Abstract
This is one of the first documented cases of metal-on-metal (MoM) total hip arthroplasty cobalt cardiac toxicity that has led to patient death. A 69-year-old female presented to our institution with cardiac failure secondary to cobalt toxicity resulting from bilateral MoM total hip arthroplasty. Her presenting metal ion levels were a cobalt level of 199 ppb and a chromium level of 77 ppb. She underwent bilateral femoral revisions to remove the source of cobalt. On postoperative day 7, the patient sustained a cerebral infarct and eventually expired because of the insult. This case represents one of the first documented fatalities related to MoM cobalt cardiac toxicity.
Keywords: Cardiac cobaltism, Metal-on-metal (MoM), Total hip arthroplasty (THA)
Introduction
Metal-on-metal (MoM) total hip arthroplasty (THA) is no longer used at most institutions throughout the United States secondary to increased risks of implant failure [1]. This is largely after numerous studies documenting the adverse effects and high revision rates secondary to adverse local tissue reactions such as pseudotumors associated with this implant 2, 3, 4. Initially, MoM bearing surfaces were believed to be a method of eliminating osteolysis secondary to improved wear characteristics [5]. However, with the use of modern bearing surfaces (highly crosslinked polyethylene), we have witnessed improved wear characteristics and decreased osteolysis [6].
MoM THA can lead to elevated cobalt (Co) and chromium (Cr) metal ion levels; however, no specific reference values have been shown to identify all implants at risk of failure 3, 7. These studies have suggested that metal ion levels may serve as a surrogate for a poor-functioning MoM THA and likely require further investigation [8]. Increased metal ion levels have been associated with metallosis, adverse local tissue reactions, including pseudotumor formation [9]. Although rare, severe systemic side effects can result after MoM THA 10, 11, 12. Co toxicity may be a cause of many of these severe systemic manifestations including neurologic and cardiac symptomatology 13, 14, 15. Two case reports of Co toxicity resulting in a fatality have been reported previously to our knowledge 16, 17. We review a rare case of a bilateral MoM THA resulting in cardiac failure and subsequent mortality.
Case history
The patient was a previously healthy 64-year-old female who presented to an outside institution with a chief complaint of bilateral hip pain and a preoperative diagnosis of bilateral hip osteoarthritis. She underwent right THA in 2008 followed by left THA 10 months later. In each case, a posterior approach was used, with a MoM bearing surface consisting of a 50-mm acetabular component, +2-mm metal liner, and a 36-mm Co alloy head (DePuy Synthes, Warsaw, Indiana). On follow-up (2-years post-op) with her primary surgeon, metal ions levels were obtained secondary to isolated increasing hip pain in both hips. Her Co levels were found to be markedly elevated to 192 ppb (normal, <1 ppb; Fig. 1). Her acetabular components were in approximately 45° of abduction each. No prior metal ion levels were available for comparison. Revision surgery was being scheduled to address her hip pain in the setting of elevated metal ion levels at the outside facility. However, she presented with an impaired renal function (createnine = 4.1 mg/dL, normal 0.50-1.30 mg/dL) and slowly began to have increasing shortness of breath. An echocardiogram was performed documenting severely abnormal right and left ventricular function with an ejection fraction estimated at 10%-15%. Additionally, a cardiac magnetic resonance imaging was performed, which showed “an unusual delayed enhancement pattern and hyperenhancement of the atria.” The outside cardiologist proceeded with a cardiac biopsy to rule out Co cardiotoxicity given the echo and MRI abnormalities in the setting of severely elevated Co levels.
Figure 1.
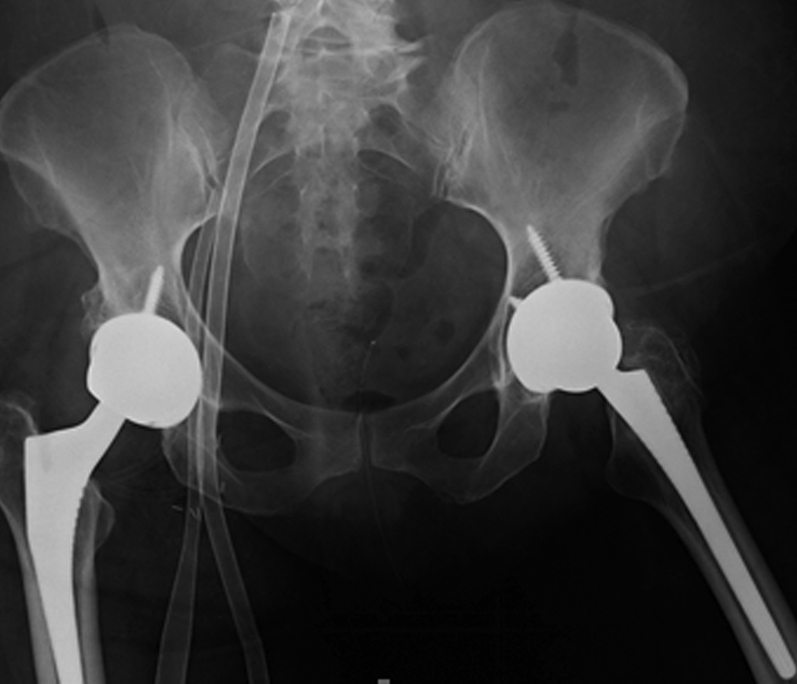
Preoperative revision anteroposterior pelvis radiograph.
The patient was transferred to our institution in critical condition and required the initiation of a biventricular assist device and extra corporeal circulation. A care conference, including orthopaedics, cardiology, and critical care, was held with the patient, and it was decided that the patient would undergo surgical intervention instead of nonoperative modalities. The patient underwent bilateral hip revision arthroplasty to exchange the MoM articulation for a ceramic-on-polyethylene bearing surface. An expeditious debridement was performed for removal of the damaged tissue secondary to the patient's medical condition and that she underwent bilateral surgeries under one anesthetic. No substantial abductor damage was noted. In each case, ceramic heads were used with a titanium sleeve (Fig. 2). On the right side, the acetabular component was found to be clinically overly anteverted, and a lipped liner was used as opposed to revising the acetabular component. Intraoperative findings included evidence of metallosis with gray-stained fluid and tissue on the right side more so than the left (Figs. 3 and 4). Intraoperative cultures were negative. A cystic mass was also identified which infiltrated each psoas bilaterally and was not debrided to expedite the surgical procedure. Postoperative radiographs are shown in Figure 5. Her postoperative course was complicated by increased drain output (1200 cc of sanguineous drainage over 8 hours) on postoperative day 3 after reinitiation of therapeutic anticoagulation and was subsequently held. Anticoagulation was resumed with intravenous heparin amidst concern regarding clot formation in the ventricular device lines. Renal function (increasing creatinine) continued to decline requiring the initiation of continuous venovenous hemofiltration, and she had a transient episode of acute onset hemiparesis and aphasia, which was thought to be an embolic event from her ventricular device. A computed tomography of her head at that time ruled out an intracranial bleed. She subsequently required reintubation, and on postoperative day 7 (hospital day 22), she suffered a hemorrhagic conversion of the previous embolic cerebrovascular event. At that point, the decision was made to transition to comfort care. The patient died the following day.
Figure 2.
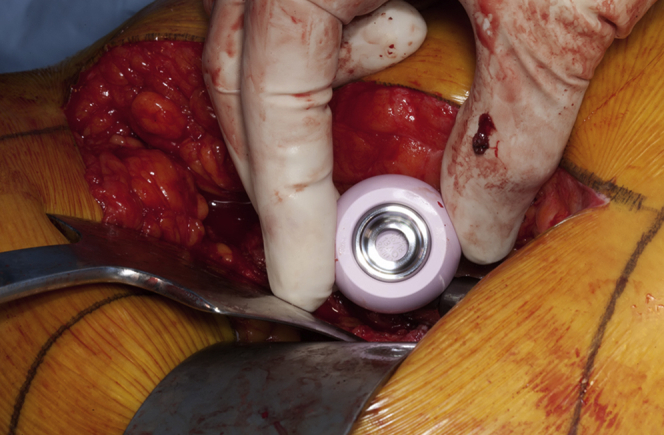
Intraoperative photo demonstrating placement of a ceramic femoral head with a titanium sleeve.
Figure 3.
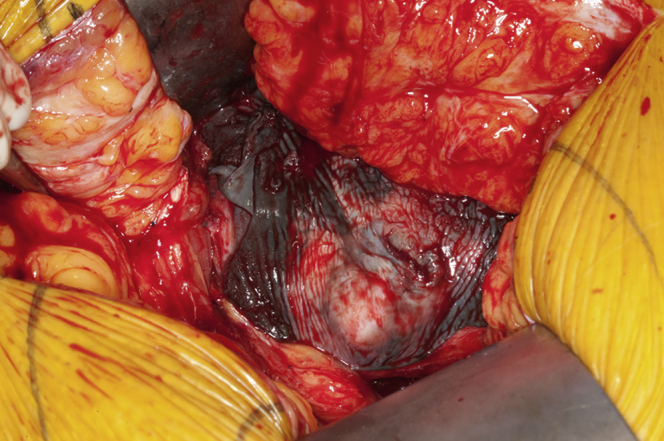
Intraoperative photograph showing dark staining of the surrounding soft tissue around the acetabulum consistent with metallosis.
Figure 4.
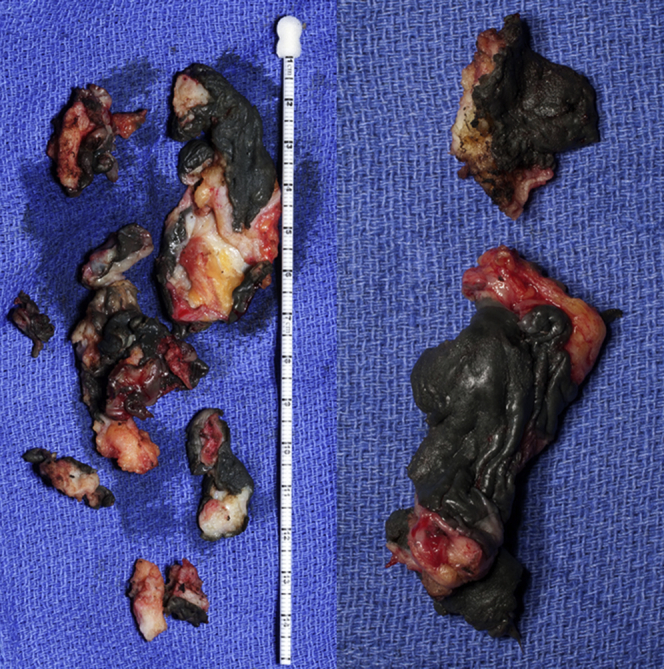
The dark-stained tissue was consistent with severe metallosis and pseudotumor tissue after debridement.
Figure 5.
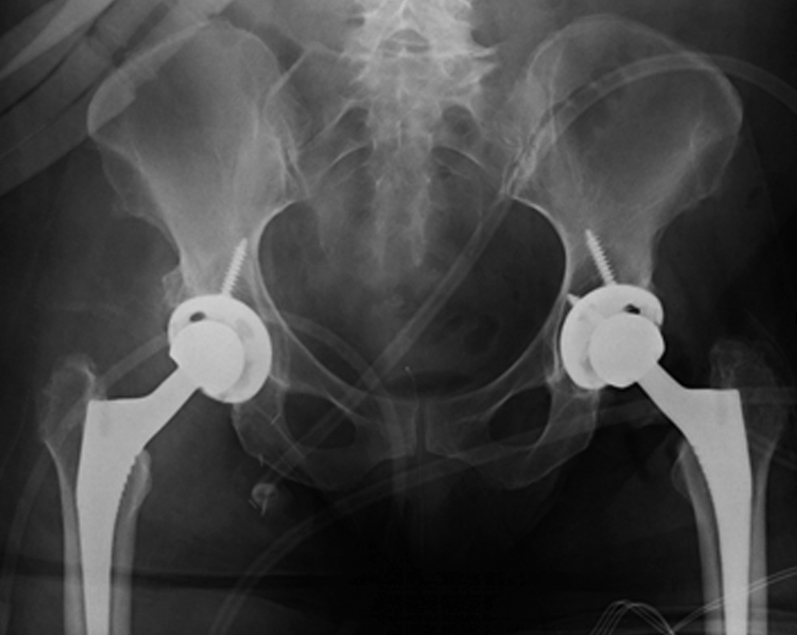
Postoperative anteroposterior pelvis radiograph.
A postmortem autopsy was performed on our patient at the request of the family. The report was consistent with diffuse Co deposition throughout the entirety of her body including the heart, liver, and kidneys. The gross pathology demonstrated evidence of severe metal staining in the soft tissue samples (Fig. 4). Additionally, the cardiac Co level was elevated to 4.75 mcg/gm of tissue (normal limit = 0.19 mcg/gm of tissue). The autopsy also confirmed a hemorrhagic stroke as the final insult that led to the patient's demise.
Discussion
Rarely, patients with MoM THA present with elevated metal ion levels (Co, >20 ppb). Elevated Co levels are currently believed to be the main cause of patient symptomatology in these cases [14]. Co toxicity has been well documented in the literature and can cause cardiomyopathy, hypothyroidism, polycythemia, and neuropathy complications 12, 15, 17. These features were initially described in beer drinkers in Quebec secondary to a Co product used for stabilizing the foam layer in beer [13]. The cardiovascular complications associated with Co toxicity can be as severe as a dilated cardiomyopathy with patients presenting with fatigue and shortness of breath approximately 8-40 weeks with elevated Co metal ion levels [18]. Luckily, there have been only a few cases of documented cardiotoxicity related to Co toxicity in the orthopaedic literature, and this case represents the first attempt to remove the source of Co toxicity by performing revision surgery in this setting 17, 19.
There are numerous proposed treatment options for Co toxicity; however, no uniform treatment algorithm exists. Isolated Co toxicity may be treated with metal ion chelators such as edetate calcium disodium, sodium 2,3-dimercaptopropane sulfonate, dimercaprol, and N-acetyl-cysteine 19, 20. Chelators work by binding metal ions and are then excreted from the body, lowering the overall metal ion levels. However, in total hip replacement, the source of Co is not removed and Co levels will likely return to baseline when the metal chelation is discontinued unless the source is removed. We believe revision and subsequent removal of CoCr metal heads and liners or cups in the setting of Co toxicity appear to be the best option at definitively lowering Co levels because it completely removes the source of Co.
The pathology report demonstrated elevated cardiac and serum Co levels in our patient. Our patient presented with a dilated cardiomyopathy likely secondary to Co toxicity. Her cardiac biopsy sample revealed a Co level 25 times higher than the normal limits. The electron microscopy from the cardiac tissue biopsy showed increased lipofuscin and vacuolar spaces consistent with Co toxicity (Fig. 6). In addition, our pathologists were able to confirm our clinical impression that Co toxicity likely contributed to the death of this patient secondary to cardiac cobaltism.
Figure 6.
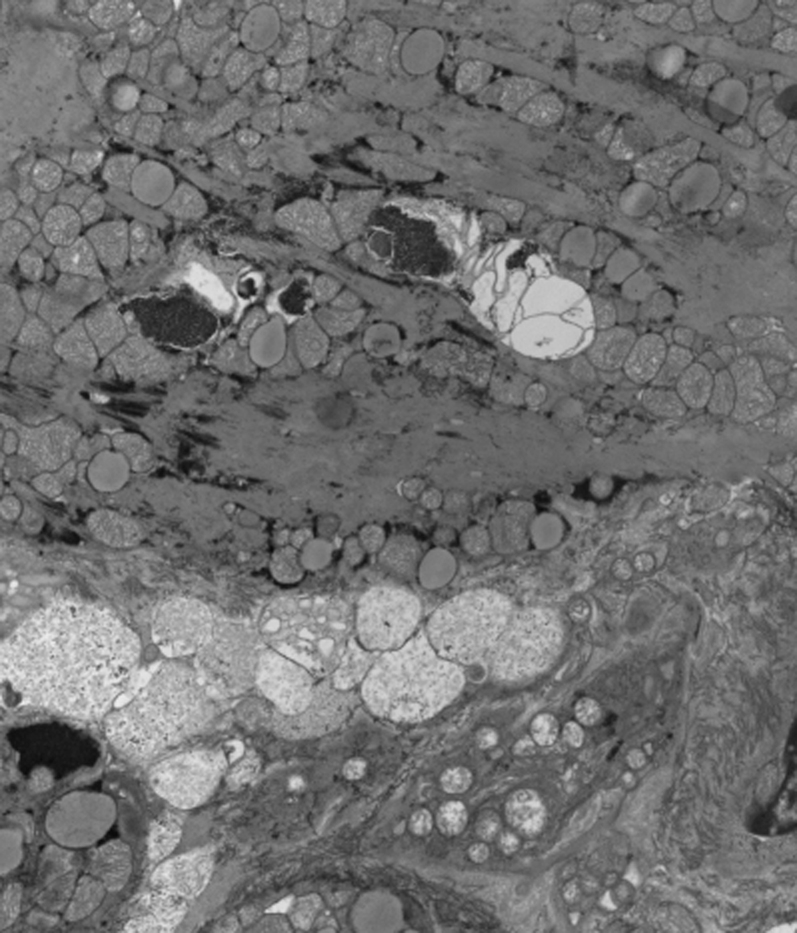
An electron microscopy of the cardiac biopsy that shows increased lipofuscin and vacuolar spaces consistent with cardiac cobalt toxicity.
MoM THA has largely disappeared from use in the United States. Fatigue and shortness of breath in the setting of MoM THA may be an indication of Co toxicity, although we acknowledge that this is exceedingly rare. At this time, we are unable to comment on screening protocols for patients with similar presentations. A reasonable algorithm for evaluating and managing patients with an MoM THA was published by Kwon et al. [4]. There is currently no cutoff Co level that indicates systemic toxicity, but levels >100 ppb may require further evaluation. In addition, definitive treatment should include replacement of the CoCr head with a ceramic head to remove the source of Co. Adjunctive treatment with chelators may be necessary in the acute period for patient stabilization but do not represent a definitive management strategy.
Summary
This case represents one of the first reports of a mortality associated with Co toxicity secondary to bilateral MoM THA. Co toxicity has been well defined in the literature and may present as cardiomyopathy, hypothyroidism, polycythemia, and neuropathy. These presentations in the setting of THA are rare, and the majority of reported complications associated with MoM THA are adverse local tissue reactions. Patients should be worked up with metal ion levels and possible confirmatory biopsy if clinically indicated. Treatment should consist of replacement of the CoCr head with a revision ceramic head and titanium sleeve and possibly the use of chelators in the setting of acute Co toxicity.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2015.10.002.
Appendix. Supplementary data
References
- 1.Kwon Y.M., Lombardi A.V., Jacobs J.J. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96:e4. doi: 10.2106/JBJS.M.00160. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann A., Hannemann F., Lützner J. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing—systematic review of clinical and epidemiological studies. PLoS One. 2013;8:e70359. doi: 10.1371/journal.pone.0070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Weegen W., Hoekstra H.J., Sijbesma T. Survival of metal-on-metal hip resurfacing arthroplasty: a systematic review of the literature. J Bone Joint Surg Br. 2011;93:298. doi: 10.1302/0301-620X.93B3.25594. [DOI] [PubMed] [Google Scholar]
- 4.Streicher R.M., Semlitsch M, Schön R, Weber H, Rieker C. Metal-on-metal articulation for artificial hip joints: laboratory study and clinical results. Proc Inst Mech Eng H. 1996;210:223. doi: 10.1243/PIME_PROC_1996_210_416_02. [DOI] [PubMed] [Google Scholar]
- 5.Dion N.T., Bragdon C., Muratoglu O., Freiberg A.A. Durability of highly cross-linked polyethylene in total hip and total knee arthroplasty. Orthop Clin North Am. 2015;46(3):321. doi: 10.1016/j.ocl.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs J.J., Skipor A.K., Patterson L.M. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Daniel J., Ziaee H., Pynsent P.B., McMinn D.J.W. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br. 2007;89:736. doi: 10.1302/0301-620X.89B6.18141. [DOI] [PubMed] [Google Scholar]
- 8.Clayton R.A., Beggs I., Salter D.M. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report. J Bone Joint Surg Am. 2008;90:1988. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 9.Rizzetti M.C., Ziaee H., Pynsent P.B., McMinn D.J.W. Loss of sight and sound. Could it be the hip? Lancet. 2009;373:1052. doi: 10.1016/S0140-6736(09)60490-6. [DOI] [PubMed] [Google Scholar]
- 10.Tower S.S. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 11.Devlin J.J., Pomerleau A.C., Brent J.M. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. J Med Tox. 2013;9:405. doi: 10.1007/s13181-013-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seghizzi P., D'Adda F., Borleri D., Barbic F., Mosconi G. Cobalt myocardiopathy. A critical review of literature. Sci Total Environ. 1994;150:105. doi: 10.1016/0048-9697(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 13.Mao X., Wong A.A., Crawford R.W. Cobalt toxicity—an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194:649. doi: 10.5694/j.1326-5377.2011.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 14.Morin Y.L., Foley A.R., Martineau G., Roussel J. Quebec beer-drinkers' cardiomyopathy: forty-eight cases. Can Med Assoc J. 1967;97:881. [PMC free article] [PubMed] [Google Scholar]
- 15.Zywiel M.G., Brandt J-M., Overgaard C.B. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. BJJ. 2013;95:31. doi: 10.1302/0301-620X.95B1.30060. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert C.J., Cheung A., Butany J. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29:639. doi: 10.1016/j.cjca.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Apel W., Denis S., Anthony O.S., Ling J. Cobalt-chromium toxic retinopathy case study. Documenta ophthalmologica. Adv Ophthalmol. 2013;126:69. doi: 10.1007/s10633-012-9356-8. [DOI] [PubMed] [Google Scholar]
- 18.Paustenbach D.J., Tvermoes B.E., Unice K.M., Finley B.L., Kerger B.D. A review of the health hazards posed by cobalt. Crit Rev Toxicol. 2013;43(4):316. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 19.Devlin J.J., Schwartz M., Brent J. Chelation in suspected prosthetic hip-associated cobalt toxicity. Can J Cardiol. 2013;29:1533. doi: 10.1016/j.cjca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Giampreti A., Lonati D., Locatelli C.A. Chelation in suspected prosthetic hip-associated cobalt toxicity. Can J Cardiol. 2014;30:465. doi: 10.1016/j.cjca.2013.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


