Abstract
Aim
Aim of this study is to find out clinical relevance of estimating PON1 arylesterase activity, total oxidative stress (TOS), nitric oxide (NO), and vitamin C levels in maternal serum for prediction of birth weight of newborn.
Methods
We have investigated the PON1 arylesterase activity, TOS, NO, vitamin C, total protein, and albumin levels in 56 postnatal clinic patients having newborn weighing <2500 gm (low birth weight) and compared with 56 postnatal clinic patients having newborn weighing >2500 gm. Samples were collected immediately after delivery.
Results
PON1 arylesterase activity levels show significant decrease in cases as compared to controls (93.27 ± 13.76 kU/l vs. 112.77 ± 9.42 kU/l). Nitric oxide (nitrate + nitrite) levels are also found to be significantly decreased in cases with respect to controls (22.89 ± 2.65 umol/l vs. 24.73 ± 3.80 umol/l). Total oxidative stress is significantly increased in cases than in control subjects (23.34 ± 2.64 μmol H2O2 equiv./l vs. ± 21.43 ± 2.47 μmol H2O2 equiv/l). Vitamin C levels are also significantly decreased in cases as compared to controls (1.23 ± 0.25 mg/dl vs. 1.34 ± 0.28 mg/dl). Positive correlation between neonatal birth weight and maternal serum PON1 arylesterase activity (r = 0.682, p < 0.05) while negative correlation is obtained between neonatal birth weight and maternal serum oxidative stress (r = −0.478, p < 0.05). Logistic regression analysis is applied for assessing predictive utility which demonstrated a significant association of birth weight with PON1 arylesterase activity (AUC = 0.960, Naglekerke’s R2 = 0.793, p < 0.05)
Conclusion
Decreased arylesterase activity and antioxidant vitamin C levels with increased total oxidative stress in maternal serum may be considered as the additional risk factors for the development of low birth weight newborn.
Keywords: Low birth weight, Arylesterase, Total oxidative stress
Introduction
The weight of the infant at birth is a powerful predictor of infant growth and survival and which depends on maternal health and nutrition throughout the pregnancy [1]. Low birth weight has been defined by the World Health Organization (WHO) as weight at birth of less than 2500 g (5.5 lb) [2]. In India prevalence of low birth weight neonate is 25–30 % [3]. Maternal, fetal, placental, and external factors along with genetic growth potential throughout pregnancy are the major determinant of the normal growth of fetus. Impairments in one or more of these factors affect the fetal growth [4]. There are various studies that revealed the maternal as well as fetal risk factors for intrauterine growth retardation; however, intrauterine growth retardation, which sometimes occurs without any risk factors, and etiopathogenesis could not be fully demonstrated. This can be understood by the thought that, in the pathophysiology of intrauterine growth retardation, placental failure, which is contributed by oxidative stress, plays an important role [5].
The family of paraoxonase enzymes comprises three members paraoxonase 1 (PON1), paraoxonase 2 (PON2), and paraoxonase 3 (PON3) and genes for which are aligned adjacent to each other on the 7q 21.3–22.1 chromosome [6, 7]. Paraoxonase 1 [PON1, (EC 3.1.8.1)] is a glycoprotein of 354 amino acid residues with a molecular weight of 43 kDa [8].
PON1 is located in a subfraction of high-density lipoprotein that contains apoA-I and clusterin (apo J) which may function to protect cell membranes generally against lipid peroxidation and other toxic effects. It is hypothesized that PON1 is mainly responsible for the breakdown of lipid peroxides before they could accumulate on LDL [9].
PON1 has been reported to have a number of enzymatic activities including arylesterase, lactonase, peroxidase, and phospholipase A2-like activities [10].
Oxidative stress occurs when the generation of reactive oxygen species and other radical species exceeds the scavenging capacity by antioxidants [11]. Placental oxidative stress can cause deterioration of the syncytiotrophoblast; this may give rise to variety of pregnancy complications out of which intrauterine growth retardation is one of the important complications. Patients with intrauterine growth retardation develop oxidative stress because of placental ischemia/reperfusion injury secondary to improper spiral arteriole development [12].
The human fetoplacental circulation is not innervated and vasoactive factors such as NO are likely to be important in maintaining fetoplacental blood flow [13].
Nitric oxide (NO) plays an important role in the control of systemic blood pressure. NO is synthesized from the nonessential amino acid l-arginine by the action of enzyme nitric oxide synthase [14]. Superoxide and oxidized low-density lipoprotein can lead to endothelial dysfunction by inactivation of nitric oxide (NO), which regulates arterial tone, and inhibits local inflammation, coagulation, and cell proliferation. Oxidative stress and oxidized low-density lipoprotein impair endothelial functioning and vasodilatation by reducing nitric oxide bioavailability in the artery wall, events that possibly could be prevented by l-ascorbic acid [15]. In addition to its antiscorbutic action, vitamin C is a potent reducing agent and scavenger of free radicals in biological systems [16].
Here we had hypothesized that low maternal PON1 arylesterase activity results in the low birth weight newborn taking in consideration the pathophysiological feature of intrauterine growth retardation endothelial damage and vascular dysfunctioning in which antioxidant enzyme PON1 arylesterase is considered as protective. We had also measured maternal oxidative stress and vitamin C levels to asses the oxidant and antioxidant status of mother and relating both to the birth weight of newborn. We had also determined the maternal nitric oxide levels which is thought to play an important role in uteroplacental blood flow in pregnancy and related them to birth outcome in the form of weight of newborn, and by assessing maternal nutritional status by measuring total protein and albumin in maternal serum which is also considered as important determinant of maternal and fetal health, we had determined its effect on birth outcome in the form of birth weight of newborn.
Materials and Methods
Study Population
This is a hospital-based case control study. In this study, 112 postpartum female patients admitted to Medical College Hospital are included; out of which 56 female patients, having low birth weight baby, were selected as cases and control population that consisted of 56 postpartum female patients having normal birth weight baby and who are matched for age and gender. None of the women from cases and control had a positive medical history of cardiac and metabolic disease. No participants smoked, used caffeine or alcohol, and had history of thyroid disease, diabetes mellitus, and hypertension. Written informed consent was obtained from all the participants, and the study was approved by institutional Ethics Committee.
Exclusion criteria included multiple pregnancies, preeclampsia, preterm delivery, cesarean delivery, and maternal chronic disease (hypertension, endocrine diseases, hyperlipidemia, acute or chronic hepatic diseases).
Sample Collection
Blood samples were obtained from antecubital veins of the subjects in the cases and control groups. Blood samples were collected immediately after the delivery. The blood was allowed to clot at room temperature in plain bulb for one hour, and serum was collected by centrifugation at 1500×g for 10 min. Serum was analyzed within 24 h of collection.
Biochemical Investigation
Serum PON1 arylesterase activity is estimated using phenylacetate as substrate, and product phenol is measured at 270 nm using spectrophotometer [17].
Serum total oxidative stress is measured colorimetrically at 530 nm [18], while determination of serum nitric oxide (nitrate + nitrite) is based on the chemical conversion of nitrate to nitrite by vanadium chloride. The reaction is followed by a colorimetric detection of nitrite as an azo dye product of the Griess reaction [19].
Vitamin C is estimated using its reducing property in which phosphotungstic acid is reduced to phosphotungstic blue and measured at 700 nm [20, 21].
Statistical Analysis
Results are presented as mean ± SD. The continuous variables are tested for normality with Shapiro–Wilk test. Student’s unpaired t test is used for statistical analysis between cases and controls for numerical variables in Gaussian distribution. The strength of association between two parameters is expressed by the Pearson’s correlation coefficient.
The logistic regression analysis is used for prediction of risk of low birth weight contributed by various risk factors.
The three models prepared in the logistic regression for the analysis of data are
Model 1 Albumin + total protein + vitamin C + nitric oxide,
Model 2 All parameters in model 1 + TOS, and
Model 3 All parameters in model 2 + PON1 arylesterase activity.
At each step, variable in the model is assessed for its contribution to the model reflected by the Naglekerke R2 value and p value of the model. Odds ratios (ORs) and 95 % CIs are calculated. p < 0.05 was considered as statistically significant.
Results
In Table 1 PON1 arylesterase activity, nitric oxide, and vitamin C levels are significantly decreased in cases as compared to controls while TOS is significantly increased in cases as compared to control.
Table 1.
PON1 arylesterase activity, TOS, nitric oxide, and vitamin C levels in cases as compared to controls
| Parameter | Cases (n = 56) | Control (n = 56) | p value |
|---|---|---|---|
| Age of mother (years) | 23.66 ± 3.22 | 22.66 ± 2.77 | 0.07 |
| Birth weight of newborn (kg) | 2.16 ± 0.17 | 2.95 ± 0.42 | <0.001* |
| Serum PON1 arylesterase activity (kU/l) | 93.27 ± 13.76 | 112.77 ± 9.42 | <0.001* |
| Serum total oxidative stress (μmol H2O2 equiv./l) | 23.34 ± 2.64 | 21.43 ± 2.47 | <0.001* |
| Serum nitric oxide (nitrate + nitrite) (umol/l) | 22.89 ± 2.65 | 24.73 ± 3.80 | <0.001* |
| Serum vitamin C (mg/dl) | 1.23 ± 0.25 | 1.34 ± 0.28 | 0.021* |
| Serum total protein (gm/dl) | 6.96 ± 0.80 | 7.12 ± 0.61 | 0.24 |
| Serum albumin (gm/dl) | 3.83 ± 0.46 | 3.92 ± 0.33 | 0.23 |
* p < 0.05
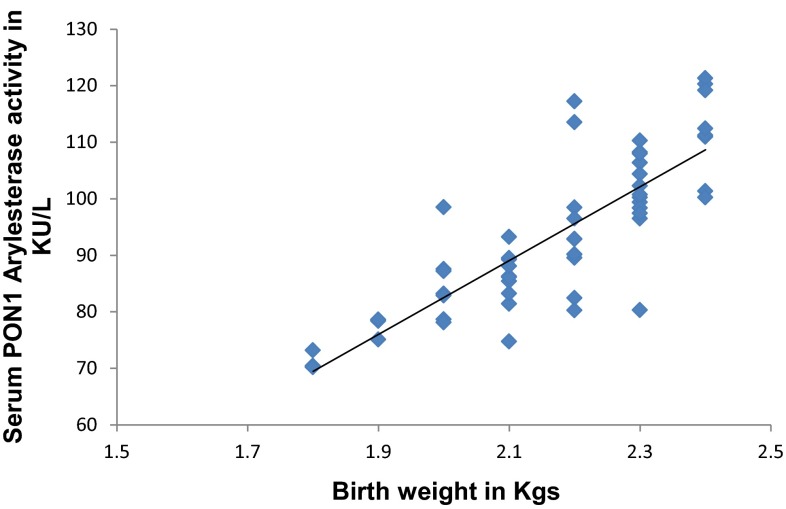
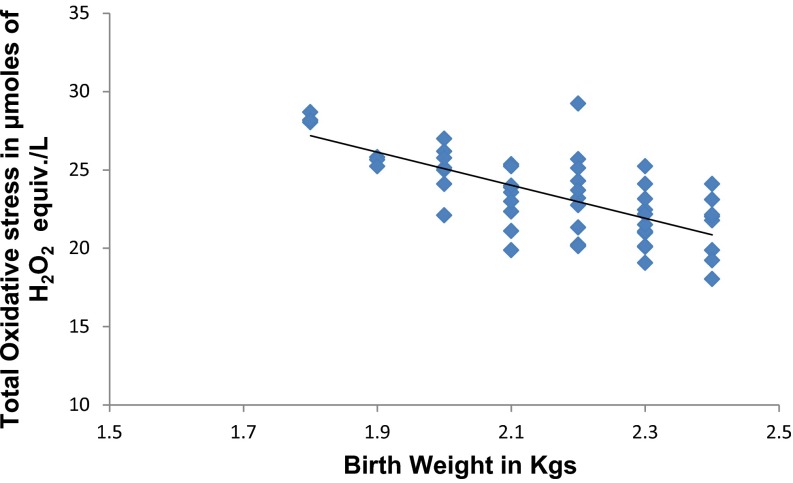
In Fig. 1 PON1 arylesterase activity has significant strong positive (r = 0.682, p < 0.05) correlation with birth weight of newborn. In Fig. 2 TOS has significant strong negative (r = −0.478, p < 0.05) correlation with birth weight of newborn.
Fig. 1.
Correlation between birth weight and PON1 arylesterase activity
Fig. 2.
Correlation between birth weight and TOS
Multivariate Logistic Regression Analysis
It is used for prediction of risk of low birth weight contributed by various risk factors. In each of the next three models, the adjusted values for nitric oxide, total oxidative stress, and PON1 arylesterase are shown. These variables are assessed for their contribution which is reflected by Naglekerke’s R2, area under curve (AUC), and p value of the model.
In Table 2 in the first model, the serum albumin, total protein, vitamin C, and nitric oxide are taken. For this model, Naglekerke’s R2 out to be 0.121 and area under curve is 0.668. In the second model when total oxidative stress is added, to this parameters the result shows increase in Naglekerke’s R2 from 0.121 to 0.182 and area under curve (AUC) also increased from 0.668 to 0.707. In the last model, PON1 Arylesterase activity is added which gives Naglekerke’s R2 as 0.793 and AUC increased to 0.960. All the models are statistically significant with p < 0.05.
Table 2.
Comparison of models 1, 2, and 3
| Parameter | Odds ratio | SE | 95 % CI |
|---|---|---|---|
| Model 1 (Naglekerke’s R 2 = 121, AUC = 0.668, p = 0.031) | |||
| Albumin | 1.407 | 0.718 | 0.518–3.824 |
| Total protein | 0.780 | 0.264 | 0.402–1.513 |
| Vitamin C | 2.694 | 2.363 | 0.483–15.030 |
| Nitric oxide | 1.171 | 0.086 | 1.015–1.351 |
| Model 2 = model 1 + TOS (Naglekerke’s R 2 = 0.182, AUC = 0.707, p = 0.006) | |||
| TOS | 0.783 | 0.083 | 0.636–0.964 |
| Model 3 = model 2 + PON1 arylesterase activity (Naglekerke’s R 2 = 0.793, AUC = 0.960, p = 0.000) | |||
| PON1 arylesterase activity | 1.696 | 0.188 | 1.365–2.107 |
All the models are statistically significant with p < 0.05
Regressions model containing albumin, total protein, and vitamin C is not significant (data not shown) when nitric oxide, total oxidative stress, and PON1 arylesterase added risk prediction is progressively increased and the models were significant. Thus, these models underscore the contribution of these parameters to low birth weight either as a cause or as effect.
This finding suggests that PON1 arylesterase activity, total oxidative stress, and nitric oxide levels are independent predictors of LBW. Multivariate logistic regression analysis after adjustment of other established risk factors for LBW demonstrates that decreased PON1 arylesterase activity is associated with greatest risk for the development of LBW.
Discussion
Results suggested that PON1 arylesterase activity, nitric oxide, and vitamin C levels are significantly decreased in case group compared to control group and TOS is significantly increased in case group as compared to control group. We also estimated maternal serum total protein and albumin levels to evaluate nutritional status of mother which is also considered as a known risk factor for the development of low birth newborn, but our results show that both groups did not differ with respect to serum total protein and albumin levels so the confounding by maternal nutritional status is nullified.
Various studies were conducted to reveal the correlation between the parameters of oxidative stress and fetal weight [22–24].
It was demonstrated that PON1 prevents LDL oxidation by hydrolyzing lipid peroxides and to have protective role against cellular oxidative damage [25]. Based on this, we evaluated markers of oxidative stress like TOS and nitric oxide. We simultaneously estimated the markers of antioxidant defences like PON1 arylesterase activity and vitamin C levels.
Previously, association of PON1 activity and birth weight was studied taking cord blood as sample [26, 27]. To the best of our knowledge, this is the first study done for the association of maternal PON1 arylesterase activity and neonatal birth weight.
In our study, as the case group females were delivered at term and having the LBW babies, they belong to the small for gestational age or IUGR group of LBW newborn.
The placenta is the major source of prooxidant agents, antioxidant enzyme systems, and hormones, and is able to keep the lipid peroxidation under control in normal pregnancy [28]. The balance between prooxidative and antioxidative metabolites affects the LDL-C oxidation. Estradiol inhibits LDL-C oxidation, whereas progesterone increases LDL-C oxidation in primary tissue cultures of term human placenta [29]. PON1 is an antioxidant enzyme found in the high-density lipoprotein and prevents oxidative modification of LDL-C [30].
Defective maternal spiral artery conversion is considered as an important factor predisposing the placenta to malperfusion and results in atherosclerotic changes in placental vasculature [31].
Oxidative stress in maternal circulation is responsible for oxidation of LDL to oxidized LDL (OxLDL) which plays an important role in triggering and accelerating the development of atherosclerotic changes resulting in vascular malperfusion which leads to endoplasmic reticulum stress. ER stress inhibits placental protein synthesis, eventually triggering apoptosis. Evidence of the unfolded protein response (UPR) is observed in both IUGR and preeclampsia + IUGR placentas, but to a greater extent in the latter [31–33].
In present study, PON1 arylesterase activity was significantly higher in mothers having IUGR babies group, and this result was considered as a compensatory response rather than a reason. It was thought that the evaluation of this response especially during the first trimester could play a role in the prediction of IUGR.
In present study, significantly decreased levels of maternal serum NO are observed in cases compared to control group this is supported by the results of Urban et al. They demonstrated that serum increase in homocysteine levels in pregnancies complicated with IUGR were accompanied by decreased levels of serum total nitrite. Homocysteine and NO both are the exponents of vessel endothelium function. Further they suggested that inadequate uteroplacental blood flow results in fetal growth restriction and the control of fetoplacental circulation is dependent on locally produced and circulating vasoactive factors. Nitric oxide is one of them, levels of which get decreased in intrauterine growth retardation [34].
However, maternal neutrophil superoxide release is increased in placental pathologies like preeclampsia and IUGR. Tissues such as macrophages and epithelial, endothelial, and interstitial cells may be induced to simultaneously produce both NO and superoxide to form peroxynitrite. In the process, any beneficial effect of NO is lost and replaced by the deleterious effect of peroxynitrite [35].
In present study, significantly decreased levels of Vitamin C were observed in cases as compared to controls. Vitamin C is a hydrophilic antioxidant; it counteracts several hydroxyl radicals and may contribute in protecting the fetus from oxygen-free radical damage during pregnancy [36].
Our findings are in concordance with Lee et al. who demonstrated that pregnant women utilize a defence mechanism, which is composed of antioxidant-enzymes and nutrients including vitamins C and E, against oxidative stress and free radical damage and imbalance between increased oxidative stress and decreased antioxidant defences impairs fetal growth [37].
To conclude, we can say that in case of mothers having IUGR newborn have reduced PON1 arylesterase activity, nitric oxide, and vitamin C levels while increased TOS as compared to control group. Such reduced activity of PON1 and reduced concentration of vitamin C is obtained probably because of its utilization for combating excess oxidative stress in case of persons having type 2 DM with complication. Present study also shows the utility of PON1 arylesterase activity for prediction of low birth weight.
Acknowledgments
Compliance with Ethical Requirements and Conflict of Interest
This is a hospital-based case control study. In this study, 112 postpartum female patients admitted to Medical College Hospital are included. Written informed consent was obtained from all the participants, and study was approved by institutional Ethics Committee. The authors declare that they have no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Dr. Mukund Ramchandra Mogarekar
M.D. (Biochemistry) is presently working as Professor and Head of Department of Biochemistry at Swami Ramanand Teerth Government Medical College Ambajogai. He is a founder member of medical research society at Swami Ramanand Teerth Government Medical College Ambajogai. He is also a member of Institutional Ethical Committee and Institutional Animal Ethical Committee. He is also a member of Maharashtra Medical Research Council Directorate of Medical Education and Research, Mumbai and Board of Studies for Undergraduate and Postgraduate at Maharashtra University of Health Sciences Nashik. He has Guided 10 MD Biochemistry students. His field of research is paraoxonase in health and diseases and lipidology. He is having many international and national publications on his credit some of which are cited in textbook. He is the secretary (west zone) of Association of Medical Biochemistry of India.
Footnotes
Dr. Mukund Ramchandra Mogarekar M.D. (Biochemistry) is a Professor and the Head of Department of Biochemistry at Swami Ramanand Teerth Government Medical College; Dr. Mahendra G. Dhabe is Associate Professor and Dr. Chanchal C. Gujrathi is JR III in the Department of Biochemistry at Swami Ramanand Teerth Government Medical College.
Contributor Information
Mukund Ramchandra Mogarekar, Email: mrmogarekar@hotmail.com.
Chanchal C. Gujrathi, Email: gujrathic@gmail.com
References
- 1.Muthayya S. Maternal nutrition and low birth weight—what is more important? IndianJ Med Res. 2009;130:600–608. [PubMed] [Google Scholar]
- 2.World Health Organization . International statistical classification of diseases and related health problems, tenth revision. Geneva: World Health Organization; 1992. [PubMed] [Google Scholar]
- 3.Park K. Park’s textbook of preventive and social medicine. 21. Jabalpur: M.S.Banarsidas Bhanot; 2011. p. 494. [Google Scholar]
- 4.Bernstein P, Divon M. Etiologies of fetal growth restriction. Clin Obstet Gynecol. 1997;40:723–729. doi: 10.1097/00003081-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran S, Kyle PM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol. 2009;23:765–777. doi: 10.1016/j.bpobgyn.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 7.Draganov DI, Teiber JF, Speelman A, et al. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;466:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Mackness MI, Mackness B, Durrington PN, et al. Paraoxonase: biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7:69–76. doi: 10.1097/00041433-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480. doi: 10.1161/01.ATV.21.4.473. [DOI] [PubMed] [Google Scholar]
- 10.Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–253. doi: 10.1016/0021-9150(94)05524-M. [DOI] [PubMed] [Google Scholar]
- 11.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/S0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myatt L, Brockman DE, Eis ALW, et al. Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta. 1993;14:487–495. doi: 10.1016/S0143-4004(05)80202-4. [DOI] [PubMed] [Google Scholar]
- 14.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology physiology and pathophysiology of reproduction. Hum Reprod. 1998;4(1):3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Tousoulis D, Davies G, TouTouzas P. Vitamin C increases nitric oxide availability in coronary atherosclerosis. Ann Intern Med. 1999;131(2):156–157. doi: 10.7326/0003-4819-131-2-199907200-00022. [DOI] [PubMed] [Google Scholar]
- 16.Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Rad Res. 2000;39(7):671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 17.Mogarekar MR, Chawan SS. The determination of Q192R polymorphism of paraoxonase 1 by using nontoxic substrate p-nitrophenyacetate. Indian J Hum Genet. 2013;19(2):71–77. doi: 10.4103/0971-6866.112897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 20.Morano JP, Cohen L, Xu G, et al. Plasma ascorbic acid concentration relate inversely to blood pressure in human subjects. Am J Clin Nutr. 1993;57(2):213–217. doi: 10.1093/ajcn/57.2.213. [DOI] [PubMed] [Google Scholar]
- 21.Kiondo P, Tumwesigye NM, Wandabwa J, et al. Plasma vitamin C assay in women of reproductive age in Kampala Uganda using a colorimetric method. Trop Med Int Health. 2012;17(2):191–196. doi: 10.1111/j.1365-3156.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 22.Min J, Park B, Kim YJ, et al. Effect of oxidative stress on birth sizes: consideration of window from mid pregnancy to delivery. Placenta. 2009;30:418–423. doi: 10.1016/j.placenta.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Potdar N, Singh R, Mistry V, et al. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG. 2009;116:637–642. doi: 10.1111/j.1471-0528.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim YJ, Hong YC, Lee KH, et al. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 25.La Du BN, Aviram M, Billecke S, et al. On the physiological role(s) of the paraoxonases. Chem Biol Interact. 1999;119–120:379–388. doi: 10.1016/s0009-2797(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 26.Mogarekar M, et al. Correlation of paraoxonase1 activities with birth weight. Indian J Pediatr. 2014;81(8):760–761. doi: 10.1007/s12098-013-1228-z. [DOI] [PubMed] [Google Scholar]
- 27.Mogarekar MR, Rojekar MV. Harbingers of neonatal birth weight: the PON1 arylesterase and lactonase activities. Turk J Biochem. 2014;39(1):25–29. doi: 10.5505/tjb.2014.72473. [DOI] [Google Scholar]
- 28.Zhu XD, Bonet B, Knopp RH. 17β-estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am J Obstet Gynecol. 1997;177(1):196–209. doi: 10.1016/S0002-9378(97)70462-9. [DOI] [PubMed] [Google Scholar]
- 29.Mueller A, Koebnick C, Binder H, et al. Placental defence is considered sufficient to control lipid peroxidation in pregnancy. Med Hypotheses. 2005;64(3):553–557. doi: 10.1016/j.mehy.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Mackness MI, Arrol A, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 31.Burton GJ, Yung HW, Cindrova-Davies T, et al. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(suppl A (Trophoblast Research)):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redman CWG, Sargent IL. The pathogenesis of preeclampsia. Gynécologie Obstétrique & Fertilité. 2001;29:518–522. doi: 10.1016/S1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 33.Yung HW, Calabrese S, Hynx D, et al. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban J, Jarocki SŁAWOMIR, Bielecki DARIUSZ, et al. Serum homocysteine and nitric oxide levels in pregnancy complicated with IUGR. Arch Perinat Med. 2007;13(3):27–29. [Google Scholar]
- 35.Tsukimori K, Maeda H, Ishida K, et al. The superoxide generation of neutrophils in normal and preeclamptic pregnancy. Obstet Gynecol. 1993;81:536–540. [PubMed] [Google Scholar]
- 36.Rose RC. Ascorbic acid metabolism in protection against free radicals: a radiation model. Biochem Biophys Res Commun. 1990;169:430–436. doi: 10.1016/0006-291X(90)90349-R. [DOI] [PubMed] [Google Scholar]
- 37.Lee BE, Hong YC, Lee KH. Influence of maternal serum levels of vitamins C and E during the second trimester on birth weight and length. Eur J Clin Nutr. 2004;58:1365–1371. doi: 10.1038/sj.ejcn.1601976. [DOI] [PubMed] [Google Scholar]