Abstract
Achondroplasia (ACH) is the most common form of hereditary dwarfism and presents with multiple musculoskeletal anomalies but is not normally associated with premature hip arthritis. Developmental dysplasia of the hip (DDH) is a spectrum of disease resulting in shallow acetabular depth and a propensity for chronic femoral subluxation or dislocation; it is among the most common causes of premature arthritis. This case report describes the diagnosis of symptomatic DDH in a patient with ACH and highlights difficulties of primary total hip arthroplasty (THA) as a treatment option. Intraoperative radiographic imaging is advised to ensure proper prosthesis placement. Femoral osteotomy may aid visualization, reduction, and avoidance of soft tissue injury. Concomitant ACH and DDH is a challenging problem that can be successfully treated with modified THA.
Keywords: Achondroplasia, Developmental dysplasia of the hip, Total hip arthroplasty
Introduction
Achondroplasia (ACH) is the most common form of hereditary dwarfism 1, 2. The incidence is estimated to range between 1 and 4.5/30,000 live births 2, 3. Achondroplasia is marked by numerous changes in musculoskeletal anatomy and joint mechanics, but has not been found to be a significant cause of early hip degeneration [4]. Features commonly associated with ACH include a long and narrow trunk, shortened extremities, particularly along the proximal (rhizomelic) segments, bowing of the tibia, and deepening and flattening of the acetabulum 5, 6, 7. Joint laxity is common, most frequently in the knee and shoulder, and manifests as progressive hyperextension of the knees and chronic inferior dislocation of the shoulder, respectively 8, 9.
Developmental dysplasia of the hip (DDH) is also characterized by aberrant joint laxity, encompassing a spectrum of pathology culminating in shallow acetabular depth and a propensity for hip subluxation or dislocation. The incidence of DDH is estimated to range between 1 and 5 per 1000 live births 10, 11. DDH is among the leading causes of premature hip osteoarthritis [12]. The hip-specific features of DDH are summarized by Crowe 13, 14 and Hartofilakidis [15] (Table 1). Crowe described the range of subluxation of the femoral head as extending from less than 50% (group I) to greater than 100% (group IV) 13, 14. Hartofilakidis described three types of DDH, ranging from only mild acetabular dysplasia to a severe dysplasia of the acetabulum and chronic dislocation of the hip with a pseudo-articulation between the femur and a hollow in the iliac wing [15].
Table 1.
Crowe and Hartofilakidis staging of developmental dysplasia of the hips.
| Classification | Description |
|---|---|
| Crowe13, 14 | |
| I | Femoral head subluxation <50% or proximal displacement <10%a |
| II | Femoral head subluxation between 50% and 75% or proximal displacement between 10% and 15%a |
| III | Femoral head subluxation between 75% and 100% or proximal displacement between 15% and 20%a |
| IV | Femoral head subluxation >100% or proximal displacement >20%a |
| Hartofilakidis[15] | |
| Dysplasia | Femoral head exhibits chronic subluxation but located within true acetabulum |
| Low disarticulation | Femoral head articulates with false acetabulum partially covering the true acetabulum. Inferior of false acetabulum overlies superior lip of true acetabulum |
| High disarticulation | Femoral head articulates with a hollow of the iliac wing superior and posterior to true acetabulum. No direct contact between false and true acetabulum |
Proximal displacement calculated as distance between medial femoral head–neck junction and the inferior margin of the acetabulum divided by the height of the pelvis.
Concomitant DDH in the patient with ACH is a rare occurrence. Here, we present a case of a patient with ACH undergoing total hip arthroplasty (THA) due to osteoarthritis secondary to Crowe IV DDH.
Case history
A 36-year-old female with ACH and bilateral DDH was referred for evaluation due to severe pain and discomfort in both hips (Fig. 1). The pain had increased in severity for the past five months and was greatly exacerbated by activity. As a result of her debilitating pain, the patient was unable to walk a distance of greater than one block. She had tried anti-inflammatory medications, ambulatory assistive devices, and low-impact exercises, for more than 3 months, none of which had provided substantial pain improvement. The patient's medical history was significant only for ACH and bilateral DDH. Both her father and son have ACH; there was no history of DDH among first-degree relatives reported.
Figure 1.

Anteroposterior pelvis view of 36-year-old female with achondroplasia and high hip dislocation, Crowe group IV developmental dysplasia of the hip.
On examination, the patient measured 122 cm (4 ft, 0 in) in height. She had an intact neuromuscular examination of bilateral lower extremities. Examination of both hips demonstrated full passive range of motion. There was significant pain in both hips upon passive internal and external rotation as well as with flexion greater than 90°. Radiographic evaluation demonstrated chronically dislocated hips, with severe degenerative changes about the hip joints bilaterally and pseudo-articulation with the iliac crests consistent with a Crowe IV classification (Fig. 1).
Treatment options were discussed with the patient, with the recommendation being bilateral total hip arthroplasty (THA) in a staged manner starting with the left hip, as it was the more symptomatic side. Intraoperatively, the patient was placed in the right lateral decubitus position and a posterior approach utilized. The pseudocapsule was identified and tagged. The location of the under developed native acetabulum was identified visually, verified with intraoperative imaging, and reamed to accept a 40 mm acetabular cup (Fig. 2). A 40 mm cup was impacted into place with 45° of abduction and 20° anteversion. Two screws were placed to secure the cup. A 22 mm highly cross-linked polyethylene liner was used. The proximal femur was identified and the femoral canal established and reamed to 11 mm. A 4 cm segment of the proximal femur was removed via a shortening subtrochanteric osteotomy to enable reduction (Fig. 3). Prophylactic cables were paced both on the proximal and distal segments to prevent fracture propagation. A modular 11 mm stem was impacted into place and a 22 mm head was used. The resected proximal femur was halved and used as a sleeve over the osteotomy site and held with a 2 mm cable. After reduction, the hip was found to be stable in all planes.
Figure 2.

Intraoperative radiograph of the pelvis with an acetabular reamer placed in the underdeveloped native acetabulum prior to acetabular reaming.
Figure 3.

4 cm of subtrochanteric femur resection. Solid black lines demarcate 1 cm intervals. Dashed black lines demarcate 1 in intervals.
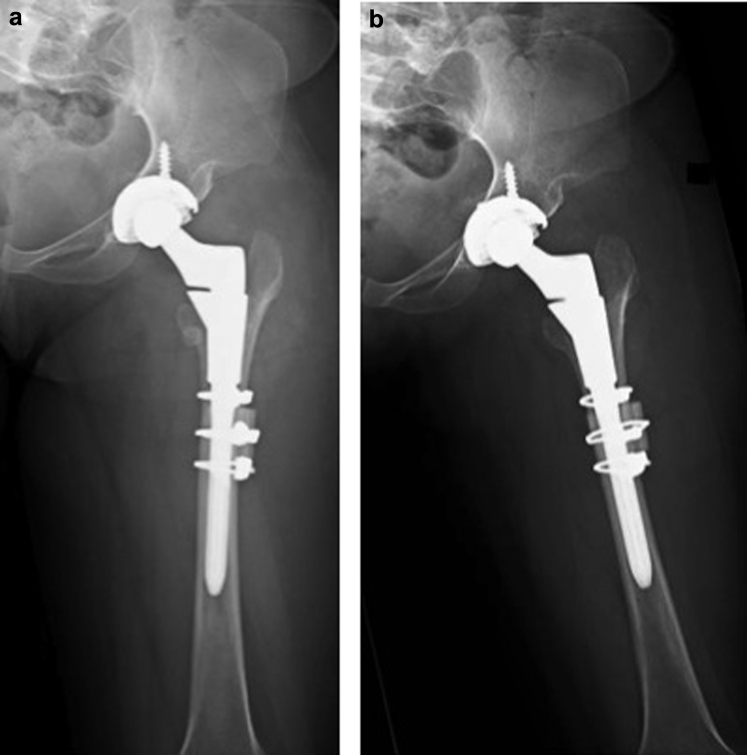
The patient was admitted on the standard total joint replacement pathway and discharged on the second postoperative day with instructions to be partial weight bearing. The first clinic visit was at two weeks after surgery. By that time, the patient was participating well with physical therapy. Radiologic and clinical examination confirmed a stable left hip with no evidence of infection, loosening, periprosthetic fracture, subluxation, or dislocation (Fig. 4a and b). She was kept partial weight bearing on the left hip. At six weeks, the patient was still participating in physical therapy without difficulty or complication; she was instructed to begin weight bearing as tolerated and received a temporary lift for her right shoe to correct the 2 cm leg length discrepancy resulting from left hip restoration. At three months, the patient was ambulating without difficulty and had no complaints at the left hip or thigh; all components were well seated, without evidence of loosening, fracture, or wear. At this time, the patient was scheduled for right primary THA, which was subsequently completed without any complications with a similar protocol (Fig. 5).
Figure 4.
(a) Anteroposterior and (b) lateral x rays of the left hip two weeks after surgery.
Figure 5.

Standing anteroposterior x-ray of the pelvis at the 3-month postoperative visit.
Discussion
In this case report we describe a patient concurrently affected by two musculoskeletal disorders with significantly different pathogenesis.
Achondroplasia
Achondroplasia results from a fully penetrant, autosomal dominant disorder of fibroblast growth factor receptor-3 (FGFR3), phenotypically characterized by sequela of deficient endochondral ossification [16]. More than 95% of cases are thought to stem from a gain of function Gly380Arg amino acid substitution in the transmembrane domain of FGFR3 [16]. FGFR3 has been shown to inhibit chondrocyte differentiation and proliferation largely through its effects on MAPK and Stat1 signaling pathways, respectively 17, 18, 19. Effects of chondrocyte inhibition via FGFR3 are enacted at the physeal plate which are characteristically delayed in maturation and thin until at least the age of puberty 20, 21.
Anatomic features of ACH persisting into adulthood include rhizomelic short stature, frontal bossing of the skull, spinal stenosis, pronounced lumbosacral lordosis, shortened lumbar pedicles, flared and splayed metaphysis, and joint laxity 5, 22. Pelvis- and hip-specific characteristics of ACH include a shortened and broadened pelvic cavity leading to a “champagne-glass” pelvis, squaring of the iliac wings, a flattened acetabular roof, a decreased acetabular beta-angle, increased acetabular coverage of the femoral head, genu varum, and a short and broad femoral neck 6, 7.
Despite aberrant joint mechanics, premature osteoarthritis is only infrequently associated with ACH [4]. Musculoskeletal complaints are common, but most often of spinous etiology [23]. End-stage hip disease has been described in the literature for ACH patients [24]. Chiavetta et al. described 37 patients that were short in stature, four of whom had ACH, that underwent 62 THAs secondary to osteochondrodysplasia [24]. However, no comments were made that addressed the uniqueness of ACH patients in this study. The authors did note that DDH was present in 18 hips but did not comment if it was present among the ACH patients [24].
Developmental dysplasia of the hip
DDH is most commonly associated with decreased appositional force between the femoral head and the acetabulum 25, 26. Risk factors for DDH include joint laxity, female gender, large birth weight for gestational age, breach delivery, oligohydramnios, and infant swaddling 27, 28, 29, 30. While genetic determinants are poorly described, multiple authors have found an increased risk associated among family members harboring this disorder 31, 32. Lee et al. found more than half of patients diagnosed with DDH had a first-degree family member with DDH [31]. Kramer et al. reported that for those affected with DDH, the odds of having a mother with DDH was higher than a sibling or father [32].
While DDH may be related to underlying systemic ligamentous laxity, DDH is a disorder localized to the hip joint. Whereas the pelvis is grossly normal, the hip is dysplastic. The acetabulum is shallow with deficiencies typically anterolaterally and superiorly. The femur often demonstrates increased anteversion, a shorter neck, decreased intramedullary canal size, and coxa vara or valga angulation [33]. Among adults with DDH, there is a range of dysplastic features and associated subluxation/dislocation.
DDH significantly increases the risk of premature osteoarthritis 12, 34. Engesaeter et al. found that DDH increased by 2.6 fold the risk of undergoing total hip arthroplasty (THA) in patients under the age of 37 years old [34]. DDH is estimated to be account for as much as 30% of adult total hip arthroplasty [12]. Hartofilakidis et al. described the natural history of 202 hips affected by DDH [15]. Three stages of arthritic disease progression were described: I: abnormal obliquity of the weight-bearing surface of the acetabulum with a normal femoral head; II: narrowed articular space with an elliptical femoral head and progressive subluxation; III: advanced degenerative arthritic changes of the hip with large cysts and osteophytes of both the acetabulum and femoral head and further subluxation of the femoral head [15]. The rate of disease progression was found to be dependent on baseline subluxation/dislocation. Patients with chronic dislocation since childhood, on average, underwent THA by the age of 48.8 [15].
Current controversies and future considerations
Common comorbidities seen in patients with ACH must be addressed preoperatively to ensure a safe outcome. Craniofacial deformities can increase the difficulty of mask ventilation and intubation; however, a resultant increase in airway complications has not been reported [35]. The spine and related neurologic causes of pain should always be addressed prior to surgery in ACH patients with hip symptoms. The spinal canal is reported to be 40% narrower among those with ACH than those without, despite preservation of the size of the spinal cord and associated nerves [36].
Detailed preoperative planning and intraoperative radiographic imaging are important due to the anatomical changes caused by ACH. The surgeon should verify during preoperative planning that the femoral implant will successfully engage the proximal femur and the distal femur segments as well as successfully engage the femoral bow. In a study of THA in 62 patients with varying causes of dwarfism, almost a quarter of femoral components were found to be in a suboptimal position thus potentially contributing to the high rate of revision THA (i.e., 29%) reported in this study [24]. In addition, acetabular diameter size may be extremely small in ACH patients, and the surgeon should be prepared with proper acetabular implants.
The surgical challenges of DDH Crowe type 4 include severe proximal femoral migration and chronic contraction of soft tissues traversing the hip joint [37]. In cases whereby restoration of the hip center to the true acetabulum may increase the length of the operative limb by 4 cm or more, a subtrochanteric osteotomy is advised [37]. After the subtrochanteric osteotomy is completed, the remaining femoral shaft may limit the surgeon in relation to stem type and length. Other surgical challenges of DDH include hypoplasia and exaggerated anteversion of the acetabulum, and narrowing of the femoral canal [37]. These challenges are accentuated in the achondroplastic patient, with changes in acetabular size and femoral shaft length and diameter. Intraoperative imaging should be used as required to locate the native acetabulum and to ensure accurate restoration of the hip joint.
Summary
Developmental hip dysplasia in the patient with achondroplasia is a rare and challenging condition. Primary total hip arthroplasty is complicated by the chronic anatomical changes and standard surgical landmarks may be altered or absent. This case report illustrates the difficulties of treating Crowe IV DDH in a person with ACH, the importance of preoperative evaluation and planning, and the techniques used to optimize a successful outcome.
Key points.
-
•
A subtrochanteric osteotomy is advised if reduction of the operative leg will increase its length by 4 cm or more, in order to avoid sciatic nerve neuropathy.
-
•
Standard surgical landmarks may be altered or absent in patients with Crowe IV DDH. A concomitant diagnosis of ACH adds another layer of difficulty to these cases.
-
•
Proper preoperative planning and templating is imperative due to the short stature and challenging anatomy of patients with ACH and DDH.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2015.03.001.
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.artd.2015.03.001.
Appendix. Supplementary data
References
- 1.Stoll C., Dott B., Roth M.P., Alembik Y. Birth prevalence rates of skeletal dysplasias. Clin Genet. 1989;35(2):88. doi: 10.1111/j.1399-0004.1989.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 2.Orioli I.M., Castilla E.E., Barbosa-Neto J.G. The birth prevalence rates for the skeletal dysplasias. J Med Genet. 1986;23(4):328. doi: 10.1136/jmg.23.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waller D.K., Correa A., Vo T.M. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am J Med Genet A. 2008;146A(18):2385. doi: 10.1002/ajmg.a.32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton W.A. Bone and joint dysplasias. In: Klippel J.H., Stone J.H., Crofford L.J., White P.H., editors. Primer on the rheumatic diseases. Springer; New York: 2008. p. 559. [Google Scholar]
- 5.Matsui Y., Yasui N., Kimura T. Genotype phenotype correlation in achondroplasia and hypochondroplasia. J Bone Joint Surg Br. 1998;80(6):1052. doi: 10.1302/0301-620x.80b6.9277. [DOI] [PubMed] [Google Scholar]
- 6.Panda A., Gamanagatti S., Jana M., Gupta A.K. Skeletal dysplasias: a radiographic approach and review of common non-lethal skeletal dysplasias. World J Radiol. 2014;6(10):808. doi: 10.4329/wjr.v6.i10.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pellegrin M., Moharamzadeh D. Ultrasound hip evaluation in achondroplasia. J Pediatr Orthop. 2008;28(4):427. doi: 10.1097/BPO.0b013e3181653b87. [DOI] [PubMed] [Google Scholar]
- 8.Richette P., Bardin T., Stheneur C. Achondroplasia: from genotype to phenotype. Joint Bone Spine. 2008;75(2):125. doi: 10.1016/j.jbspin.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Kopits S.E. Orthopedic aspects of achondroplasia in children. Basic Life Sci. 1988;48:189. doi: 10.1007/978-1-4684-8712-1_28. [DOI] [PubMed] [Google Scholar]
- 10.Barlow T.G. Early diagnosis and treatment of congenital dislocation of the hip. J Bone Joint Surg Br. 1962;44-B(2):292. [Google Scholar]
- 11.Bialik V., Bialik G.M., Blazer S. Developmental dysplasia of the hip: a new approach to incidence. Pediatrics. 1999;103(1):93. doi: 10.1542/peds.103.1.93. [DOI] [PubMed] [Google Scholar]
- 12.Feldman D.S. How to avoid missing congenital dislocation of the hip. Lancet. 1999;354(9189):1490. doi: 10.1016/S0140-6736(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 13.Jawad M.U., Scully S.P. In Brief: Crowe's Classification: arthroplasty in developmental dysplasia of the hip. Clin Orthop. 2011;469(1):306. doi: 10.1007/s11999-010-1316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe J.F., Mani V.J., Ranawat C.S. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61(1):15. [PubMed] [Google Scholar]
- 15.Hartofilakidis G., Karachalios T., Stamos K.G. Epidemiology, demographics, and natural history of congenital hip disease in adults. Orthopedics. 2000;23(8):823. doi: 10.3928/0147-7447-20000801-16. [DOI] [PubMed] [Google Scholar]
- 16.Bellus G.A., McIntosh I., Smith E.A. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995;10(3):357. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- 17.Eswarakumar V.P., Lax I., Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Sahni M., Ambrosetti D.C., Mansukhani A. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13(11):1361. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton W.A., Hall J.G., Hecht J.T. Achondroplasia. Lancet. 2007;370(9582):162. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.-H., Modi H.N., Song H.-R. Deceleration in maturation of bone during adolescent age in achondroplasia – a retrospective study using RUS scoring system. Skelet Radiol. 2009;38(2):165. doi: 10.1007/s00256-008-0544-2. [DOI] [PubMed] [Google Scholar]
- 21.Pannier S., Mugniery E., Jonquoy A. Delayed bone age due to a dual effect of FGFR3 mutation in Achondroplasia. Bone. 2010;47(5):905. doi: 10.1016/j.bone.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Oberklaid F., Danks D.M., Jensen F., Stace L., Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J Med Genet. 1979;16(2):140. doi: 10.1136/jmg.16.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahomed N.N., Spellmann M., Goldberg M.J. Functional health status of adults with achondroplasia. Am J Med Genet. 1998;78(1):30. doi: 10.1002/(sici)1096-8628(19980616)78:1<30::aid-ajmg7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Chiavetta J.B., Parvizi J., Shaughnessy W.J., Cabanela M.E. Total hip arthroplasty in patients with dwarfism. J Bone Joint Surg Am. 2004;86-A(2):298. doi: 10.2106/00004623-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ponseti I.V. Growth and development of the acetabulum in the normal child. Anatomical, histological, and roentgenographic studies. J Bone Joint Surg Am. 1978;60(5):575. [PubMed] [Google Scholar]
- 26.Harrison T.J. The influence of the femoral head on pelvic growth and acetabular form in the rat. J Anat. 1961;95(Pt 1):12. [PMC free article] [PubMed] [Google Scholar]
- 27.Stein-Zamir C., Volovik I., Rishpon S., Sabi R. Developmental dysplasia of the hip: risk markers, clinical screening and outcome. Pediatr Int. 2008;50(3):341. doi: 10.1111/j.1442-200X.2008.02575.x. [DOI] [PubMed] [Google Scholar]
- 28.Lapunzina P., Camelo J.S.L., Rittler M., Castilla E.E. Risks of congenital anomalies in large for gestational age infants. J Pediatr. 2002;140(2):200. doi: 10.1067/mpd.2002.121696. [DOI] [PubMed] [Google Scholar]
- 29.Chan A., McCaul K.A., Cundy P.J., Haan E.A., Byron-Scott R. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed. 1997;76(2):F94. doi: 10.1136/fn.76.2.f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamuro T., Ishida K. Recent advances in the prevention, early diagnosis, and treatment of congenital dislocation of the hip in Japan. Clin Orthop. 1984;184:34. [PubMed] [Google Scholar]
- 31.Lee C.B., Mata-Fink A., Millis M.B., Kim Y.-J. Demographic differences in adolescent-diagnosed and adult-diagnosed acetabular dysplasia compared with infantile developmental dysplasia of the hip. J Pediatr Orthop. 2013;33(2):107. doi: 10.1097/BPO.0b013e3182745456. [DOI] [PubMed] [Google Scholar]
- 32.Kramer A.A., Berg K., Nance W.E. Familial aggregation of congenital dislocation of the hip in a Norwegian population. J Clin Epidemiol. 1988;41(1):91. doi: 10.1016/0895-4356(88)90013-3. [DOI] [PubMed] [Google Scholar]
- 33.Noble P.C., Kamaric E., Sugano N. Three-dimensional shape of the dysplastic femur: implications for THR. Clin Orthop. 2003;417:27. [PubMed] [Google Scholar]
- 34.Engesaeter IØ, Lie S.A., Lehmann T.G. Neonatal hip instability and risk of total hip replacement in young adulthood: follow-up of 2,218,596 newborns from the Medical Birth Registry of Norway in the Norwegian Arthroplasty Register. Acta Orthop. 2008;79(3):321. doi: 10.1080/17453670710015201. [DOI] [PubMed] [Google Scholar]
- 35.Monedero P., Garcia-Pedrajas F., Coca I. Is management of anesthesia in achondroplastic dwarfs really a challenge? J Clin Anesth. 1997;9(3):208. doi: 10.1016/s0952-8180(97)00033-0. [DOI] [PubMed] [Google Scholar]
- 36.Lutter L.D., Langer L.O. Neurological symptoms in achondroplastic dwarfs – surgical treatment. J Bone Joint Surg Am. 1977;59(1):87. [PubMed] [Google Scholar]
- 37.Krych A.J., Howard J.L., Trousdale R.T., Cabanela M.E., Berry D.J. Total hip arthroplasty with shortening subtrochanteric osteotomy in Crowe type-IV developmental dysplasia: surgical technique. J Bone Joint Surg Am. 2010;92(Suppl. 1 Pt 2):176. doi: 10.2106/JBJS.J.00061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.