Abstract
Cobalt metallosis after revision metal-on-polyethylene total hip arthroplasty for catastrophic failure of ceramic components is uncommon but a potentially devastating complication. Common findings associated with heavy metal toxicity include cardiomyopathy, hypothyroidism, skin rashes, visual disturbances, hearing changes, polycythemia, weakness, fatigue, cognitive deterioration, and neuropathy. We report a case of a 57-year-old woman who presented with complaints of progressively worsening hip pain, fatigue, memory loss, lower extremity sensory loss, persistent tachycardia, and ocular changes 5 years after synovectomy and revision of a failed ceramic-on-ceramic total hip arthroplasty to metal-on-polyethylene components. A cobalt level of 788.1 ppb and chromium level of 140 ppb were found on presentation and subsequently decreased to 468.8 ppb and 105.9 ppb, respectively, 2 weeks after revision to a ceramic-on-polyethylene total hip arthroplasty. Improvement of symptoms accompanied this decrease in cobalt and chromium levels. Revision of failed ceramic arthroplasties with later-generation ceramics to avoid this potential complication is recommended.
key words: Ceramic, Cobalt, Metal, Revision, Total hip arthroplasty, Toxicity
Introduction
Ceramic-on-ceramic total hip arthroplasties (THAs) were first developed in the 1970s, with the hope that the low linear wear rates of ceramics would reduce revision rates 1, 2 Unfortunately, ceramic components can catastrophically fracture because of their brittleness and steep Young's modulus curve [3]. The femoral head component most often fails because of a traumatic event, in contrast to the acetabular liner which often fails without history of trauma [4].
Recent data from the manufacturer, CeramTec GmbH, Plochingen, Germany, have shown a significant decrease in fracture rate of the fourth-generation Biolox delta ceramic heads (0.002%) compared to the third-generation ceramic heads (0.021%) [5]. The fracture rates of the third- and fourth-generation ceramic liners have remained relatively constant at 0.032% and 0.028%, respectively [5]. When discussing revision component options after catastrophic failure, patients are often reluctant to accept another ceramic device for fear of another component fracture. For this reason among others, metal-on-polyethylene is often chosen in conjunction with synovectomy to mitigate the residual ceramic particles increasing wear rates. However, it has been demonstrated that reliably removing all particulate ceramic debris without an extensive dissection with anterior and posterior synovectomies is extremely difficult [6]. These residual particles embedded in the polyethylene rapidly increase wear rates on the femoral head and expose the patient to potential heavy metal toxicities.
We report a case of a 57-year-old woman with cobalt toxicity after revision of a fractured ceramic-on-ceramic THA to a metal-on-polyethylene THA. At the time of the initial revision surgery, the ceramic head was found to be intact and the acetabular liner fractured. The events leading to presentation at our hospital and placing her in our care are described. Radiographic and intraoperative findings are discussed. Finally, recommendations for management and prevention of this complication are reviewed.
Case history
Our patient is a 57-year-old woman referred to our office with a painful right hip and a diagnosis of cobalt toxicity. She underwent a ceramic-on-ceramic primary hip arthroplasty in 2004 and revision to metal-on-polyethylene components 3 years later for mechanical failure. She had been doing well until 1 year before presentation to our office in early 2015, when she began experiencing right hip pain. Other ailments included worsening fatigue, memory loss, lower extremity sensory loss, new onset tachycardia, hypothyroidism, and vision changes. On examination, she had pain with leg raise and decreased sensation to the dorsum of her foot. Laboratory values were significant for a chromium level of 140 ppb (normal, ≤1.8 ppb) and a cobalt level of 788.1 ppb (normal, ≤3.6 ppb). Plain radiographs revealed an obviously aspherical head component of her right THA, as well as multiple radiographically hyperdense loci surrounding her implant and in her thigh (Fig. 1a and b). Magnetic resonance imaging revealed extensive foreign body reaction surrounding the right THA (Fig. 2).
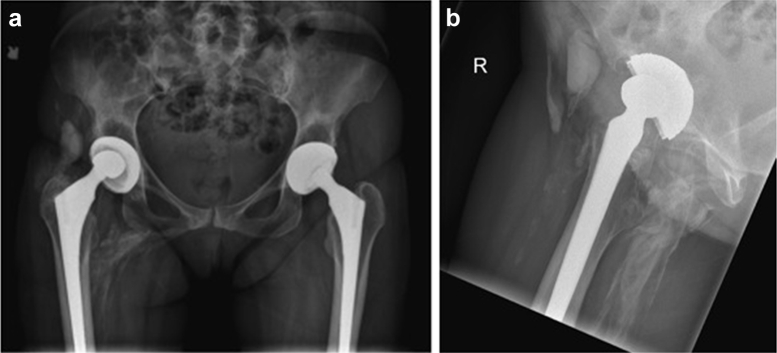
Figure 1.
(a) Prerevision anteroposterior radiograph pelvis and (b) lateral view showing misshapen head, “debris cloud,” and osteolysis.
Figure 2.
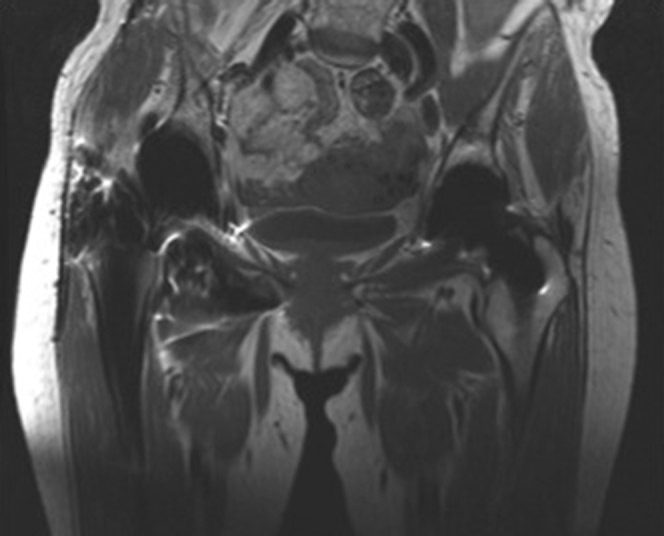
Prerevision T1 coronal pelvis magnetic resonance imaging demonstrating extensive foreign body reaction.
We performed a revision right THA with a 32-mm ceramic head on a polyethylene liner (Fig. 3a and b). On entering the relatively thin, attenuated pseudocapsule, a large amount of black fluid was encountered; fluid and tissue samples were sent for culture and Gram stain. Ultimately, cultures demonstrated no growth. The surrounding tissues and pseudocapsule were of poor quality with obvious black staining and areas of osteolysis posterior and superolaterally around the acetabular component. The bone otherwise appeared healthy and viable. The acetabular component was easily removed owing to the surrounding osteolysis with only one area of bony ingrowth measuring <1 cm × 1 cm. The shell was revised from a 52-mm shell to a Depuy Pinnacle revision 58-mm titanium shell (Depuy, Warsaw IN) with a 10-degree-elevated rim liner and placement of 5 titanium screws. The titanium Stryker secure-fit femoral component (Stryker, Kalamazoo MI) was well fixed and retained, but the femoral head was profoundly misshapen and removed (Fig. 4). The trunnion showed minor corrosion, and a CeramTec 32-mm ceramic head (CeramTec, Plochingen Germany) with a titanium revision sleeve was placed. After thorough debridement and betadine lavage, the wound was primarily closed [7].
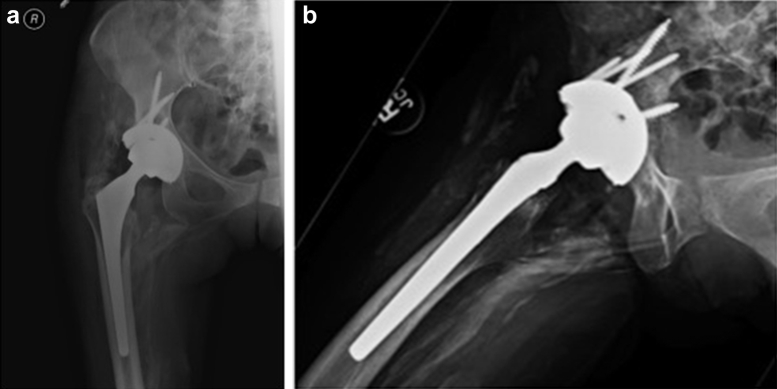
Figure 3.
(a) Postrevision anteroposterior pelvis radiograph and (b) lateral view showing a well-placed 58-mm Depuy Pinnacle revision cup with 5 screws and 32-mm ceramic head.
Figure 4.

Photograph of a retrieved, misshapen, 28-mm, cobalt-chrome, modular femoral head (left) next to an unused head of the same size (right).
Postoperatively, neurology, ophthalmology, and cardiology were consulted for management of new onset sinus tachycardia, visual changes, and distal lower extremity neuropathy with no acute intervention. She was discharged on postoperative day 3 without incident. Over the course of the following 4 months, the patient's symptoms steadily improved with cobalt levels of 468.8, 282.2, and 180.0 ppb at 2 weeks, 2 months, and 3 months, respectively. At her most recent follow-up visit, she has minimal pain with right hip range of motion and is able to ambulate comfortably. The sinus tachycardia resolved, although she reports occasional palpitations while sleeping and continues to smoke 1 pack of cigarettes per day. The cardio-reactive protein level has decreased from 19.16 (normal: 0-10mg/L) on initial presentation to 1.69 mg/L at 8 months postoperatively. The levothyroxine requirement for hypothyroidism remained constant at 25 mcg daily, whereas the thyroid-stimulating hormone level has steadily declined from 2.510 (0.350-5.500 uIU/mL) 1 month after surgery to 1.845 and 1.160 uIU/mL at 7 and 8 months postoperatively. Visual acuity and lower extremity neuropathy continue to slowly improve, and overall, she is content with her recovery and progress.
Discussion
Our patient presented with the most common symptoms associated with cobalt toxicity. From prior reports of metallosis, symptoms of toxicity present within 2 years after revision surgery to metal-on-polyethylene components from failed ceramic-on-ceramic components 8, 9, 10, 11, 12. However, our patient presented 8 years after revision with memory, sensory, ocular, and cardiac symptoms of roughly 1-year duration due to heavy metal toxicity, well outside the normal temporal onset of heavy metal toxicity.
It has been well established in the literature that revision of a fractured ceramic total hip replacement with metallic components can result in metallosis. Several authors have reported symptomatic cobalt toxicity after revision of a failed ceramic head component to a metal-on-polyethylene articulation, but there is a paucity of literature describing the failure of ceramic acetabular components 8, 10, 11, 12. These reports showed whole blood serum cobalt levels ranged from 398 to 6521 ppb, with one case resulting in fatal cardiomyopathy [8]. Our patient presented with a cobalt level of 788.1 ppb. In each case, a thick black fluid was encountered on entering the capsule, and an obviously misshapen metal femoral head was retrieved. Further analysis of the components showed ceramic material embedded in the polyethylene and surrounding tissues, which resulted in third-body wear on the femoral head.
Reports of cobalt toxicity from failed metal-on-metal THAs have reported whole blood levels in the range of 15-83 ppb, significantly less compared to third-body wear from revision failed ceramic components 13, 14, 15. This is likely related to both the volume and mechanism of how the metal particles are generated.
Given the multiple reports of cobalt toxicity after revision for failed ceramic components, it is recommended that revisions should consist of a harder material or a newer-generation ceramic replacement. Removal of all ceramic particles during revision requires extensive dissection including posterior and anterior synovectomies with no guarantee of complete success [6]. Avoiding this unnecessarily morbid dissection and addressing any malpositioning at the time of revision mitigates the potential for the complication of metallosis. If a metal head was implanted at the time of revision, careful clinical follow-up and monitoring of cobalt blood levels are warranted.
Summary
Failure of THA ceramic components, although less common since recent advances in manufacturing, is a difficult problem to address but must be approached appropriately [5]. Revision metal components create the potential for metallosis given the inability to reliably eliminate third-body wear by removing all ceramic fragments. Revision THA must consist of extensive debridement with incorporation of later-generation ceramic components and placement of a titanium revision sleeve if a previously used taper is retained to prevent the debilitating complication of heavy metal toxicity.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2015.09.002.
Appendix. Supplementary data
References
- 1.D'Antonio J.A., Capello W.N., Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop. 2012;470(2):373. doi: 10.1007/s11999-011-2076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher J., Jin Z., Tipper J., Stone M., Ingham E. Tribology of alternative bearings. Clin Orthop. 2006;453:25. doi: 10.1097/01.blo.0000238871.07604.49. [DOI] [PubMed] [Google Scholar]
- 3.Willmann G. Ceramic femoral head retrieval data. Clin Orthop. 2000;379:22. doi: 10.1097/00003086-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Whittingham-Jones P., Mann B., Coward P., Hart A.J., Skinner J.A. Fracture of a ceramic component in total hip replacement. J Bone Joint Surg Br. 2012;94(4):570. doi: 10.1302/0301-620X.94B4.28013. [DOI] [PubMed] [Google Scholar]
- 5.Massin P., Lopes R., Masson B., Mainard D., French Hip & Knee Society (SFHG) Does Biolox Delta ceramic reduce the rate of component fractures in total hip replacement? Orthop Traumatol Surg Res. 2014;100(6 Suppl):S317. doi: 10.1016/j.otsr.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Sharma V., Ranawat A.S., Rasquinha V.J. Revision total hip arthroplasty for ceramic head fracture: a long-term follow-up. J Arthroplasty. 2010;25(3):342. doi: 10.1016/j.arth.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Brown N.M., Cipriano C.A., Moric M., Sporer S.M., Della Valle C.J. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Zywiel M.G., Brandt J.M., Overgaard C.B. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95-B(1):31. doi: 10.1302/0301-620X.95B1.30060. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M., Sudo A., Uchida A. Cobalt-chromium head wear following revision hip arthroplasty performed after ceramic fracture—a case report. Acta Orthop. 2006;77(5):833. doi: 10.1080/17453670610013088. [DOI] [PubMed] [Google Scholar]
- 10.Matziolis G., Perka C., Disch A. Massive metallosis after revision of a fractured ceramic head onto a metal head. Arch Orthop Trauma Surg. 2003;123(1):48. doi: 10.1007/s00402-002-0449-9. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg M., Wegner R., Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009;24(5):825.e15. doi: 10.1016/j.arth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda T., Takahashi K., Kabata T. Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve. 2010;42(1):140. doi: 10.1002/mus.21638. [DOI] [PubMed] [Google Scholar]
- 13.Mao X., Wong A.A., Crawford R.W. Cobalt toxicity—an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649. doi: 10.5694/j.1326-5377.2011.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 14.Tower S.S. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 15.Tower S.S. Arthroprosthetic cobaltism associated with metal on metal hip implants. BMJ. 2012;344:e430. doi: 10.1136/bmj.e430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.