Abstract
Adverse local tissue reaction associated with total hip replacement may occur when mechanically assisted crevice corrosion occurs at metal-metal modular junctions in which at least one of the components is fabricated from cobalt-chromium alloy. Complete removal of components may be associated with significant morbidity; when components are well fixed and in acceptable position, it may be appropriate to consider modular rather than complete revision. We have diagnosed mechanically assisted crevice corrosion in total hip arthroplasty patients with noncontemporary but well-fixed femoral components and found that modular conversion to a ceramic femoral head to remove a source of CoCr corrosion and fretting products was only possible by having a custom titanium sleeve manufactured. Surgical implantation with a revision style Biolox ceramic head (CeramTec, Plochingen, Germany) was then achieved.
keywords: Adverse local tissue reaction, Total hip arthroplasty, Hip, Mechanically assisted crevice corrosion
Introduction
Serious adverse local tissue reactions (ALTRs) have become associated with metal-on-metal (MoM) joint failures, modular femoral neck components, and more recently, mechanically assisted crevice corrosion (MACC) at the taper interface of metal-on-polyethylene (MoP) total hip replacements 1, 2, 3. Although some contemporary surgeries seem to have a higher prevalence of this issue [1], nonmodern implants may also fail in this manner, particularly after many years in service or after prior femoral head revision.
We present an approach to patients with MACC and noncontemporary total hip arthroplasties (THAs). When only “off-the-shelf” cobalt-chromium (Co-Cr) alloy femoral head options for revision of their well-fixed femoral components are available, we describe commissioning a custom titanium sleeve to be made that could be used with an already manufactured Biolox ceramic femoral head (CeramTec, Plochingen, Germany) to remove the source of Co alloy at revision. In our experience, metal ion levels decrease postoperatively, and patients are satisfied and improved at follow-up. This is the first report, to our knowledge, that describes the use of a custom titanium sleeve for surgical treatment of MACC in conjunction with a well-fixed nonmodern stem in THA.
Surgical technique
Once revision THA for MACC is contemplated, the exact implant is researched, preferably by obtaining the implant identification stickers. The manufacturer is then contacted to confirm the availability of a revision femoral head other than one made from a Co alloy (eg, ceramic with a titanium revision sleeve, BioBall Adapter System, and ceramic head [Merete, Germany] or zirconium alloy metal substrate that transitions into a ceramic zirconium oxide outer surface [Oxinium; Smith & Nephew, Inc., Memphis, TN]).
When no other options are available, in the case of a well-fixed and well-positioned femoral component, we have requested a custom titanium sleeve to be manufactured that works in conjunction with an “off-the-shelf” revision Biolox ceramic femoral head (Biolox Option; CeramTec, Plochingen, Germany). We have requested that the femoral stem manufactures make this product, as they have the exact specifications of the femoral trunnion. Table 1 summarizes the steps necessary to manufacture such a custom product. Note that if the company has made <5 such custom products in the last year, the Federal Food, Drug, and Cosmetics act was amended to allow the company to manufacture these for compassionate use without institutional review board approval. Our institutional review board, however, noted that the Food and Drug Administration recommends that physicians should follow as many of the patient protection procedures as possible (Table 2).
Table 1.
General process for obtaining manufacture of a custom titanium sleeve to allow implantation with a revision style Biolox ceramic head (CeramTec, Plochingen, Germany).
| Confirm that ceramic or Oxinium (Smith & Nephew, Inc., Memphis, TN) revision femoral head component is not currently available for fixed femoral stem. Request prosthesis trunnion specifications from femoral stem manufacturer. Confirm that the custom titanium sleeve will work with implanted prosthesis (fixed femoral stem). Obtain an assessment from a physician (orthopaedic surgeon) who is not biased concurring with plan to use the custom component. Obtain compassionate use device documentation through hospital or practice IRB if indicated. Submit device description including planned neck length and head size (Special products Implant Request Form). Review manufacturing plan. Forward purchase order to manufacturer for two devices (in case second urgent surgery is needed or the first implant is contaminated). Obtain patient consent after reviewing risks, benefits, goals, and alternatives. Sign informed risk document for surgery. Proceed with surgery when device is available but have appropriate backup plan for revision if device is not suitable. |
Table 2.
Patient protection procedures recommended by the Food and Drug Administration for use of a custom device in joint replacement surgery.
| Informed consent from the patient or a legal representative. Clearance from the institution as specified by institutional policies. Concurrence by the IRB chairperson. Assessment from a physician (orthopaedic surgeon) who is not biased concurring with plan to use the custom component. Authorization from the investigational device exemption (IDE sponsor) if an IDE exists for the device. |
Of note, the surgeon should consider the exact femoral neck length that is being sought when manufacturing the custom sleeve. We choose the same or slightly longer length of the implanted neck if the leg length is acceptable to ensure stability at the time of revision. Also, we have had two sleeves per patient made, in case one is inadvertently contaminated during surgery or a repeat revision is needed.
The technique itself is exactly as for the Biolox Option (CeramTec, Plochingen, Germany) technique [4]. The ceramic femoral head is placed on the head adapter, and pressure is applied until resistance is felt. The ceramic femoral head must be placed straight down on the sleeve. The system components are then assembled on the femoral stem; no washing or cleaning is necessary.
Case example
The patient is a 52-year-old man who reported newly onset groin and buttock pain of the left hip 18 years after total hip replacement surgery for osteonecrosis and 5 years postrevision total hip replacement for instability. A 36-mm, medium-plus Co-Cr head was used on the 6° taper of the patient's titanium fiber metal ingrowth stem (Zimmer, Inc., Warsaw, IN). Physical examination of the patient demonstrated no gait impairment, and abductor strength was found to be satisfactory. Medical history was positive for diabetes mellitus, type 1.
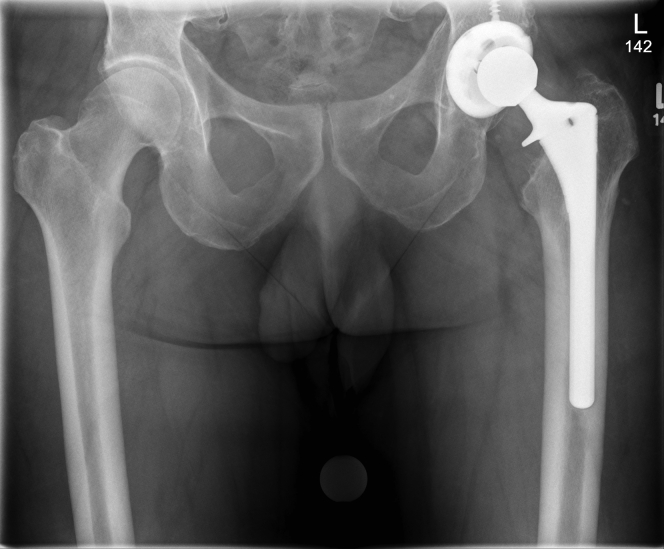
Radiographic examination showed no obvious evidence of osteolysis or loosening and that hip components were satisfactorily positioned. The calcar osteolysis associated with his prior surgery had not increased (Fig. 1). Laboratory tests conducted approximately 3 months after the onset of hip pain revealed serum Co (2.2 ppb; normal, <0.3 ppb) and Cr (2.4 ppb; normal, 0.0-0.9 ppb) ions in the blood. Complete blood count revealed a white blood cell count of 5600 cells/mm3 (normal, 4200-9900); C-reactive protein was found to be normal at 0.9 mg/L (normal, 0.0-8.0 mg/L), as was erythrocyte sedimentation rate at 8 mm/h (normal, 0-10 mm/h). Axial, coronal, and sagittal sequencing performed using metal artifact reduction sequence magnetic resonance imaging found no large volume of synovitis or effusion. There was a small collection of heterogeneous fluid detected in the periprosthetic soft tissues anteriorly measuring 1 × 1.8 cm, with no decomposition into the trochanteric or iliopsoas bursa. No evidence was found of muscle edema or tear of the muscle detachment. Joint aspiration demonstrated very high Co and Cr levels of 322 and 598.8 ppb, respectively. Gram stain and culture showed no bacteria present.
Figure 1.
Anteroposterior pelvis radiograph of a 52-year-old man with a revision metal-on-polyethylene bearing surface THA and new diagnosis of MACC. The acetabulum and femoral stem appear to be well fixed. Osteolysis associated with his prior surgery has not increased over time.
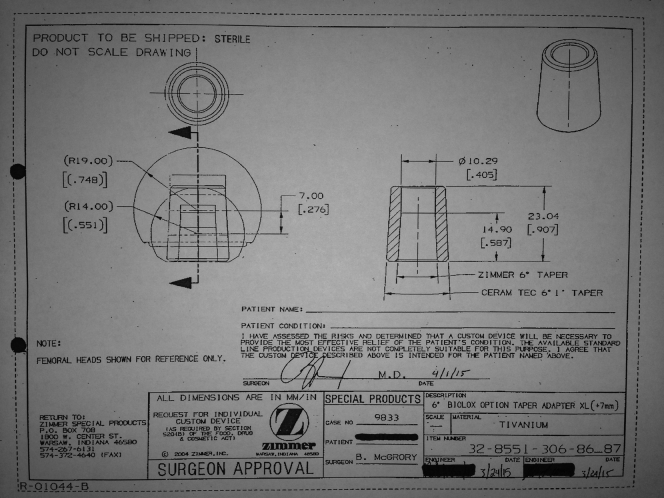
MACC of the trunnion was diagnosed. After shared decision-making discussion, the patient opted for revision surgery of his well-fixed, well-positioned total hip replacement. After contacting Zimmer, Inc. (Warsaw, IN), it was determined that a non-Co alloy head was not available for the 6° taper of the stem, so a custom titanium sleeve for use with a Biolox Option ceramic head was commissioned (Fig. 2).
Figure 2.
Drawing sent for surgeon approval of a custom titanium sleeve.
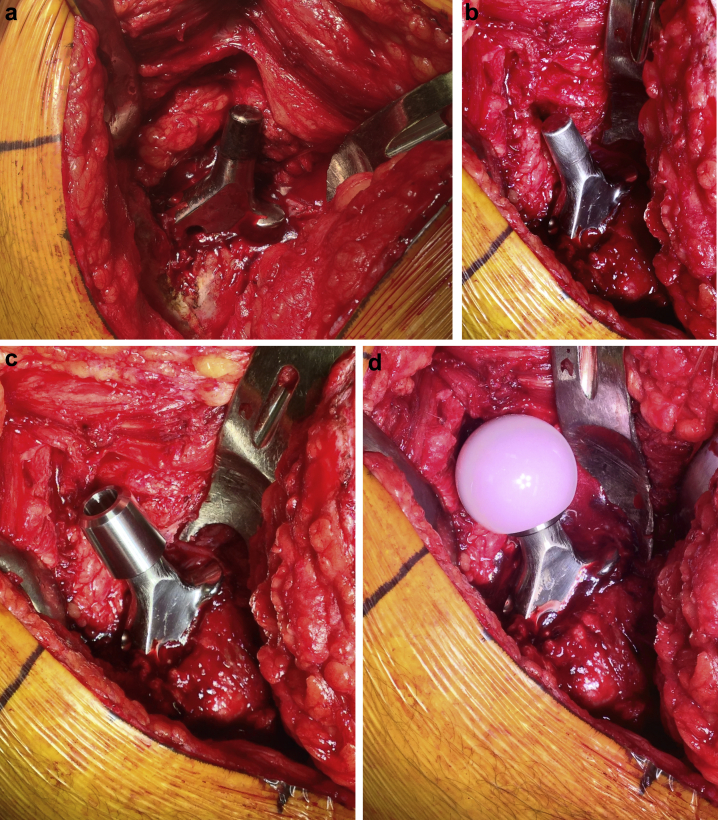
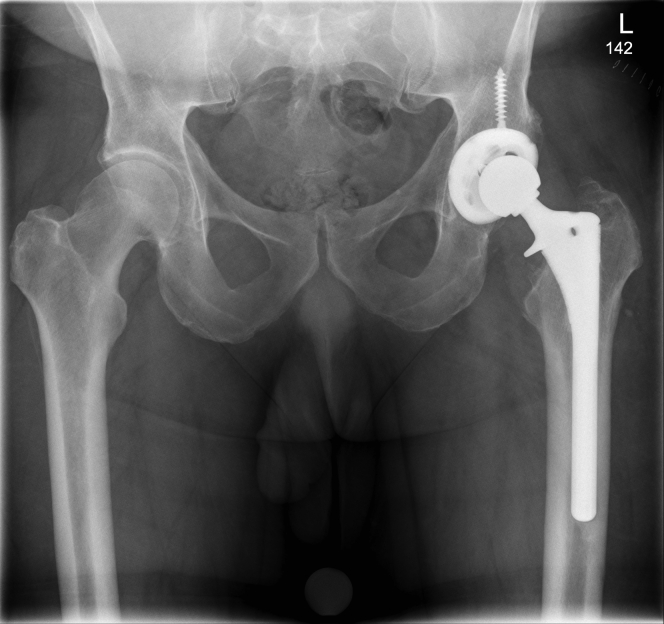
At surgery, MACC was confirmed with black discoloration and debris of the trunnion and inside of the femoral head (Fig. 3a). The trunnion was cleaned (Fig. 3b) after irrigation and debridement and polyethylene revision to treat third-body debris [5]. The sleeve was placed on the clean trunnion (Fig. 3c) until resistance was felt. The ceramic femoral head was then placed straight down on the sleeve and impacted as per the Zimmer protocol [4] (Fig. 3d). The hip was carefully reduced, and the hip capsule was reconstructed before closure. The patient recovered and is pain free with full function at 3 months postoperatively (Fig. 4). Serum Co is undetectable and serum Cr is 1.9 ppb.
Figure 3.
Intraoperative photograph of blackened trunnion associated with MACC (a), trunnion after cleaning and removal of discolored material (b), placement of a custom titanium sleeve onto the cleaned trunnion (c), and placement of Biolox Option (CeramTec, Plochingen, Germany) ceramic femoral head (d).
Figure 4.
Anteroposterior pelvis radiograph of the patient after revision using a custom titanium sleeve for conversion to a ceramic femoral head.
Discussion
MACC has been linked to a number of adverse outcomes in THA, including mechanical deficiency of the implant and ALTRs or adverse reaction to metal debris, so-called “pseudotumor” formation, osteolysis, and muscle and tissue necrosis and deficiency 1, 2, 3. Development of appropriate diagnosis and treatment guidelines for MACC is important, but until these are available, minimizing patient morbidity while removing or minimizing the intra-articular and serum Co and Cr metal ions seems prudent. This technique may be considered in select patients, where removal of a well-fixed, well-positioned stem is difficult or unwise, and the stem is noncontemporary without an option of an “off-the-shelf” non-Co alloy replacement head.
Our current definition of MACC agrees with that recently reported by the Rush University group [3]. They found that patients who present with new onset of postoperative pain, in whom the implants are well fixed and infection has been ruled out, should be evaluated with serum Co and Cr levels. Because previous work from their institution suggested that the serum Co level should be <1 ppb in a well-functioning THA with a MoP bearing [6], they used a Co level of >1 ppb as a diagnostic cutoff. We have also found that intra-articular serum levels of Co and Cr, although not mandatory, can be helpful in confirming MACC. We have found that they are commonly elevated 50 to 100 times or more the serum levels.
The Co-to-Cr ratio may also be helpful but is not pathognomonic. Although Plummer et al. [3] found that the abnormal serum Co level was significantly elevated above the serum Cr level (by a mean 11-to-2 ppb ratio) in MACC, less dramatic but asymmetrical ratios have been described with MoM total hip replacements 7, 8. On the other hand, the patient diagnosed with MACC presented here had a Co:Cr ratio of approximately 1, although his implant was a MoP articulation. Furthermore, Fehring et al. [9] have found that the Co:Cr ratio is not a predictive biomarker for ALTRs (they looked at MoM total hip replacement only.)
The logic of using only a ceramic head with a titanium sleeve in revision settings is based on manufacturer recommendations [10], and the fact that deformed areas of the previously used taper may create stress risers that can lead to ceramic crack initiation and propagation [11]. Although placement of a ceramic head directly on an undamaged but previously used trunnion has been successful at short-term follow-up in one study of 61 hips [11], case reports of ceramic head fracture in this situation have been reported 12, 13. Conversely, we could find no reports of a fractured third-generation ceramic head used with a revision sleeve, and the fracture rate for Biolox femoral heads (CeramTec, Plochingen, Germany) manufactured after 1994 has been reported to be 0.004% [10].
A thorough discussion with the patient before accepting the risks associated with the use of a custom implant should be undertaken. The patient should understand the risk of ceramic fracture and the ramifications of such a fracture. In cases where the well-fixed stem is made from a Co alloy, MACC may theoretically continue despite removing the Co alloy femoral head. On the other hand, the technique of revising the femoral head only, even for Co-Cr stems, is successful at significantly reducing serum Co and Cr levels at an average of 2.7 years [3]. Next, there are significant challenges for the surgeon in pursuing this approach including painstaking preoperative workup and the need for multiple surgical plans if the custom implant does not fit properly or instability is noted after reconstruction.
Summary
ALTR associated with MACC occurs at metal-metal modular junctions in which at least one of the components is fabricated from Co-Cr alloy. Complete removal of THA components may be associated with significant morbidity, and it may be appropriate to consider modular rather than complete revision in select patients. We describe the surgical technique of using a custom titanium sleeve with an “off-the-shelf” revision style Biolox ceramic head (CeramTec, Plochingen, Germany) for revision THA for MACC.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2015.10.001.
Appendix A. Supplementary data
References
- 1.McGrory B.J., MacKenzie J., Babikian G. A high prevalence of corrosion at the head-neck taper with contemporary Zimmer non-cemented hip components. J Arthroplasty. 2015;30:1265. doi: 10.1016/j.arth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Cooper H.J., Della Valle C.J., Berger R.A. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg. 2012;94-A:1655. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plummer D.R., Berger R.A., Paprosky W.G. Diagnosis and management of adverse local tissue reactions secondary to corrosion at the head-neck junction in patients with metal on polyethylene bearings. J Arthroplasty. 2015 doi: 10.1016/j.arth.2015.07.039. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Zimmer I. 2015. Zimmer Option Technique.www.productcompatibility.zimmer.com Available at: Accessed November 15, 2015. [Google Scholar]
- 5.Urban R.M., Jacobs J.J., Gilbert J.L., Galante J.O. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994;76:1345. doi: 10.2106/00004623-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Levine B.R., Hsu A.R., Skipor A.K. Ten-year outcome of serum metal ion levels after primary total hip arthroplasty. J Bone Joint Surg. 2013;95:512. doi: 10.2106/JBJS.L.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbuz D.S., Tanzer M., Greidanus N.V. The John Charnley Award: metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty, a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilo K., Hothi H., Whittaker R.K. et al.: Large diameter metal-on-metal arthroplasty: modularity effects blood metal ion levels ratio. Presented at the AAOS 2015 Annual Meeting. March 25–27, 2015; Las Vegas, NV, 2015.
- 9.Fehring T.K., Carter J.L., Fehring K.A. Cobalt to chromium ratio is not a key marker for adverse local tissue reaction (ALTR) in metal-on-metal hips. J Arthroplasty. 2015;30:107. doi: 10.1016/j.arth.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Willmann G. Ceramic femoral head retrieval data. Clin Orthop Relat Res. 2000;379:22. doi: 10.1097/00003086-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hannouche D., Delambre J., Zadegan F. Is there a risk in placing a ceramic head on a previously implanted trunion? Clin Orthop Relat Res. 2010;468:3322. doi: 10.1007/s11999-010-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulliam I.T., Trousdale R.T. Fracture of a ceramic femoral head after a revision operation: a case report. J Bone Joint Surg. 1999;79:118. doi: 10.2106/00004623-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Tai S.M.M., Parker L., de Roeck N.J. Case report: recurrent catastrophic ceramic femoral head failure in total hip arthroplasty. Case Rep Orthop. 2014;1:2014. doi: 10.1155/2014/837954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.