS in vaginal cancer. A retrospective series from MD Anderson by Frank et al. [7] (p < 0.01) also came out with the observation that stage was the prime factor that correlated with treatment outcome. In their multivariate analysis, stage and tumor size were the only independent predictors of survival. Platta et al. [8] also opined that primary factor influencing the outcome in vaginal cancer was disease stage.
Treatment Factors
Conformal radiotherapy planning encompassing the entire tumor volume and nodal stations depending on location of the lesion is found to result in good local control and there by in predictable survival outcomes. Not only the common, external, and internal iliac groups of lymph nodes, but the inferior gluteal, pre-sacral, and peri-rectal nodes in case of posterior vaginal wall lesion, and when the distal vagina is involved, the inguinal and the femoral groups of nodes can also be involved in malignancies of vagina. Hence, accurate delineation of target volume carries importance. Frank et al. reporting from MD Anderson in 2005 had stressed the benefits of tailored individualized radiotherapy planning [7]. Better imaging modalities in recent years have also contributed significantly to the improved target delineation and optimized planning and dose delivery in radiotherapy.
Patients who had received EBRT and VBT had better 5-year OS, DFS, and DSS in our group. A 2-Gy equivalent dose (EqD2) of brachytherapy also showed an advantage for those who had received >20 Gy, especially for OS (p 0.080) (Fig. 1, 2, 3). Total dose in EqD2, of >70 Gy has been demonstrated to have a better cause specific survival by authors like Lian et al., and Kirkbride et al. [5, 14]. In our study, better 5-year OS (67.1 vs. 38.9 %; p 0.151) and DSS (80.5 vs. 65.0 %; p 0.380) were observed in patients who received a EqD2 > 70 Gy (Figs. 1, 2, 3), but the same advantage was not reflected in DFS (57.3 vs. 60.1 %; p 0.799). Stryker et al. [3] had also failed to corroborate this in their study. Kucera et al. analyzed the outcomes of HDR versus LDR brachytherapy [19] and failed to show any significant difference in terms of complications, although the precise positioning and immobilization along with shorter treatment time favored HDR. Jang et al. [18] had suggested that definitive radiotherapy is an effective treatment modality, and local control translated to disease-free and overall survival. Murakami et al. [20] concluded that combination of EBRT and HDR brachytherapy provides a good local control. Customized vaginal applicators with central and peripheral channels for differential loading and optimized dose delivery, using Iridium-192 alone, or Iridium-192 and Cesium-132 in combination have been in vogue in different centers from as early as 2004, as published by Frank et al. from MD Anderson [7].
Various authors have argued for and against concurrent chemotherapy for primary vaginal cancers. Miyomoto [9, 21, 22] on multivariate analysis, observed a significantly better prognosis for the use of concurrent chemotherapy in their series from Harvard University. A better OS was observed in chemotherapy group in general, and concurrent chemotherapy group in specific, in our series. An advantage for DSS was also noted in concurrent chemotherapy group. The same advantage was not observed in DFS, which could have been because of small number in each group. Analyzing SEER data, Ghia et al. [23] observed that use of concurrent chemoradiation had increased since 1999, but failed to observe any benefit in OS or DFS. Frank et al. [7] from MD Anderson also failed to observe any advantage for concurrent chemoradiation in their series; but, extrapolating the benefits from randomized trials on cervical cancer, they suggested that, concurrent chemoradiation could be offered for select group of high-risk vaginal cancer patients with good performance status.
Probably due to diligent conformal planning, dose limiting toxicities were minimal in our retrospective analysis. This would imply that a higher dose could be achieved for the target volume with better target delineation using high-definition imaging modalities like MRI, and conformal planning, which would ensure a good local control, thereby being a surrogate for disease-free and overall survivals. Blecharz et al. [24] have also concluded that radiotherapy is well tolerated and that late toxicities are rare.
Level of evidence from the available literature on primary vaginal cancers is limited by the absence of randomized prospective trials. Ours also is a retrospective series from a single institute. The newer prognostic factors like incidence of human papilloma virus as a causative factor in primary vaginal cancers need to be looked into prospectively.
Conclusion
Our evaluations conclude that, grade of differentiation of tumor is a statistically significant predictor of poor DSS with a trend toward poor DFS and OS. FIGO stage also emerged as a major, statistically significant factor, predicting prognosis in primary vaginal cancer. A similar trend was also noted with the increasing tumor size. Hysterectomy done earlier could also be considered as a prognostic factor. Radical radiotherapy, combining external beam therapy and high dose rate brachytherapy, has a pivotal role, and with strict and diligent care to achieve the required tumoricidal dose with respect to tolerance of adjacent structures, using spatial optimization and conformal planning offers good disease control.
Acknowledgments
Compliance with Ethical Requirements and Conflict of Interest
This is a retrospective analysis of all cases of primary vaginal cancers presented to our tertiary care institute from 2004 to 2012. Hence no ethical issues were involved. No conflict of interest.
Dr. Chelakkot Govindalayathil Prameela
completed her post-graduation in Radiation Oncology from Manipal Academy of Higher Education. She has 15 years of experience as Radiation Oncologist. She is presently an Associate Professor, in the Department of Radiation Oncology, at Amrita Institute of Medical Sciences, Kochi, Kerala. She is interested in Female health care and Head and Neck Oncology. She has performed international and national presentations on breast, stomach, vagina, and head and neck malignancies. She is involved in works on androgen receptors in breast cancer, dysphagia aspiration-related structures in head and neck radiation, and in gynecological malignancies.
Footnotes
Dr. Chelakkot G. Prameela is an Associate Professor at Amrita Institute of Medical Sciences; Dr. Rahul Ravind is a Resident at Amrita Institute of Medical Sciences; Dr. Bharath C. Gurram is a Resident at Amrita Institute of Medical Sciences; Ms. V. S. Sheejamol is a Lecturer at Amrita Institute of Medical Sciences; and Dr. Makuny Dinesh is a Professor at Amrita Institute of Medical Sciences.
Contributor Information
Chelakkot G. Prameela, Email: cgprameela@gmail.com
Rahul Ravind, Email: rahulravind@gmail.com.
Bharath C. Gurram, Email: dr.bharath9@gmail.com
V. S. Sheejamol, Email: sheejamolvs@aims.amrita.edu
Makuny Dinesh, Email: mdinesh@aims.amrita.edu.
References
- 1.Di Donato V, Bellati F, Fischeti M, et al. Vaginal cancer. J Crit Rev Oncol/Hematol. 2012;81:286–295. doi: 10.1016/j.critrevonc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Taussig FJ. Primary cancer of the vulva, vagina and female urethra: 5 year results. Surg Gynecol Obstet. 1935;60:477–488. [Google Scholar]
- 3.Stryker JA. Radiotherapy for vaginal carcinoma: a 23-year review. Br J Radiol. 2000;73:1200–1205. doi: 10.1259/bjr.73.875.11144798. [DOI] [PubMed] [Google Scholar]
- 4.Kosary CL. Cancer of the vagina. In: Ries LA, Young JL, Keel GE, et al (eds.). SEER survival monographs: cancer survival among adults: U.S.Seer Program, 1988 [NIH Pub.No07-6215]. Bethesda (MD): National Cancer Institute; 2007. p. 155–160.
- 5.Lian J, Dundas G, Carlone M, et al. Twenty year review of radiotherapy for vaginal cancer: an institutional experience. Gynecol Oncol. 2008;111:298–306. doi: 10.1016/j.ygyno.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Chyle V, Zagras GK, Wheeler JA, et al. Definitive radiotherapy for carcinoma of the vagina: outcomes and prognostic factors. Int J Radiat Oncol Biol Phys. 1996;35(5):891–905. doi: 10.1016/0360-3016(95)02394-1. [DOI] [PubMed] [Google Scholar]
- 7.Frank SJ, Jhingran A, Levenback C, et al. Definitive radiation therapy for squamous cell carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 2005;62(1):138–147. doi: 10.1016/j.ijrobp.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Platta CS, Anderson B, Geye H, et al. Adjuvant and definitive radiation therapy for primary carcinoma of the vagina using brachytherapy and external beam radiation therapy. J Contemp Brachytherapy. 2013;5(2):76–82. doi: 10.5114/jcb.2013.36177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto DT, Viswanathan AN. Concurrent chemoradiation for vaginal cancer. PLoS One. 2013;8(6):e65048. doi: 10.1371/journal.pone.0065048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creasman WT, Phillips JL, Meneck HR. The national cancer data base report on cancer of the vagina. Cancer. 1998;83(5):1033–1040. doi: 10.1002/(SICI)1097-0142(19980901)83:5<1033::AID-CNCR30>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Hellman K, Lundell M, Silfversward C, et al. Clinical and histopathologic factors related to prognosis in primary squamous cell carcinoma of the vagina. Int J Gynecol Cancer. 2006;16(3):1201–1211. doi: 10.1111/j.1525-1438.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiniker SM, Roux A, Murphy JD, et al. Primary squamous cell carcinoma of the vagina: prognostic factors, treatment patterns, and outcomes. Gynecol Oncol. 2013;131:380–385. doi: 10.1016/j.ygyno.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Fleming P, Syed AMN, Neblett D, et al. Description of an after-loading Ir-192 interstitial-intra-cavitary technique in the treatment of carcinoma of the vagina. Obstet Gynecol. 1980;55:525–530. [PubMed] [Google Scholar]
- 14.Kirkbride P, Fyles A, Rawlings GA, et al. Carcinoma of the vagina—experience at the Princess Margaret Hospital (1974–1989) Gynecol Oncol. 1995;56:435–443. doi: 10.1006/gyno.1995.1077. [DOI] [PubMed] [Google Scholar]
- 15.Kucera H, Vavra N. Radiation management of primary carcinoma of the vagina: clinical and histopathological variables associated with survival. Gynecol Oncol. 1991;40:12–16. doi: 10.1016/0090-8258(91)90076-H. [DOI] [PubMed] [Google Scholar]
- 16.Ali MM, Huang DT, Howels R, et al. Radiation alone for carcinoma of vagina: variation in response related to the location of the primary tumor. Cancer. 1996;77:1934–1939. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1934::AID-CNCR25>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Perez CA, Grigsby PW, Garipagaoglu M, et al. Factors affecting long-term outcome of irradiation in carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 1999;44:37–45. doi: 10.1016/S0360-3016(98)00530-6. [DOI] [PubMed] [Google Scholar]
- 18.Jang WI, Wu HG, Ha SW, et al. Definitive radiotherapy for treatment of primary vaginal cancer: effectiveness and prognostic factors. Int J Gynecol Cancer. 2012;22(3):521–527. doi: 10.1097/IGC.0b013e31823fd621. [DOI] [PubMed] [Google Scholar]
- 19.Kucera H, Mock U, Knocke TH, et al. Radiotherapy alone for invasive vaginal cancer: outcome with intra-cavitary high dose rate brachytherapy versus conventional low dose rate brachytherapy. Acta Obstet Gynecol Scand. 2001;80(4):355–360. doi: 10.1034/j.1600-0412.2001.080004355.x. [DOI] [PubMed] [Google Scholar]
- 20.Murakami N, Kasamatsu T, Sumi M, et al. Radiation therapy for primary vaginal carcinoma. J Radiat Res. 2013;54(5):931–937. doi: 10.1093/jrr/rrt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto DT, Tanaka CK, Viswanathan AN. Concurrent chemoradiation improves survival in patients with vaginal cancer. Int J Radiat Oncol Biol Phys. 2010;10(3):s120. doi: 10.1016/j.ijrobp.2010.07.306. [DOI] [Google Scholar]
- 22.Miyamoto DT, Viswanathan AN. Concurrent chemoradiation for vaginal cancer. PLoS One. 2013;8(6):e65048. doi: 10.1371/journal.pone.0065048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghia AJ, Gonzalez VJ, Tward JD, et al. Primary vaginal cancer and chemoradiotherapy: a pattern-of-care analysis. Int J Gynecol Cancer. 2011;21(2):378–384. doi: 10.1097/IGC.0b013e3182072e44. [DOI] [PubMed] [Google Scholar]
- 24.Blecharz P, Reinfuss M, Jakubowicz J, et al. Radiation therapy complications in patients with primary invasive vaginal carcinoma. Ginekol Pol. 2013;84(3):206–210. doi: 10.17772/gp/1564. [DOI] [PubMed] [Google Scholar]