Abstract
The rising incidence of Clostridium difficile infections (CDIs) in adults is partly related to the global spread of fluoroquinolone-resistant strains, namely, BI/NAP1/027. Although CDIs are also increasingly diagnosed in children, BI/NAP1/027 is relatively uncommon in children. Little is known about the antibiotic susceptibility of pediatric CDI isolates. C. difficile was cultured from tcdB-positive stools collected from children diagnosed with CDI between December 2012 and December 2013 at an academic children's hospital. CDI isolates were grouped by restriction endonuclease analysis (REA). MICs were measured by agar dilution method for 7 antibiotics. Susceptibility breakpoints were based on guidelines from CLSI and/or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). MICs and REA groupings of C. difficile isolates from 74 adult patients (29 isolates underwent REA) from a temporally and geographically similar adult cohort were compared to those of pediatric isolates. Among 122 pediatric and 74 adult isolates, respectively, the rates of resistance were as follows: metronidazole, 0% and 0%; vancomycin, 0% and 8% (P = 0.003); rifaximin, 1.6% and 6.7% (P = 0.11); clindamycin, 18.9% and 25.3% (P = 0.29); and moxifloxacin, 2.5% and 36% (P = <0.0001). Only 1 of 122 (0.8%) BI/NAP1/027 isolates was identified among the children, compared to 9 of 29 (31%) isolates identified among the adults (P = <0.0001). The 3 moxifloxacin-resistant pediatric isolates were of REA groups BI and CF and a nonspecific group. The 2 rifaximin-resistant pediatric isolates were of REA groups DH and Y. The 21 clindamycin-resistant pediatric isolates were distributed among 9 REA groups (groups A, CF, DH, G, L, M, and Y and 2 unique nonspecific REA groups). These data suggest that a diverse array of relatively antibiotic-susceptible C. difficile strains predominate in a cohort of children with CDI compared to adults.
INTRODUCTION
The increased frequency, morbidity, and mortality of Clostridium difficile infections (CDI) in adults over the past 2 decades are primarily attributable to the emergence and global spread of a fluoroquinolone-resistant strain known as BI/NAP1/027 (1, 2). CDI is now the most frequently encountered health care-associated infection in the United States (3), causing nearly 500,000 infections and 30,000 deaths each year (4). These troubling trends in the clinical and molecular epidemiology of CDI recently prompted the Centers for Disease Control and Prevention (CDC) to classify CDI among the most serious immediate antibiotic-resistant diseases representing “public health threats that require urgent and aggressive action” (5).
The molecular epidemiology (6–8) and antibiotic resistance (7–9) patterns of CDI in adults over the past 3 decades have been well described. Although the prevalence of BI/NAP1/027 is declining, recent data from the United States (4) and the United Kingdom (10) indicate that BI/NAP1/027 is still an important cause of CDI in adults. Limited investigation of the molecular epidemiology of pediatric CDI suggests that BI/NAP1/027 is much less common in pediatric populations (11, 12). In addition, preceding antibiotic use is relatively infrequent among pediatric patients with CDI (13). Very little is known about the antibiotic resistance patterns of C. difficile isolates in pediatric patients. The primary objective of this study was to describe the antibiotic susceptibility patterns of pediatric CDI isolates that were previously characterized by restriction endonuclease analysis (REA) (11). Secondarily, we compared the molecular epidemiology and antibiotic susceptibility patterns of CDI isolates derived from our pediatric cohort to those determined for a geographically and temporally related adult cohort.
MATERIALS AND METHODS
Study setting, patients, and bacterial isolates.
This retrospective cohort study was performed at Ann & Robert H. Lurie Children's Hospital of Chicago (Lurie Children's), an urban tertiary-care academic children's hospital. The pediatric cohort included all children ≥12 months old diagnosed with CDI by tcdB PCR between December 2012 and December 2013, as well as a small convenience sample of children diagnosed with CDI between March 2011 and October 2012. A geographically (i.e., Chicago area) and temporally (i.e., year 2013) similar adult cohort was assembled using data collected as part of an unrelated study and included only adult patients diagnosed with CDI at Hines Veterans Affairs (VA) Hospital in 2013. The Institutional Review Board at Lurie Children's and the Institutional Review Board at Hines VA Hospital waived informed consent.
As previously described, frozen stools from patients with CDI were cultured anaerobically for C. difficile (14), and C. difficile isolates were grouped by REA (15). All pediatric isolates included in this study underwent REA, but only a subset of randomly selected adult isolates underwent REA. Using REA, each isolate from an individual patient with multiple CDI episodes during the study period was classified as either a relapse isolate (the isolate belonged to the same REA group as the previously collected isolate) or a reinfection isolate (the isolate belonged to a REA group different from the group to which the previously collected isolate belonged) as previously described (16).
Antibiotic agents and agar susceptibility testing.
For the C. difficile isolates from pediatric patients, the following 7 antibiotics were tested at the Loyola University Chicago Stritch School of Medicine: metronidazole, vancomycin, rifaximin, fidaxomicin, surotomycin, clindamycin, and moxifloxacin. The antibiotics were tested using the following mean inhibitory concentration (MIC) ranges: metronidazole, 0.06 to 16 μg/ml; vancomycin, 0.125 to 16 μg/ml; rifaximin, 0.002 to 32 μg/ml; fidaxomicin, 0.0078 to 32 μg/ml; surotomycin, 0.015 to 16 μg/ml; clindamycin, 0.125 to 64 μg/ml; and moxifloxacin, 0.06 to 32 μg/ml. Antibiotics were dissolved and diluted according to Clinical Laboratory and Standards Institute (CLSI) guidelines (17, 18). For the C. difficile isolates from adult patients, all of the antibiotics listed above, with the exception of surotomycin, were tested at Tufts University School of Medicine. The tested MIC ranges were the same as those determined for the pediatric isolates, with the following exceptions: rifaximin, 0.004 to 4 μg/ml; fidaxomicin, 0.0078 to 4 μg/ml; and clindamycin, 0.5 to 16 μg/ml.
At both laboratories, the CLSI-recommended reference agar dilution method for anaerobes (guidelines M11-A8 and M100-S25) was used for susceptibility testing (17, 18). The interpretation of endpoints was conducted according to CLSI guidelines M11-A8 and M100-S25 (17, 18). Breakpoints were set for metronidazole (≥32 μg/ml), clindamycin (≥8 μg/ml), and moxifloxacin (≥8 μg/ml) based on CLSI breakpoints (17) and for vancomycin (≥4 μg/ml) based on the epidemiological cutoff value recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (19). For pediatric isolates, the rifaximin resistance breakpoint (≥32 μg/ml) was based on previously published methods (7). For the adult isolates, rifaximin resistance was defined as ≥4 μg/ml because that was the maximum MIC tested for isolates from that cohort. No recommended breakpoints are available for fidaxomicin and surotomycin.
Data collection and statistical analysis.
For the pediatric patients, antibiotic exposures in the previous 90 days were electronically extracted from the medical record. Geometric mean (GM) MIC, MIC50, and MIC90 values were calculated for each antibiotic. For antibiotics with MIC values above or below the range of testing, the following adjustments were made to calculate the GM MIC: MIC values less than or equal to the minimum concentration tested were adjusted to the minimum concentration tested, and MIC values exceeding the maximum concentration tested were adjusted to a value representative of 1 dilution higher than the maximum concentration tested. The proportions of isolates resistant to each antibiotic were calculated.
Proportions were compared using Fisher's exact test, and GM MICs were compared using the Wilcoxon rank sum test for independent samples and the Wilcoxon signed-rank test for related samples. Because of the differences between the ranges of drug MICs tested for the pediatric and adult isolates for rifaximin and clindamycin, drug MICs for the pediatric isolates that exceeded the maximum MIC tested for adult isolates were adjusted to the adult isolate ranges prior to statistical comparisons of adult and pediatric GM MICs. Two-sided P values of <0.05 were considered statistically significant. Analyses were performed using Stata/IC statistical software, version 12.1 (StatCorp, College Station, TX).
RESULTS
Antibiotic susceptibility of pediatric isolates.
A total of 143 pediatric isolates underwent REA and antibiotic susceptibility testing. Of the 143 isolates, there were 21 CDI relapse isolates (i.e., the subsequent isolate from an individual patient belonged to the same REA group as the previous CDI isolate) among 16 patients; 4 patients had multiple CDI relapse isolates. In addition, 13 isolates were classified as representing C. difficile reinfections (i.e., the subsequent isolate from an individual patient belonged to a REA group different from that to which the previous CDI isolate belonged). To construct an antibiogram of the pediatric C. difficile isolates at Lurie Children's, the 21 CDI relapse isolates were excluded. Thus, 122 CDI isolates collected from 110 individual patients were analyzed. The MIC data for the 7 antibiotics tested are listed in Tables 1 and 2.
TABLE 1.
Distribution of drug MICs among 122 clinical C. difficile isolates from children
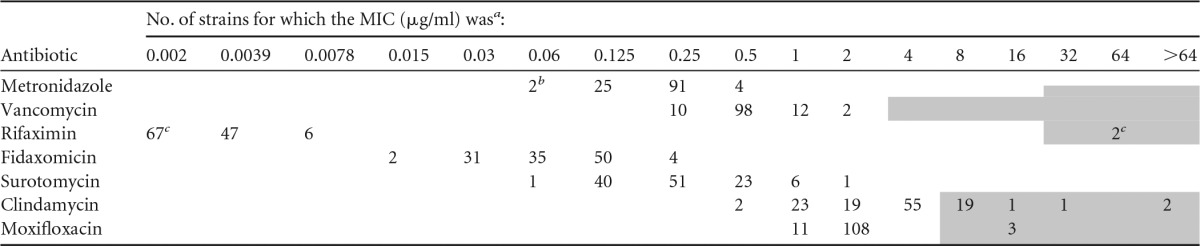
a Shaded cells indicate resistance-level MICs for which recommended breakpoints/cutoffs are available.
b Two isolates had metronidazole MICs of ≤0.06 μg/ml.
c Two isolates had rifaximin MICs of >32 μg/ml, and 65 isolates had MICs of ≤0.002 μg/ml.
TABLE 2.
Summary of MIC data among 122 clinical C. difficile isolates from children and comparisons to 74 isolates from adultsa
| Antibiotic | Pediatric isolates |
Adult isolates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) |
Susceptibility profile |
GM MIC (μg/ml) | P | % Res | P | ||||||
| MIC range | GM MIC | MIC50 | MIC90 | % Sens | % Int | % Res | |||||
| Metronidazole | ≤0.06 to 1 | 0.22 | 0.25 | 0.25 | 100 | 0 | 0 | 0.94 | <0.0001 | 0 | |
| Vancomycin | 0.25 to 2 | 0.52 | 0.5 | 1 | 100 | NA | 0 | 1.23 | <0.0001 | 8 | 0.0032 |
| Rifaximin | ≤0.002 to >32 | 0.0033 | 0.002 | 0.0039 | 98.4 | NA | 1.6 | 0.021 | <0.0001 | 6.7 | 0.11 |
| Fidaxomicin | 0.015 to 0.25 | 0.070 | 0.06 | 0.125 | NA | NA | NA | 0.16 | <0.0001 | NA | NA |
| Surotomycin | 0.06 to 2 | 0.24 | 0.25 | 0.5 | NA | NA | NA | ND | ND | ND | ND |
| Clindamycin | 0.5 to >64 | 3.24 | 4 | 8 | 36 | 45.1 | 18.9 | 3.42 | 0.37 | 25.3 | 0.29 |
| Moxifloxacin | 1 to 16 | 1.98 | 2 | 2 | 97.5 | 0 | 2.5 | 3.93 | <0.001 | 36 | <0.0001 |
Bolded P values indicate statistical significance. GM, geometric mean; Sens, sensitive; Int, intermediate; Res, resistant; NA, recommended breakpoints/cutoffs are not available; ND, assay not done for the adult isolates.
Overall, with the exception of clindamycin (18.9% resistance rate), the rate of antibiotic resistance was low (<3%) among the pediatric isolates. Clindamycin exposure in the previous 90 days was infrequent, and the levels of exposure did not significantly differ between patients with CDI caused by clindamycin-resistant strains and those with CDI caused by clindamycin-susceptible strains (6/23 [26%] versus 14/99 [14%]; odds ratio, 2.1; 95% confidence interval, 0.59 to 7.0; P = 0.21). The REA groups of the 23 clindamycin-resistant isolates were as follows: A (n = 1), CF (n = 4), DH (n = 6), G (n = 1), L (n = 1), M (n = 2), Y (n = 6), and nonspecific (n = 2). Only 2 (1.6%) rifaximin-resistant isolates were identified in the pediatric cohort (REA groups DH and Y), and only 1 of these patients had had exposure to a rifamycin-class antibiotic in the previous 90 days. In total, only 3 (2.5%) patients in the pediatric cohort had had exposure to a rifamycin-class antibiotic in the previous 90 days, and only 1 of them had developed CDI with a rifaximin-resistant strain. Only 3 (2.5%) moxifloxacin-resistant isolates (REA groups BI, CF, and nonspecific) were identified in the pediatric cohort, and none of the patients had had exposure to a fluoroquinolone antibiotic in the previous 90 days. In total, 13 (10.7%) patients in the pediatric cohort had had exposure to a fluoroquinolone antibiotic (ciprofloxacin) in the previous 90 days, and none of them developed CDI with a fluoroquinolone-resistant strain.
To assay for acquisition of antibiotic resistance with subsequent CDIs, the original and subsequent C. difficile isolates from patients with CDI relapse were compared. The GM drug MICs for the original isolate and the first relapse isolate among pediatric patients did not significantly differ for any of the 7 antibiotics tested (Table 3).
TABLE 3.
Comparison of GM MICs between the original isolate and the first relapse isolate in 16 pediatric patients with multiple C. difficile infections
| Antibiotic | GMa MIC (μg/ml) |
P | |
|---|---|---|---|
| Original isolate | Relapse isolate | ||
| Metronidazole | 0.20 | 0.24 | 0.42 |
| Vancomycin | 0.55 | 0.59 | 0.56 |
| Rifaximin | 0.0058 | 0.0090 | 0.54 |
| Fidaxomicin | 0.91 | 0.87 | 0.68 |
| Surotomycin | 0.26 | 0.32 | 0.13 |
| Clindamycin | 4.36 | 4.76 | 0.71 |
| Moxifloxacin | 1.92 | 1.92 | 1 |
GM, geometric mean.
Molecular epidemiology and antibiotic susceptibility of adult and pediatric isolates.
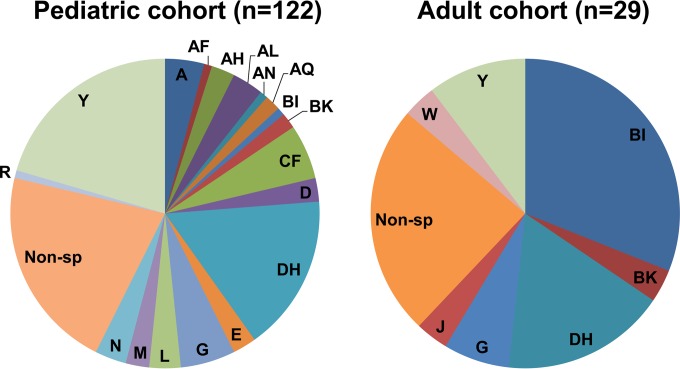
Antibiotic susceptibility testing was performed on 74 clinical C. difficile isolates derived from a geographically and temporally similar adult cohort. REA was performed on all (n = 122) of the pediatric isolates and on 29/74 (39%) randomly selected adult isolates. The REA groupings in each cohort are illustrated in Fig. 1. Isolates from adult patients were more frequently resistant to vancomycin and moxifloxacin and had significantly higher GM MICs of all antibiotics tested, with the exception of clindamycin, than isolates from the pediatric cohort (Table 2). All vancomycin-resistant adult isolates had a vancomycin MIC equal to 4 μg/ml, which is the EUCAST epidemiologic cutoff for vancomycin resistance (19). The differences in the frequencies of moxifloxacin resistance were related to the differences in the proportions of REA group BI strains between the cohorts. The pediatric cohort had a statistically significant lower proportion of isolates belonging to REA group BI than the adult cohort (1/122 [0.8%] versus 9/29 [31%], P < 0.0001). Of the 29 adult isolates undergoing REA, 10 (34%) were moxifloxacin resistant, and 8/10 (80%) belonged to REA group BI (other moxifloxacin-resistant isolates belonged to the REA G group and a nonspecific group). In total, 8/9 (89%) of the BI isolates in the adult cohort were moxifloxacin resistant.
FIG 1.
REA groupings of C. difficile isolates in the pediatric and adult cohorts. Non-sp, nonspecific.
DISCUSSION
Clinical C. difficile isolates obtained from a large single-center pediatric cohort revealed a wide variety of strain types causing CDI, and only 1 (<1%) pediatric isolate was identified as epidemic strain BI/NAP1/027. This diverse set of isolates from children with CDI demonstrated 100% susceptibility to first-line CDI therapies (i.e., metronidazole and vancomycin) (20); a very low rate of resistance to rifaximin, a second-line therapy typically reserved for children with multiply recurrent CDI (20); and favorable MICs of fidaxomicin and surotomycin, both of which are new CDI therapies currently being investigated in phase 3 clinical trials in pediatric patients (21).
With the exception of clindamycin, the GM MICs of all antibiotics tested for isolates from pediatric patients were significantly lower than those seen with isolates from a temporally and geographically similar adult patient cohort. However, the proportions of isolates with drug MICs greater than the breakpoints for resistance were significantly higher in adult patients only for vancomycin and moxifloxacin. Because vancomycin had a MIC equal to 4 μg/ml, which is the EUCAST epidemiologic cutoff for vancomycin resistance, for all adult isolates resistant to vancomycin (19), and because vancomycin has poor systemic absorption and achieves fecal concentrations well above this MIC (22), this difference is unlikely to be clinically significant. The significantly higher proportion of moxifloxacin-resistant isolates in adult patients is likely related to the significantly higher proportion of BI/NAP1/027 strains in the adult cohort. The reasons for the substantially different proportions of BI/NAP1/027 are not entirely clear but are likely related to the generally infrequent use of fluoroquinolones in children.
The relatively infrequent rate of antibiotic resistance among pediatric C. difficile clinical isolates may be related to differences in antibiotic exposure between adult and pediatric patients with CDI. In our pediatric cohort, 51% of patients had had documented antibiotic exposure in the previous 30 days (11), and this is consistent with other pediatric studies (13). Among the patients in our cohort (11), the proportions of children with CDI who had received clindamycin, a fluoroquinolone, a first- or second-generation cephalosporin, a third- or fourth-generation cephalosporin, or a carbapenem within 30 days prior to their CDI diagnosis were 8%, 4%, 8%, 24%, and 3%, respectively. Whereas recent antibiotic use is reported in >85% of adult patients with CDI, recent antibiotic exposure is less frequently (∼35% to ∼75%) reported among children with CDI (23). Antibiotic exposure is particularly infrequent among children with community-associated CDI (CA-CDI). For example, we previously reported antibiotic exposure in the previous 30 days among 26% of children with CA-CDI (compared to 88% of children with hospital-onset health care facility-associated [HO-HCFA] CDI), and Tschudin-Sutter et al. reported antibiotic exposure in the previous 30 days among 42% of children with CA-CDI (compared to 76% of children with HCFA-CDI). Furthermore, we previously reported that CA-CDI is the most common type of CDI in a pediatric cohort (11), and other population-based studies support this; CA-CDI accounted for 60% to 80% of CDIs, depending on age, in a U.S. surveillance study (24) and for 75% of cases in a population-based study in Minnesota (25). Thus, because of the predominance of CA-CDI in children and the relatively infrequent rate of antibiotic exposure preceding CA-CDI in children, this may account for the favorable susceptibility profile among our pediatric isolates.
As with adults, recurrent CDI is frequent among children, occurring in 12% to 22% of pediatric patients (11, 26, 27). Despite the high frequency of CDI recurrence, the optimal method of management of children with multiple recurrences is unknown. In this study, GM MICs of drugs did not significantly differ between initial and subsequent isolates from patients with CDI relapse (i.e., initial and subsequent infection caused by the same REA group). These data suggest that acquisition of strains with reduced susceptibility to metronidazole or vancomycin likely does not contribute to recurrences. These data may reinforce the previously reported importance of host factors, such as immunocompromising conditions (26, 28) and continued perturbation of the normal intestinal microbiota through concomitant antibiotic exposure (27), as the primary risk factors for CDI recurrence in children. Other factors contributing to recurrence may be related to the inability of antibiotic therapy to eradicate C. difficile spores from the intestinal tract or to reexposure to spores that persist in the patient's environment. Because of the favorable susceptibility profile of rifaximin identified in the present study, these data support the use of rifaximin as a potential therapy in children with multiply recurrent CDI. Furthermore, although susceptibility breakpoints of fidaxomicin and surotomycin are not yet available, MICs of these antibiotics were relatively low among the pediatric isolates in this study. Pending results of pediatric phase 3 clinical trials of these new CDI therapies, fidaxomicin and surotomycin may also emerge as potential therapies for CDI in children.
This report provides insight about the antibiotic susceptibility profiles and molecular epidemiology of C. difficile in children, both of which are poorly understood. However, this study was limited by its having been performed at a single center, which reduces generalizability. Further assessment of C. difficile isolates from children at other centers and geographical locations is needed. Although access to C. difficile isolates obtained from an adult cohort in the same year (i.e., 2013) and geographical location (i.e., the Chicago area) as the pediatric cohort was a strength of this study, this convenience sample of isolates from adults at a VA hospital may not necessarily reflect the general adult population in the Chicago area. However, in a multicenter study of adult CDI that included 925 isolates (all of which underwent antibiotic susceptibility testing and 322 [35%] of which underwent REA) collected from patients at 7 geographically diverse U.S. medical centers in 2011 to 2012 (including Hines VA Hospital), the proportion of isolates identified as belonging to REA group BI (26%) and the proportions of isolates resistant to clindamycin and moxifloxacin (24% and 34%, respectively) were similar to those identified in the 2013 Hines VA Hospital adult cohort in the present study (29). More-recent data from this same multicenter cohort from 2013 suggest that, while there is significant variability among U.S. medical centers, CDI isolates at Hines VA Hospital are similar to those collected at many other hospitals in the United States. In this multicenter cohort, the proportions of isolates that were of REA group BI, clindamycin resistant, and moxifloxacin resistant were 20%, 21%, and 27%, respectively. The proportions of isolates identified as belonging to REA group BI at Hines VA Hospital in 2013 were very similar to those identified at Tufts Medical Center and Virginia Medical College (27% and 33%), respectively, which are medical centers that include more generalizable adult populations (30).
In summary, our data suggest that, compared to those found in adults, a diverse array of relatively antibiotic-susceptible C. difficile strains predominate in a cohort of children with CDI. Further assessment of molecular epidemiology and antibiotic susceptibility patterns of C. difficile isolates from a more geographically diverse cohort of children is needed to fully understand the spectrum of CDI in children.
ACKNOWLEDGMENTS
L.K.K. has received research grants from Merck and research supplies from Alere/Techlab and is a scientific advisor for Actelion. D.N.G. holds patents for the prevention of C. difficile infection and is a consultant for Sanofi Pasteur, DaVolterra, MGB, and Pfizer and an advisory board member of Merck, Rebiotix, Summit, and Actelion. S.J.P. has received a research grant from Merck. D.R.S. has received research support from Summit, Actelion, Merck, Optimer, Cubist, Tetraphase, and Genentech and is a scientific advisor for BioK Plus, Merck, Shire, Novartis, Takeda, Genentech, and Summit.
This study was supported by investigator-initiated grants from Merck (to L.K.K.) and Optimer (to D.R.S.), who had had no role in study design, data collection, data interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Kelly CP, Lamont JT. 2008. Clostridium difficile—more difficult than ever. N Engl J Med 359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 2.Kociolek LK, Gerding DN. 2016. Clinical utility of laboratory detection of Clostridium difficile strain BI/NAP1/027. J Clin Microbiol 54:19–24. doi: 10.1128/JCM.02340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- 6.Tenover FC, Akerlund T, Gerding DN, Goering RV, Bostrom T, Jonsson AM, Wong E, Wortman AT, Persing DH. 2011. Comparison of strain typing results for Clostridium difficile isolates from North America. J Clin Microbiol 49:1831–1837. doi: 10.1128/JCM.02446-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenover FC, Tickler IA, Persing DH. 2012. Antimicrobial-resistant strains of Clostridium difficile from North America. Antimicrob Agents Chemother 56:2929–2932. doi: 10.1128/AAC.00220-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tickler IA, Goering RV, Whitmore JD, Lynn AN, Persing DH, Tenover FC, Healthcare Associated Infection Consortium. 2014. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother 58:4214–4218. doi: 10.1128/AAC.02775-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother 51:2716–2719. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox MH, Shetty N, Fawley WN, Shemko M, Coen P, Birtles A, Cairns M, Curran MD, Dodgson KJ, Green SM, Hardy KJ, Hawkey PM, Magee JG, Sails AD, Wren MWD. 2012. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 55:1056–1063. doi: 10.1093/cid/cis614. [DOI] [PubMed] [Google Scholar]
- 11.Kociolek LK, Patel SJ, Shulman ST, Gerding D. 2015. Molecular epidemiology of Clostridium difficile infections in children: a retrospective cohort study. Infect Control Hosp Epidemiol 36:445–451. doi: 10.1017/ice.2014.89. [DOI] [PubMed] [Google Scholar]
- 12.Stoesser N, Crook DW, Fung R, Griffiths D, Harding RM, Kachrimanidou M, Keshav S, Peto TE, Vaughan A, Walker AS, Dingle KE. 2011. Molecular epidemiology of Clostridium difficile strains in children compared with that of strains circulating in adults with Clostridium difficile-associated infection. J Clin Microbiol 49:3994–3996. doi: 10.1128/JCM.05349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kociolek LK, Gerding DN. 2014. Is pediatric Clostridium difficile infection associated with prior antibiotic exposure? Future Microbiol 9:825–828. doi: 10.2217/fmb.14.51. [DOI] [PubMed] [Google Scholar]
- 14.Wilson KH, Kennedy MJ, Fekety FR. 1982. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clabots CR, Johnson S, Bettin KM, Mathie PA, Mulligan ME, Schaberg DR, Peterson LR, Gerding DN. 1993. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J Clin Microbiol 31:1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. 2011. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis 53:1003–1006. doi: 10.1093/cid/cir643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard— 8th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf.
- 20.Schutze GE, Willoughby RE; Committee on Infectious Diseases; American Academy of Pediatrics. 2013. Clostridium difficile infection in infants and children. Pediatrics 131:196–200. doi: 10.1542/peds.2012-2992. [DOI] [PubMed] [Google Scholar]
- 21.Kociolek LK, Gerding DN. 2016. Breakthroughs in the treatment and prevention of Clostridium difficile infections. Nat Rev Gastroenterol Hepatol 13:150–160. doi: 10.1038/nrgastro.2015.220. [DOI] [PubMed] [Google Scholar]
- 22.Gonzales M, Pepin J, Frost EH, Carrier JC, Sirard S, Fortier LC, Valiquette L. 2010. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis 10:363. doi: 10.1186/1471-2334-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma PD, Sandora TJ. 2012. Clostridium difficile infection in children: current state and unanswered questions. J Pediatric Infect Dis Soc 1:230–243. doi: 10.1093/jpids/pis071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wendt JM, Cohen JA, Mu Y, Dumyati GK, Dunn JR, Holzbauer SM, Winston LG, Johnston HL, Meek JI, Farley MM, Wilson LE, Phipps EC, Beldavs ZG, Gerding DN, McDonald LC, Gould CV, Lessa FC. 2014. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 133:651–658. doi: 10.1542/peds.2013-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, Harmsen WS, Pardi DS. 2013. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 56:1401–1406. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM. 2015. Novel risk factors for recurrent Clostridium difficile infection in children. J Pediatr Gastroenterol Nutr 60:18–22. doi: 10.1097/MPG.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tschudin-Sutter S, Tamma PD, Milstone AM, Perl TM. 2014. Predictors of first recurrence of Clostridium difficile infections in children. Pediatr Infect Dis J 33:414–416. doi: 10.1097/INF.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 28.Kociolek LK, Palac HL, Patel SJ, Shulman ST, Gerding DN. 2015. Risk factors for recurrent Clostridium difficile infection in children: a nested case-control study. J Pediatr 167:384–389. doi: 10.1016/j.jpeds.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Snydman DR, McDermott LA, Jacobus NV, Thorpe C, Stone S, Jenkins SG, Goldstein EJ, Patel R, Forbes BA, Mirrett S, Johnson S, Gerding DN. 2015. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile-associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 59:6437–6443. doi: 10.1128/AAC.00845-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snydman DR, McDermott LA, Jacobus NV, Chang J, Stone S, Wick J, Thorpe CM, Goldstein EJC, Patel R, Jenkins SG, Forbes BA, Johnson S, Gerding DN. 2016. A US-based National Sentinel Surveillance study for the susceptibility and epidemiology of Clostridium difficile-associated diarrheal isolates: 2013–2014, abstr MONDAY-442. Abstr ASM Microbe 2016, Boston, MA, 16–20 June. American Society of Microbiology, Washington, DC. [Google Scholar]