Abstract
Satellite cells are tissue resident muscle stem cells required for postnatal skeletal muscle growth and repair through replacement of damaged myofibers. Muscle regeneration is coordinated through different mechanisms, which imply cell-cell and cell-matrix interactions as well as extracellular secreted factors. Cellular dynamics during muscle regeneration are highly complex. Immune, fibrotic, vascular and myogenic cells appear with distinct temporal and spatial kinetics after muscle injury. Three main phases have been identified in the process of muscle regeneration; a destruction phase with the initial inflammatory response, a regeneration phase with activation and proliferation of satellite cells and a remodeling phase with maturation of the regenerated myofibers. Whereas relatively minor muscle injuries, such as strains, heal spontaneously, severe muscle injuries form fibrotic tissue that impairs muscle function and lead to muscle contracture and chronic pain. Current therapeutic approaches have limited effectiveness and optimal strategies for such lesions are not known yet. Various strategies, including growth factors injections, transplantation of muscle stem cells in combination or not with biological scaffolds, anti-fibrotic therapies and mechanical stimulation, may become therapeutic alternatives to improve functional muscle recovery.
Keywords: Skeletal muscle, Injury, Regeneration, Stem cell, Fibrosis, Scaffolds, Growth factors
Introduction
Human skeletal muscle is about 40 % of the body mass and is formed by bundle of contractile multinucleated muscle fibers, resulting from the fusion of myoblasts. Satellite cells (SC) are skeletal muscle stem cell located between the plasma membrane of myofibers and the basal lamina. Their regenerative capabilities are essential to repair skeletal muscle after injury (Hurme and Kalimo 1992; Lipton and Schultz 1979) (Sambasivan et al. 2011; Dumont et al. 2015a). In adult muscles, SC are found in a quiescent state and represent, depending on species, age, muscle location, and muscle type, around 5 to 10 % of skeletal muscle cells (Rocheteau et al. 2015). After injury, SC become activated, proliferate and give rise to myogenic precursor cells, known as myoblasts. After entering the differentiation process, myoblasts form new myotubes or fuse with damaged myofibers, ultimately mature in functional myofibers.
Skeletal muscle injuries can stem from a variety of events, including direct trauma such as muscle lacerations and contusions, indirect insults such as strains and also from degenerative diseases such as muscular dystrophies (Huard et al. 2002; Kasemkijwattana et al. 2000; Kasemkijwattana et al. 1998; Menetrey et al. 2000; Menetrey et al. 1999; Crisco et al. 1994; Garrett et al. 1984; Lehto and Jarvinen 1991; Jarvinen et al. 2005; Cossu and Sampaolesi 2007). Skeletal muscle can regenerate completely and spontaneously in response to minor injuries, such as strain. In contrast, after severe injuries, muscle healing is incomplete, often resulting in the formation of fibrotic tissue that impairs muscle function. Although researchers have extensively investigated various approaches to improve muscle healing, there is still no gold standard treatment.
This concise review provides a sight about the various phases of muscle repair and regeneration, namely degeneration, inflammation, regeneration, remodeling and maturation. We also give an overview of research efforts that have focused on the use of stem cell therapy, growth factors and/or biological scaffolds to improve muscle regeneration and repair. We also address the therapeutic potential of mechanical stimulation and of anti-fibrotic therapy to enhance muscle regeneration and repair.
Review
Muscle healing process
Skeletal muscle has a robust innate capability for repair after injury through the presence of adult muscle stem cells known as satellite cells (SC). The disruption of muscle tissue homeostasis, caused by injury, generates sequential involvement of various players around three main phases (Fig. 1).
(1, 2) Degeneration/inflammation phase: characterized by rupture and necrosis of the myofibers, formation of a hematoma and an important inflammatory reaction.
(3) Regeneration phase: phagocytosis of damaged tissue, followed by myofibers regeneration, leading to satellite cell activation.
(4, 5) Remodeling phase: maturation of regenerated myofibers with recovery of muscle functional capacity (4) and also fibrosis and scar tissue formation (5).
Fig. 1.
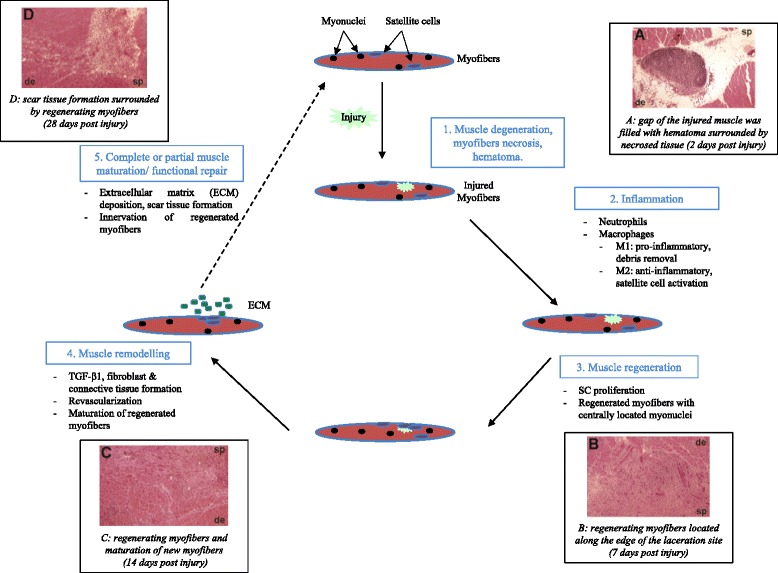
Sequential cycle of muscle healing phases after laceration. Histological images adapted from Menetrey et al, Am J Sports Med 1999. (sp: superficial portion, de: deepest part)
Muscle degeneration and inflammation
Active muscle degeneration and inflammation occur within the first few days after injury. The initial event is necrosis of the muscle fibers, which is triggered by disruption of local homeostasis and particularly by unregulated influx of calcium through sarcolemma lesions (Tidball 2011). Excess in cytoplasmic calcium causes proteases and hydrolases activation that contribute to muscle damage and also causes activation of enzymes that drive the production of mitogenic substances for muscle and immune cells (Tidball 2005). After muscle degeneration, neutrophils are the first inflammatory cells infiltrating the lesion. A large number of pro-inflammatory molecules such as cytokines (TNF-α, IL-6), chemokine (CCL17, CCL2) and growth factors (FGF, HGF, IGF-I, VEGF; TGF-β1) are secreted by neutrophils in order to create a chemoattractive microenvironment for other inflammatory cells such as monocytes and macrophages (Tidball 1995; Toumi and Best 2003). Two types of macrophages are identified during muscle regeneration (McLennan 1996), which appear sequentially during muscle repair (Arnold et al. 2007). M1 macrophages, defined as pro-inflammatory macrophages, act during the first few days after injury,. contribute to cell lysis, removal of cellular debris and stimulate myoblast proliferation. Conversely, M2 macrophages, defined as anti-inflammatory macrophages, act 2 to 4 days after injury, attenuate the inflammatory response and favor muscle repair by promoting myotubes formation (Tidball and Wehling-Henricks 2007; Chazaud 2014; Chazaud et al. 2003). Macrophages, infiltrating injured muscle, are key players of the healing process (Zhao et al. 2016), able to participate in the muscle regeneration process or to favor fibrosis (Munoz-Canoves and Serrano 2015; Lemos et al. 2015).
Muscle regeneration, remodeling and maturation
Muscle regeneration usually starts during the first 4–5 days after injury, peaks at 2 weeks, and then gradually diminishes 3 to 4 weeks after injury. It’s a multiple steps process including activation/proliferation of SC, repair and maturation of damaged muscle fibers and connective tissue formation. A fine balance between these mechanisms is essential for a full recovery of the contractile muscle function.
Muscle fibers are post-mitotic cells, which do not have the capacity to divide. Following an injury, damaged muscle fibers can’t be repaired without the presence of adult muscle stem cells, the satellite cells (SC) (Relaix and Zammit 2012; Sambasivan et al. 2011). Following activation, SC proliferate and generate a population of myoblasts that can either differentiate to repair damaged fibers or, for a small proportion, self-renew to maintain the SC pool for possible future demands of muscle regeneration (Collins 2006; Dhawan and Rando 2005). SC cycle progression and cell fate determination are control by complex regulatory mechanisms in which, intrinsic and extrinsic factors are involved (Dumont et al. 2015a; Dumont et al. 2015b).
Connective tissue/fibrosis
Connective tissue remodeling is an important step of the regenerative muscle process. Rapidly after muscle injury, a gap is formed between damaged muscle fibers and filled with a hematoma. Muscle injuries can be clinically classified depending of the nature of the hematoma (size, location). Late elimination of the hematoma is known to delay skeletal muscle regeneration, to improve fibrosis and to reduce biomechanical properties of the healing muscle (Beiner et al. 1999). In rare complication, major muscle injuries may lead to the development of myositis ossificans that will impair muscle regeneration and repair (Beiner and Jokl 2002) (Walczak et al. 2015).
The presence of fibrin and fibronectin at the injury site, initiate the formation of an extracellular matrix that is rapidly invaded by fibroblasts (Darby et al. 2016; Desmouliere and Gabbiani 1995). Fibrogenic cytokines such as transforming growth factor β1 (TGF-β1) participate to excessive fibroblasts/myofibroblasts proliferation and to an increase in type I/III collagens, laminin and fibronectin production (Lehto et al. 1985). In its initial phase, the fibrotic response is beneficial, stabilizing the tissue and acting as a scaffold for myofibers regeneration. Nevertheless, an excessive collagen synthesis post injury, often result in an increase of scar tissue size over time that can prevent normal muscle function (Mann et al. 2011). Many growth factors are involved in the development of fibrosis, such as Connective Tissue Growth Factor (CTGF), Platelet-Derived Growth Factor (PDGF) or myostatin. TGF-β1, by stimulating fibroblasts/myofibroblasts to produce extracellular proteins such as fibronectin and type I/III collagen, has been identified as the key element in this process (Mann et al. 2011),. Although fibroblasts are the major collagen-producing cells in skeletal muscle, TGF-β1 have also an effect directly on myoblasts causing their conversion to myofibroblasts. Thus myoblasts initially acting to repair damaged myofibers, will produce significant level of collagen and will contribute to muscle fibrosis (Li and Huard 2002).
Revascularization
The restoration of the blood supply in the injured skeletal muscle is one of the first signs of muscle regeneration and is essential to its success. Without revascularization, muscle regeneration is incomplete and a significant fibrosis occurs (Best et al. 2012; Ota et al. 2011). After muscle trauma, blood vessels rupture induces tissue hypoxia at the injury site (Jarvinen et al. 2005). New capillaries formation quickly after injury is therefore necessary (Scholz et al. 2003) for a functional muscle recovery. Secretion of angiogenic factors such as vascular endothelial growth factor (VEGF) at the lesion site is important and several studies have shown that VEGF, by favoring angiogenesis, improve skeletal muscle repair (Deasy et al. 2009; Frey et al. 2012).
Innervation
Muscle repair is complete when injured myofibers are fully regenerated and become innervated. The synaptic contact between a motor neuron and its target muscle fiber, often take place at a specific site in the central region of myofibers, the neuromuscular junction (NMJ) (Wu et al. 2010). NMJ are essential for maturation and functional activity of regenerating muscles. Within 2–3 weeks after muscle damage, the presence of newly formed NMJ is observed in regenerative muscle (Rantanen et al. 1995; Vaittinen et al. 2001).
Strategies to improve muscle regeneration and repair
Growth factors
Growth factors play a variety of roles in the different stages of muscle regeneration (Grounds 1999; Menetrey et al. 2000). These biologically active molecules, synthetized by the injured tissue or by other cell types present at the inflammatory site, are release in the extracellular space and modulate the regenerative response (Table 1). Although hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) are of interest because of their capacity to stimulate satellite cells (Sheehan et al. 2000; Allen and Boxhorn 1989; Yablonka-Reuveni et al. 1990), insulin like growth factor-1 (IGF-I) appears to be of particular importance for the muscle regeneration process. IGF-I stimulates myoblasts proliferation and differentiation (Engert et al. 1996) and is implicated in the regulation of muscle growth (Schiaffino and Mammucari 2011). In a mouse model, direct injections of human recombinant IGF-I at two, five, and seven days after injury enhanced muscle healing in lacerated, contused, and strain-injured muscles (Menetrey et al. 2000; Kasemkijwattana et al. 2000). However, the efficacy of direct injection of recombinant proteins is limited by the high concentration of the factor typically required to elicit a measurable effect. This is mainly due to the bloodstream’s rapid clearance of these molecules and their relatively short biological half-lives. Gene therapy may be an effective method by which to deliver high, maintainable concentrations of growth factor to injured muscle (Barton-Davis et al. 1998; Barton et al. 2002; Musaro et al. 2001). Although IGF-I improved muscle healing, histology of the injected muscle revealed fibrosis within the lacerated site, despite high level of IGF-I production (Lee et al. 2000). Another growth factor, VEGF, by favoring angiogenesis, is known to enhance skeletal muscle repair (Deasy et al. 2009; Frey et al. 2012; Messina et al. 2007). By targeting simultaneously angiogenesis and myogenesis, it was shown that combined delivery of VEGF and IGF-I enhance muscle regenerative process (Borselli et al. 2010). In this direction, the use of platelet-rich plasma (PRP) is considered as a possible alternative approach based on the ability of autologous growth factors to improve skeletal muscle regeneration (Hamid et al. 2014; Hammond et al. 2009). Considered as safe products, autologous PRP injections are increasingly used in patients with sports-related injuries (Engebretsen et al. 2010). Nevertheless, a recent randomized clinical trial show no significant positive effects of PRP injections, as compared with placebo injections, in patients with muscle injuries, up to one year after injections (Reurink et al. 2014; Reurink et al. 2015). Customization of PRP preparation, as recently demonstrated by the use of TGF-β1 neutralizing antibodies, is a promising alternative to promote muscle regeneration while significantly reducing fibrosis (Li et al. 2016).
Table 1.
The role of growth factors in skeletal muscle regeneration
| Growth factors | Physiological effects, potential benefits | Shortcomings | Commentary |
|---|---|---|---|
| IGF-1 | - Essential for muscle growth during development and regeneration. - Promote myoblast proliferation and differentiation in vitro (Huard et al. 2002) - Hypertrophic effect of IGF-1 (Barton-Davis et al. 1999) - Serial injections of IGF-1 improve muscle healing in vivo (Menetrey et al. 2000). - Existence of a muscle specific isoform of IGF-1 (mIGF-1) (Musaro et al. 1999; Musaro et al. 2004) |
- Chemotactic for fibroblasts, increase collagen production, enhance fibrosis development | - IGF-1 play a central role in the enhancement of muscle regeneration- - Anti-inflammatory actions of IGF-1 (Mourkioti and Rosenthal 2005; Tidball and Welc 2015) |
| HGF | - Promote myoblast proliferation and inhibit myoblast differentiation (Anderson 2016; Yin et al. 2013) - Important role for satellite cell activation. Balance between the activation of satellite cells and their return to quiescence. (Chazaud 2010) - Recently, it was shown that a second set of HGF production is crucial for inflammation resolution after injury (Proto et al. 2015) |
- Injection of HGF into injured muscle increased myoblast numbers but blocked the regeneration process (Miller et al. 2000) | - HGF is important during the early phase of muscle regeneration, activate satellite cells |
| VEGF | - Important signaling protein that favor angiogenesis. - Promote myoblast migration, proliferation and survival. (Arsic et al. 2004) - VEGF administration improves muscle regeneration. (Messina et al. 2007; Deasy et al. 2009) |
- Non regulated VEGF expression promote aberrant angiogenesis and fibrosis in skeletal muscle (Karvinen et al. 2011) | - Importance of the proximity between satellite cells and the microvasculature during muscle regeneration, role of VEGF |
| FGF | - Large family of mitogen involved in cell growth and survival - FGF-6 has a muscle specific expression, stimulates satellite cell proliferation and promotes myogenic terminal differentiation (Floss et al. 1997) - FGF-2 promote satellite cell proliferation and inhibit myogenic differentiation (Menetrey et al. 2000; Kastner et al. 2000) |
- Stimulate fibroblast proliferation, | - FGF signaling plays a key role in muscle repair, blocking FGF signaling delay muscle regeneration (Saera-Vila et al. 2016). |
| TGF-β1 | - Key regulator of the balance between muscle fibrosis and muscle regeneration - Inhibits satellite cell proliferation and differentiation in vitro |
- Excessive TGFβ1-induced deposition of ECM at the site of injury, fibrosis (Garg et al. 2015). | - Anti fibrotic therapy by blocking overexpression of TGF-β1 improve muscle regeneration. (Burks et al. 2011; Hwang et al. 2016) |
| PDGF-BB | - PDGF isoforms can regulate myoblast proliferation and differentiation in vitro (Yablonka-Reuveni et al. 1990) - PDGF-BB stimulates satellite cell proliferation and inhibit their differentiation (Charge and Rudnicki 2004) |
- Potent mitogen for fibroblasts | - Release from injured vessels and platelets, PDGF stimulates early skeletal muscle regeneration |
Stem cells
Transplantation of satellite cell-derived myoblasts has long been explored as a promising approach for treatment of skeletal muscle disorders. After an initial demonstration that normal myoblasts can restore dystrophin expression in mdx mice (Partridge et al. 1989), clinical trials, in which allogeneic normal human myoblasts were injected intramuscularly several times in dystrophic young boys muscles, have not been successful (Law et al. 1990; Mendell et al. 1995). Even recently, despite clear improvement in methodologies that enhance the success of myoblast transplantation in Duchenne patients (Skuk et al. 2007), outcomes of clinical trials are still disappointing. These experiments have raised concerns about the limited migratory and proliferative capacities of human myoblasts, as well as their limited life span in vivo. It led to the investigations of other muscle stem cells sources that could overcome these limitations and outperform the success of muscle cell transplantation. Among all these non-satellite myogenic stem cells, human mesoangioblasts, human myogenic-endothelial cells and human muscle–derived CD133+ have shown myogenic potentials in vitro and in vivo (Sampaolesi et al. 2006; Zheng et al. 2007; Meng et al. 2014). The use of such myogenic progenitors cells for improving muscle healing may become an interesting therapeutic alternative (Tedesco and Cossu 2012; Tedesco et al. 2010; Chen et al. 2012). A first phase I/IIa clinical trial has recently demonstrated that intra arterial injections of human mesoangioblasts are safe but display only very limited clinical efficacy in Duchenne patients (Cossu et al. 2015).
Scaffolds
Myogenic precursor cell survival and migration is greatly increased by using appropriate scaffold composition and growth factor delivery (Hill et al. 2006) (Boldrin et al. 2007). Controlling the microenvironment of injected myogenic cells using biological scaffolds enhance muscle regeneration (Borselli et al. 2011). Ideally, using an appropriate extracellular matrix (ECM) composition and stiffness, scaffolds should best replicate the in vivo milieu and mechanical microenvironment (Gilbert et al. 2010) (Engler et al. 2006). A combination of stem cells, biomaterial-based scaffolds and growth factors may provide a therapeutic option to improve regeneration of injured skeletal muscles (Jeon and Elisseeff 2016).
Anti-fibrotic therapy
TGF-β1 is expressed at high levels and plays an important role in the fibrotic cascade that occurs after the onset of muscle injury (Bernasconi et al. 1995; Li et al. 2004). Therefore, neutralization of TGF-β1 expression in injured skeletal muscle should inhibit the formation of scar tissue. Indeed, the use of anti-fibrotic agents (ie decorin, relaxin, antibody against TGF-β1…) that inactivate TGF-β1 signaling pathways reduces muscle fibrosis and, consequently, improve muscle healing, leading to a near complete recovery of lacerated muscle (Fukushima et al. 2001; Li et al. 2007). Losartan, an angiotensin II receptor antagonist, neutralize the effect of TGF-β1 and reduce fibrosis, making it the treatment of choice, since it already has FDA approval to be used clinically (Bedair et al. 2008; Park et al. 2012; Terada et al. 2013). Suramin, also approved by the FDA, blocks TGF-β1 pathway and reduces muscle fibrosis in experimental model (Chan et al. 2003; Taniguti et al. 2011).
Mechanical stimulation
Mechanical stimulation may offer a simple and effective approach to enhance skeletal muscle regeneration. Stretch activation, mechanical conditioning but also massage therapy or physical manipulation of injured skeletal muscles have shown multiple benefit effects on muscle biology and function in vitro and in vivo (Tatsumi et al. 2001);(Best et al. 2012) (Crane et al. 2012; Kumar et al. 2002; Gilbert et al. 2010; Powell et al. 2002). Recently, Cezar and colleagues demonstrates that mechanical forces are as important biological regulators as chemicals and genes, and underlines the immense potential of developing mechano-therapies to treat muscle damage (Cezar et al. 2016). A recent study also demonstrated that a treatment based on ultrasound-guided intra-tissue percutaneous electrolysis (EPI technique) enhances the treatment of muscle injuries (Abat et al. 2015). Altogether, these results suggest that mechanical stimulation should be considered as a possible therapy to improve muscle regeneration and repair.
Conclusions
Skeletal muscle injuries are very frequently present in sports medicine and pose challenging problems in traumatology. Despite their clinical importance, the optimal rehabilitation strategies for treating these injuries are not well defined. After a trauma, skeletal muscles have the capacity to regenerate and repair in a complex and well-coordinated response. This process required the presence of diverse cell populations, up and down-regulation of various gene expressions and participation of multiples growth factors. Strategies based on the combination of stem cells, growth factors and biological scaffolds have already shown promising results in animal models. A better understanding of the cellular and molecular pathways as well as a better definition of the interactions (cell-cell and cell-matrix) that are essential for effective muscle regeneration, should contribute to the development of new therapies in humans. In this direction, a recent paper from Sadtler et al demonstrated that specific biological scaffold implanted in injured mice muscles trigger a pro-regenerative immune response that stimulate skeletal muscle repair (Sadtler et al. 2016).
Abbreviation
CTGF, connective tissue growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF-I, insulin like growth factor-I; NMJ, neuromuscular junction; PDGF, platelet derived growth factor; PRP, platelet rich plasma; SC, satellite cells; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TL and JM participated equally in drafting the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Thomas Laumonier, Phone: +41 22 3795393, Email: thomas.laumonier@unige.ch.
Jacques Menetrey, Email: jacques.menetrey@hcuge.ch.
References
- Abat F, Valles SL, Gelber PE, Polidori F, Jorda A, Garcia-Herreros S, Monllau JC, Sanchez-Ibanez JM. An experimental study of muscular injury repair in a mouse model of notexin-induced lesion with EPI(R) technique. BMC Sports Sci Med Rehabil. 2015;7:7. doi: 10.1186/s13102-015-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Anderson JE. Hepatocyte growth factor and satellite cell activation. Adv Exp Med Biol. 2016;900:1–25. doi: 10.1007/978-3-319-27511-6_1. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95(26):15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167(4):301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36(8):1548–1554. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- Beiner JM, Jokl P (2002) Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res (403 Suppl):S110-119 [DOI] [PubMed]
- Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999;27(1):2–9. doi: 10.1177/03635465990270011101. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96(2):1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best TM, Gharaibeh B, Huard J. Stem cells, angiogenesis and muscle healing: a potential role in massage therapies? Br J Sports Med. 2012 doi: 10.1136/bjsports-2012-091685. [DOI] [PubMed] [Google Scholar]
- Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, Gazzola MV, Messina C, Gamba P, Vitiello L, de Coppi P. Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng. 2007;13(2):253–262. doi: 10.1089/ten.2006.0093. [DOI] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32(34):8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3(82):82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ, Mooney DJ. Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A. 2016;113(6):1534–1539. doi: 10.1073/pnas.1517517113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YS, Li Y, Foster W, Horaguchi T, Somogyi G, Fu FH, Huard J. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol. 2003;95(2):771–780. doi: 10.1152/japplphysiol.00915.2002. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chazaud B. Dual effect of HGF on satellite/myogenic cell quiescence. Focus on "High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo". Am J Physiol Cell Physiol. 2010;298(3):C448–C449. doi: 10.1152/ajpcell.00561.2009. [DOI] [PubMed] [Google Scholar]
- Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219(3):172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163(5):1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Corselli M, Peault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA. Satellite cell self-renewal. Curr Opin Pharmacol. 2006;6(3):301–306. doi: 10.1016/j.coph.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13(12):520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, De Pellegrin M, Godi C, Giuliani S, Ciotti F, Tonlorenzi R, Lorenzetti I, Rivellini C, Benedetti S, Gatti R, Marktel S, Mazzi B, Tettamanti A, Ragazzi M, Imro MA, Marano G, Ambrosi A, Fiori R, Sormani MP, Bonini C, Venturini M, Politi LS, Torrente Y, Ciceri F. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015 doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Ogborn DI, Cupido C, Melov S, Hubbard A, Bourgeois JM, Tarnopolsky MA. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4(119):119ra113. doi: 10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM. A muscle contusion injury model. Biomechanics, physiology, and histology. Am J Sports Med. 1994;22(5):702–710. doi: 10.1177/036354659402200521. [DOI] [PubMed] [Google Scholar]
- Darby IA, Zakuan N, Billet F, Desmouliere A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci. 2016;73(6):1145–1157. doi: 10.1007/s00018-015-2110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009;17(10):1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Gabbiani G. Myofibroblast differentiation during fibrosis. Exp Nephrol. 1995;3(2):134–139. [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen L, Steffen K, Alsousou J, Anitua E, Bachl N, Devilee R, Everts P, Hamilton B, Huard J, Jenoure P, Kelberine F, Kon E, Maffulli N, Matheson G, Mei-Dan O, Menetrey J, Philippon M, Randelli P, Schamasch P, Schwellnus M, Vernec A, Verrall G. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med. 2010;44(15):1072–1081. doi: 10.1136/bjsm.2010.079822. [DOI] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135(2):431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997;11(16):2040–2051. doi: 10.1101/gad.11.16.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S. VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model. Clin Orthop Relat Res. 2012;470(12):3607–3614. doi: 10.1007/s11999-012-2456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29(4):394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- Garg K, Corona BT, Walters TJ. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front Pharmacol. 2015;6:87. doi: 10.3389/fphar.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WE, Jr, Seaber AV, Boswick J, Urbaniak JR, Goldner JL. Recovery of skeletal muscle after laceration and repair. J Hand Surg. 1984;9(5):683–692. doi: 10.1016/S0363-5023(84)80014-3. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD. Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol. 1999;12(5):535–543. doi: 10.1097/00019052-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Hamid MS, Yusof A, Mohamed Ali MR. Platelet-rich plasma (PRP) for acute muscle injury: a systematic review. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37(6):1135–1142. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12(5):1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–832. [PubMed] [Google Scholar]
- Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc. 1992;24(2):197–205. doi: 10.1249/00005768-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Hwang OK, Park JK, Lee EJ, Lee EM, Kim AY, Jeong KS. Therapeutic effect of losartan, an angiotensin II type 1 receptor antagonist, on CCl(4)-induced skeletal muscle injury. Int J Mol Sci. 2016;17(2):227. doi: 10.3390/ijms17020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- Jeon OH, Elisseeff J. Orthopedic tissue regeneration: cells, scaffolds, and small molecules. Drug Deliv Transl Res. 2016;6(2):105–120. doi: 10.1007/s13346-015-0266-7. [DOI] [PubMed] [Google Scholar]
- Karvinen H, Pasanen E, Rissanen TT, Korpisalo P, Vahakangas E, Jazwa A, Giacca M, Yla-Herttuala S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011;18(12):1166–1172. doi: 10.1038/gt.2011.66. [DOI] [PubMed] [Google Scholar]
- Kasemkijwattana C, Menetrey J, Somogyl G, Moreland MS, Fu FH, Buranapanitkit B, Watkins SC, Huard J. Development of approaches to improve the healing following muscle contusion. Cell Transplant. 1998;7(6):585–598. doi: 10.1016/S0963-6897(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland MS, Fu FH, Buranapanitkit B, Watkins SS, Huard J. Use of growth factors to improve muscle healing after strain injury. Clin Orthop. 2000;370:272–285. doi: 10.1097/00003086-200001000-00028. [DOI] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48(8):1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Kumar A, Chaudhry I, Reid MB, Boriek AM. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277(48):46493–46503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]
- Law PK, Bertorini TE, Goodwin TG, Chen M, Fang QW, Li HJ, Kirby DS, Florendo JA, Herrod HG, Golden GS. Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet. 1990;336(8707):114–115. doi: 10.1016/0140-6736(90)91628-N. [DOI] [PubMed] [Google Scholar]
- Lee C, Fukushima K, Usas A, Xin L, Pelinkovic D, Martinek V, Huard J. Biological intervention based on cell and gene therapy to improve muscle healing after laceration. J Musculoskelet Res. 2000;4(4):256–277. doi: 10.1142/S0218957700000264. [DOI] [Google Scholar]
- Lehto MU, Jarvinen MJ. Muscle injuries, their healing process and treatment. Ann Chir Gynaecol. 1991;80(2):102–108. [PubMed] [Google Scholar]
- Lehto M, Sims TJ, Bailey AJ. Skeletal muscle injury--molecular changes in the collagen during healing. Res Exp Med. 1985;185(2):95–106. doi: 10.1007/BF01854894. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21(7):786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161(3):895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–1019. doi: 10.1016/S0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15(9):1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- Li H, Hicks JJ, Wang L, Oyster N, Philippon MJ, Hurwitz S, Hogan MV, Huard J. Customized platelet-rich plasma with transforming growth factor beta1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials. 2016;87:147–156. doi: 10.1016/j.biomaterials.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Lipton BH, Schultz E. Developmental fate of skeletal muscle satellite cells. Science. 1979;205(4412):1292–1294. doi: 10.1126/science.472747. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J Anat. 1996;188(Pt 1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Nagaraja H, Stephens R, Lantry L, Morris GE, Burghes AH. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med. 1995;333(13):832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med. 1999;27(2):222–229. doi: 10.1177/03635465990270021801. [DOI] [PubMed] [Google Scholar]
- Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82(1):131–137. doi: 10.1302/0301-620X.82B1.8954. [DOI] [PubMed] [Google Scholar]
- Meng J, Chun S, Asfahani R, Lochmuller H, Muntoni F, Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22(5):1008–1017. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina S, Mazzeo A, Bitto A, Aguennouz M, Migliorato A, De Pasquale MG, Minutoli L, Altavilla D, Zentilin L, Giacca M, Squadrito F, Vita G. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21(13):3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol. 2000;278(1):C174–C181. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26(10):535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Munoz-Canoves P, Serrano AL. Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol Metab. 2015;26(9):449–450. doi: 10.1016/j.tem.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400(6744):581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27(2):195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2004;101(5):1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med. 2011;39(9):1912–1922. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, Rando TA, Jeong KS. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-beta and fibrosis in skeletal muscle injury. Cell Transplant. 2012;21(11):2407–2424. doi: 10.3727/096368912X637055. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283(5):C1557–C1565. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- Proto JD, Tang Y, Lu A, Chen WC, Stahl E, Poddar M, Beckman SA, Robbins PD, Nidernhofer LJ, Imbrogno K, Hannigan T, Mars WM, Wang B, Huard J. NF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen J, Ranne J, Hurme T, Kalimo H. Denervated segments of injured skeletal muscle fibers are reinnervated by newly formed neuromuscular junctions. J Neuropathol Exp Neurol. 1995;54(2):188–194. doi: 10.1097/00005072-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139(16):2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, Maas M, Tol JL, Dutch Hamstring Injection Therapy Study I Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370(26):2546–2547. doi: 10.1056/NEJMc1402340. [DOI] [PubMed] [Google Scholar]
- Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, Maas M, Tol JL, Dutch HITsI Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206–1212. doi: 10.1136/bjsports-2014-094250. [DOI] [PubMed] [Google Scholar]
- Rocheteau P, Vinet M, Chretien F. Dormancy and quiescence of skeletal muscle stem cells. Results Probl Cell Differ. 2015;56:215–235. doi: 10.1007/978-3-662-44608-9_10. [DOI] [PubMed] [Google Scholar]
- Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saera-Vila A, Kish PE, Kahana A. Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell Signal. 2016;28(9):1196–1204. doi: 10.1016/j.cellsig.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1(1):4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz D, Thomas S, Sass S, Podzuweit T. Angiogenesis and myogenesis as two facets of inflammatory post-ischemic tissue regeneration. Mol Cell Biochem. 2003;246(1-2):57–67. doi: 10.1023/A:1023403928385. [DOI] [PubMed] [Google Scholar]
- Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23(2):239–245. doi: 10.1002/(SICI)1097-4598(200002)23:2<239::AID-MUS15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, Lachance JG, Tremblay JP. First test of a "high-density injection" protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17(1):38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve. 2011;43(1):82–87. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267(1):107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25(5):597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, Witt M, Vadala G, Oyster N, Otsuka T, Fu FH, Huard J. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am. 2013;95(11):980–988. doi: 10.2106/JBJS.L.00266. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27(7):1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1(4):2029–2062. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578(Pt 1):327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Welc SS. Macrophage-Derived IGF-1 Is a Potent Coordinator of Myogenesis and Inflammation in Regenerating Muscle. Mol Ther. 2015;23(7):1134–1135. doi: 10.1038/mt.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br J Sports Med. 2003;37(4):284–286. doi: 10.1136/bjsm.37.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaittinen S, Lukka R, Sahlgren C, Hurme T, Rantanen J, Lendahl U, Eriksson JE, Kalimo H. The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle. J Neuropathol Exp Neurol. 2001;60(6):588–597. doi: 10.1093/jnen/60.6.588. [DOI] [PubMed] [Google Scholar]
- Walczak BE, Johnson CN, Howe BM. Myositis Ossificans. J Am Acad Orthop Surg. 2015;23(10):612–622. doi: 10.5435/JAAOS-D-14-00269. [DOI] [PubMed] [Google Scholar]
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137(7):1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990;111(4):1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J. 2016;30(1):380–393. doi: 10.1096/fj.14-270090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25(9):1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]


