Abstract
The Klebsiella pneumoniae carbapenemase (KPC), first described in the United States in 1996, is now a widespread global problem in several Gram-negative species. A worldwide surveillance study collected Gram-negative pathogens from 202 global sites in 40 countries during 2012 to 2014 and determined susceptibility to β-lactams and other class agents by broth microdilution testing. Molecular mechanisms of β-lactam resistance among carbapenem-nonsusceptible Enterobacteriaceae and Pseudomonas aeruginosa were determined using PCR and sequencing. Genes encoding KPC enzymes were found in 586 isolates from 22 countries (76 medical centers), including countries in the Asia-Pacific region (32 isolates), Europe (264 isolates), Latin America (210 isolates), and the Middle East (19 isolates, Israel only) and the United States (61 isolates). The majority of isolates were K. pneumoniae (83.4%); however, KPC was detected in 13 additional species. KPC-2 (69.6%) was more common than KPC-3 (29.5%), with regional variation observed. A novel KPC variant, KPC-18 (KPC-3[V8I]), was identified during the study. Few antimicrobial agents tested remained effective in vitro against KPC-producing isolates, with ceftazidime-avibactam (MIC90, 4 μg/ml), aztreonam-avibactam (MIC90, 0.5 μg/ml), and tigecycline (MIC90, 2 μg/ml) retaining the greatest activity against Enterobacteriaceae cocarrying KPC and other β-lactamases, whereas colistin (MIC90, 2 μg/ml) demonstrated the greatest in vitro activity against KPC-positive P. aeruginosa. This analysis of surveillance data demonstrated that KPC is widely disseminated. KPC was found in multiple species of Enterobacteriaceae and P. aeruginosa and has now become a global problem.
INTRODUCTION
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) contribute to attributable mortality higher than that for patients infected with carbapenem-susceptible isolates (1). The effect of CRE on morbidity and mortality can vary significantly between countries and may depend upon the β-lactam resistance mechanisms that are most problematic in certain regions (2–5). Population movements, poor infection control, and the lack of antimicrobial stewardship initiatives have perpetuated the dissemination of genes that encode carbapenemases among clinically significant bacterial species on a global scale (2, 4, 6, 7). Detection of CRE and their associated resistance mechanisms is essential in order to determine the appropriate therapeutic options required for a positive patient infection outcome (8–10).
The Klebsiella pneumoniae carbapenemase (KPC) is a class A serine carbapenemase first recognized in the northeastern United States in 1996 (11). Bacterial pathogens expressing KPC are clinically significant in that they are often multi- or pan-drug resistant, including resistance to currently available latest-in-line therapeutic options (7, 12, 13). The impact of KPC became more fully recognized as this family of enzymes became a global threat to public health, in that the gene encoding KPC (blaKPC) has now been observed in multiple Enterobacteriaceae species and has disseminated worldwide, in large part due to the spread of K. pneumoniae isolates belonging to the successful high-risk clonal complex 258 (7, 13). blaKPC is most often embedded within the Tn4401 transposon, though it has also been reported in other mobile elements, and found in plasmids belonging to 12 incompatibility groups capable of species-to-species transfer within Enterobacteriaceae and some nonfermentative Gram-negative pathogens, including Pseudomonas aeruginosa (14–17). Furthermore, these plasmids commonly also carry genes encoding aminoglycoside resistance mechanisms and additional β-lactamases, including extended-spectrum β-lactamases (ESBLs) (12, 17). blaKPC has also been found inserted into the bacterial chromosome (18, 19).
This investigation documented the distribution of KPC-producing Gram-negative bacterial pathogens isolated from a sampling of clinically significant pathogens collected during a global surveillance study.
MATERIALS AND METHODS
Nonduplicate, nonconsecutive isolates from intra-abdominal, urinary tract, skin and soft tissue, lower respiratory tract, and bloodstream infections were collected from 202 medical centers in 40 countries located in the Asia-Pacific region, Europe, Latin America, the Middle East-Africa, and North America. The medical centers were instructed to contribute a specific number of isolates of each requested species regardless of antibiotic susceptibility. Participating countries by year are listed in Table S1 in the supplemental material. Basic patient demographic data were collected but were not linked to patient identity or therapeutic outcome. Organism collection, transport, confirmation of organism identification, susceptibility testing, molecular characterization, data quality assurance, and development and management of a centralized database were coordinated by a central laboratory.
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker Daltronics, Bremen, Germany) was used to confirm the organism identification of all isolates. Antibiotic susceptibility testing was performed by broth microdilution using custom frozen panels. Ceftazidime-avibactam and aztreonam-avibactam were tested at a fixed concentration of 4 μg/ml avibactam. Panel manufacture, inoculation, incubation, interpretation, and quality control testing were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (20, 21). All P. aeruginosa isolates that were nonsusceptible to doripenem, meropenem, or imipenem and Enterobacteriaceae isolates that were nonsusceptible to those carbapenems or ertapenem using CLSI breakpoints were molecularly characterized for β-lactamase genes encoding KPC and other β-lactamases (OXA-48-like, TEM, SHV, CTX-M, VEB, PER, GES, ACT, CMY, DHA, MIR, ACC, MOX, FOX, NDM, IMP, VIM, SPM, and GIM) using a combination of microarray and multiplex PCR assays, followed by sequencing as previously described (22).
Nucleotide sequence accession number.
The sequence of the new variant KPC-18 was deposited in GenBank with accession no. KP681699.
RESULTS
A total of 38,266 isolates of Enterobacteriaceae and 8,010 isolates of P. aeruginosa were collected in 40 countries participating in a global surveillance study in 2012 to 2014. Of these, 586 (1.3%) carbapenem-nonsusceptible Gram-negative isolates collected from medical centers in 22 countries carried blaKPC. In addition, four carbapenem-susceptible KPC-positive isolates were also identified as part of the study but were excluded from analysis.
With the exception of 34 isolates collected during patient visits to an emergency room, all carbapenem-nonsusceptible, KPC-positive isolates were from patients admitted to an inpatient hospital ward, with one-third collected in intensive care units (data not shown). Patient ages ranged from <1 to 95 years, with a median age of 61 years, and more male patients (354, 60.4%) were identified with a KPC-positive isolate than females (220, 37.5%); information regarding gender was not available for 12 patients. KPC-positive bacteria were isolated from various infection sources, with the greatest number of overall isolates (189 of 586, 32.2%) collected from respiratory cultures and approximately equal numbers of isolates collected from urinary tract infections (137) and skin and soft tissue infections (132). The largest numbers of isolates were collected from urine (126), wounds (71), endotracheal aspirates (67), sputum (63), and peritoneal fluid (45) (Table 1). In cases when information regarding the length of hospital stay was available, 75.1% of patients with a KPC-positive isolate were admitted more than 48 h prior to culture, suggesting nosocomial acquisition (Table 1).
TABLE 1.
Body source distribution of 586 carbapenem-nonsusceptible KPC-positive Enterobacteriaceae and P. aeruginosa isolates collected in 2012 to 2014
| Source of infectiona | Source of culture | No. of isolates from patients hospitalized for: |
||
|---|---|---|---|---|
| <48 h | ≥48 h | NAb | ||
| Gastrointestinal tract | Peritoneal fluid | 12 | 33 | |
| Abscess | 3 | 25 | ||
| Gallbladder | 3 | 11 | ||
| Otherc | 2 | 12 | ||
| Total (%) | 20 (3.4) | 81 (13.8) | ||
| Urinary tract | Urine | 41 | 85 | |
| Ureter/urethra/bladder | 3 | 5 | ||
| Otherd | 3 | |||
| Total (%) | 44 (7.5) | 93 (15.9) | ||
| Skin and soft tissue | Wound | 22 | 48 | 1 |
| Decubitus ulcer | 7 | 22 | ||
| Abscess | 2 | 12 | ||
| Carbuncle/furuncle/cellulitis/erysipelas | 2 | 6 | ||
| Burn | 3 | 4 | ||
| Othere | 3 | |||
| Total (%) | 36 (6.1) | 95 (16.2) | 1 (0.2) | |
| Respiratory tract | Endotracheal aspirate | 14 | 51 | 2 |
| Sputum | 14 | 48 | 1 | |
| Bronchoalveolar lavage fluid | 5 | 27 | 1 | |
| Bronchial brushing | 3 | 10 | ||
| Otherf | 4 | 9 | ||
| Total (%) | 40 (6.8) | 145 (24.7) | 4 (0.7) | |
| Bloodstream | Blood | 1 | 25 | |
| Total (%) | 1 (0.2) | 25 (4.3) | ||
| Unknown | Unknown | 1 | ||
| Total (%) | 1 (0.2) | |||
| Total (%) | 141 (24.1) | 440 (75.1) | 5 (0.9) | |
Species found (number of isolates): gastrointestinal tract, C. farmeri (1), C. freundii (3), C. koseri (1), E. aerogenes (1), E. cloacae (3), E. coli (4), K. oxytoca (3), K. pneumoniae (84), and P. aeruginosa (1); urinary tract, C. freundii (2), E. cloacae (3), E. coli (10), K. oxytoca (3), K. pneumoniae (110), and P. aeruginosa (9); skin and soft tissue, C. amalonaticus (1), C. freundii (1), E. asburiae (2), E. cloacae (2), E. coli (8), K. oxytoca (3), K. pneumoniae (111), and P. aeruginosa (4); respiratory tract, C. freundii (1), C. koseri (1), E. aerogenes (2), E. asburiae (1), E. coli (2), K. oxytoca (4), K. pneumoniae (159), M. morganii (1), P. aeruginosa (15), R. ornithinolytica (1), and S. marcescens (2); bloodstream, E. cloacae (1), K. oxytoca (1), and K. pneumoniae (24); unknown, K. pneumoniae (1).
NA, not available because the hospital admission date was not provided by the investigator.
Other sources (number of isolates): appendix (4), liver (4), large colon (3), not specified (2), and pancreas (1).
Other sources (number of isolates): not specified (2), and prostate (1).
Other sources (number of isolates): not specified (3).
Other sources (number of isolates): thoracentesis (5), not specified (5), and lungs (3).
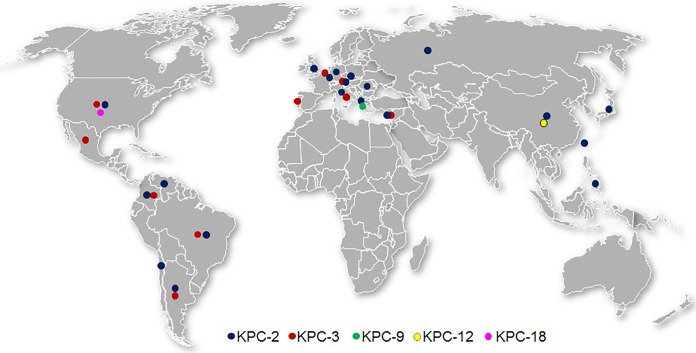
K. pneumoniae was the most commonly isolated KPC-producing species (n = 489, 83.4%), followed by P. aeruginosa (29, 4.9%), Escherichia coli (24, 4.1%), and Klebsiella oxytoca (14, 2.4%). The remaining 5% of KPC-positive isolates were composed of 10 species of Enterobacteriaceae (9 Enterobacter cloacae, 7 Citrobacter freundii, 3 each Enterobacter aerogenes and Enterobacter asburiae, 2 each Citrobacter koseri and Serratia marcescens, and 1 each Citrobacter amalonaticus, Citrobacter farmeri, Morganella morganii, and Raoultella ornithinolytica) (Table 2). KPC-positive isolates were collected in all regions, with large numbers of isolates collected in countries in which KPC-producing organisms were previously reported to be endemic (Greece, Italy, Colombia, Argentina, Brazil, the United States, China, and Israel), as well as Portugal, in which KPC-positive isolates have been reported only recently (Table 2; Fig. 1) (7, 23). It should be noted that isolates from patients in China were obtained only during 2012 to 2013 due to export restrictions of bacterial pathogens imposed in 2014.
TABLE 2.
Geographic and species distribution of 586 carbapenem-nonsusceptible KPC-positive isolates collected as part of a global surveillance program (2012 to 2014)
| Region | Country | n | Organism | KPC variant(s) (n) |
|---|---|---|---|---|
| Europe | Austria | 5 | E. aerogenes | KPC-2 (1) |
| K. oxytoca | KPC-2 (1) | |||
| K. pneumoniae | KPC-2 (2), KPC-3 (1) | |||
| Belgium | 4 | K. pneumoniae | KPC-2 (1), KPC-3 (3) | |
| Czech Republic | 1 | K. pneumoniae | KPC-2 (1) | |
| Germany | 1 | K. pneumoniae | KPC-2 (1) | |
| Greece | 134 | C. amalonaticus | KPC-2 (1) | |
| K. oxytoca | KPC-2 (3) | |||
| K. pneumoniae | KPC-2 (128), KPC-9 (2) | |||
| Italy | 87 | E. coli | KPC-2 (1) | |
| K. oxytoca | KPC-3 (1) | |||
| K. pneumoniae | KPC-2 (15), KPC-3 (70) | |||
| Portugal | 24 | C. freundii | KPC-3 (1) | |
| E. coli | KPC-3 (3) | |||
| K. oxytoca | KPC-3 (1) | |||
| K. pneumoniae | KPC-3 (19) | |||
| Romania | 6 | K. pneumoniae | KPC-2 (6) | |
| Russia | 1 | E. cloacae | KPC-2 (1) | |
| United Kingdom | 1 | K. pneumoniae | KPC-2 (1) | |
| Latin America | Argentina | 60 | E. cloacae | KPC-2 (2) |
| E. coli | KPC-2 (2) | |||
| K. oxytoca | KPC-2 (2) | |||
| K. pneumoniae | KPC-2 (50), KPC-3 (2) | |||
| M. morganii | KPC-2 (1) | |||
| P. aeruginosa | KPC-2 (1) | |||
| Brazil | 60 | E. cloacae | KPC-2 (2) | |
| E. coli | KPC-2 (1) | |||
| K. oxytoca | KPC-2 (2) | |||
| K. pneumoniae | KPC-2 (53), KPC-3 (2) | |||
| Chile | 17 | K. pneumoniae | KPC-2 (1) | |
| P. aeruginosa | KPC-2 (16) | |||
| Colombia | 60 | C. freundii | KPC-2 (3) | |
| C. koseri | KPC-2 (1) | |||
| E. cloacae | KPC-2 (2) | |||
| E. coli | KPC-2 (4) | |||
| K. oxytoca | KPC-2 (1) | |||
| K. pneumoniae | KPC-2 (24), KPC-3 (13) | |||
| S. marcescens | KPC-2 (1) | |||
| P. aeruginosa | KPC-2 (11) | |||
| Mexico | 2 | K. pneumoniae | KPC-3 (1) | |
| R. ornithinolytica | KPC-3 (1) | |||
| Venezuela | 11 | C. freundii | KPC-2 (1) | |
| K. pneumoniae | KPC-2 (10) | |||
| North America | United States | 61 | C. farmeri | KPC-3 (1) |
| C. freundii | KPC-2 (1) | |||
| E. asburiae | KPC-2 (2) | |||
| E. cloacae | KPC-2 (1) | |||
| E. coli | KPC-3 (3), KPC-18 (2) | |||
| K. pneumoniae | KPC-2 (11), KPC-3 (40) | |||
| Asia-Pacific | Chinaa | 28 | C. koseri | KPC-2 (1) |
| E. aerogenes | KPC-2 (1) | |||
| E. cloacae | KPC-2 (1) | |||
| E. coli | KPC-2 (5) | |||
| K. oxytoca | KPC-2 (3) | |||
| K. pneumoniae | KPC-2 (14), KPC-12 (1) | |||
| S. marcescens | KPC-2 (1) | |||
| P. aeruginosa | KPC-2 (1) | |||
| Japan | 1 | E. asburiae | KPC-2 (1) | |
| Philippines | 2 | K. pneumoniae | KPC-2 (2) | |
| Taiwan | 1 | K. pneumoniae | KPC-2 (1) | |
| Middle East-Africa | Israel | 19 | C. freundii | KPC-2 (1) |
| E. aerogenes | KPC-2 (1) | |||
| E. coli | KPC-2 (2), KPC-3 (1) | |||
| K. pneumoniae | KPC-2 (4), KPC-3 (10) |
No isolates were obtained from patients in mainland China in 2014 due to export restrictions.
FIG 1.
Distribution of KPC-positive Enterobacteriaceae and P. aeruginosa collected in 2012 to 2014.
Five KPC sequence variants were identified, with 99.1% of isolates carrying either KPC-2 (408, 69.6%) or KPC-3 (173, 29.5%). KPC-2 was detected in 20 of 22 countries in this investigation, whereas KPC-3 was detected in 10 countries and was the only variant found in isolates collected in Mexico and Portugal. Larger proportions of isolates from Italy (81.6%), Israel (57.9%), and the United States (72.1%) carried KPC-3 in comparison to KPC-2. A total of 93.1% of detected KPC-3 variants were carried by K. pneumoniae. In contrast, KPC-2 was found in more diverse Gram-negative species, but still 79.7% were carried by K. pneumoniae. All KPC-positive P. aeruginosa isolates carried the KPC-2 variant, and all but one were collected from countries in Latin America (Table 2). Two K. pneumoniae isolates collected in Greece carried KPC-9, and one K. pneumoniae isolate collected in China carried KPC-12. One novel variant, KPC-18 (KPC-3[V8I]), was identified during this study. KPC-18 was detected in E. coli collected from two different patients within a 2-week period in 2014 at a medical center located in suburban Chicago, IL, USA. The first isolate was cultured from a patient with a respiratory infection in an intensive care unit, whereas the second isolate was collected from peritoneal fluid during an emergency room visit. Both isolates were resistant to ampicillin, aztreonam, ceftazidime, cefepime, doripenem, imipenem, meropenem, piperacillin-tazobactam, and levofloxacin but were susceptible in vitro to ceftazidime-avibactam, amikacin, tigecycline, and colistin and showed low MIC values (0.12 μg/ml) of aztreonam-avibactam (data not shown).
A total of 96.8% of KPC-positive isolates also carried additional β-lactamases, including plasmid-encoded and presumed intrinsic chromosomally encoded enzymes. Notably, nine isolates carried a second carbapenemase belonging to Ambler class B or class D. Four K. pneumoniae isolates collected in Greece carried KPC-2 and VIM-1, two K. pneumoniae isolates collected in China carried KPC-2 and IMP-4, one P. aeruginosa isolate collected in Chile carried KPC-2 and VIM-2, and two K. pneumoniae isolates collected in Greece and Argentina carried KPC-2 and OXA-163. Of these, 7 isolates also carried ESBLs and/or AmpC β-lactamases (Table 3). The majority (291, 49.7%) of isolates carried KPC alone or with an original-spectrum β-lactamase (OSBL) (TEM-1, TEM-2, SHV-1, or SHV-11) that is not expected to significantly impact susceptibility to antimicrobial agents in clinical use, with 36% of KPC-2-positive (n = 147) and 82.1% of KPC-3-positive (n = 142) isolates found in this subset. Approximately one-third of isolates (219, 37.4%) coproduced KPC and ESBLs, whereas isolates carrying KPC and AmpC β-lactamases (25, 4.3%) or KPC plus both AmpC and one or more ESBLs (14, 2.4%) were less frequently encountered. KPC-3 was most often found with CTX-M-15 (11 isolates) or SHV-12 (15 isolates) and was not found in combination with a plasmid-mediated AmpC in any of the isolates. KPC-2 was most often cocarried with SHV-12 (104 isolates), CTX-M-15 (35 isolates), and VEB-1 (18 isolates); the last combination was detected in 17 K. pneumoniae isolates and one E. aerogenes isolate collected from Greece (16 isolates) and Austria (2 isolates) (Table 3).
TABLE 3.
Cocarriage of KPC and other β-lactamases in carbapenem-nonsusceptible Enterobacteriaceae and P. aeruginosa collected in 2012 to 2014
| β-Lactamasesa | Organism | n | Molecular variant(s) |
|---|---|---|---|
| KPC + MBL | K. pneumoniae | 1 | KPC-2, VIM-1 |
| KPC + MBL + ESBL + AmpC + OSBL | K. pneumoniae | 2 | KPC-2, VIM-1, SHV-12, CMY-13, TEM-OSBL |
| KPC + MBL + ESBL | K. oxytocab | 2 | KPC-2, IMP-4, SHV-12 |
| KPC + MBL + AmpC ± OSBL | K. pneumoniae | 1 | KPC-2, VIM-1, MOX-1, SHV-OSBL |
| P. aeruginosac | 1 | KPC-2, VIM-2 | |
| KPC + ESBL-like OXA + ESBL + OSBL | K. pneumoniae | 1 | KPC-2, OXA-163, CTX-M-2, SHV-OSBL, TEM-OSBL |
| KPC + ESBL-like OXA + OSBL | K. pneumoniae | 1 | KPC-2, OXA-163, SHV-OSBL, TEM-OSBL |
| KPC + ESBL + AmpC ± OSBL | C. freundiic | 1 | KPC-2, SHV-12, TEM-OSBL |
| 1 | KPC-2, CTX-M-15 | ||
| 1 | KPC-3, CTX-M-9, SHV-12 | ||
| C. koseric | 1 | KPC-2, CTX-M-3, TEM-OSBL | |
| E. aerogenesc | 1 | KPC-2, VEB-1, TEM-OSBL | |
| E. asburiaec | 2 | KPC-2, SHV-30, TEM-OSBL | |
| E. cloacaec | 1 | KPC-2, SHV-12, DHA-1 | |
| 3 | KPC-2, CTX-M-15, TEM-OSBL | ||
| K. pneumoniae | 2 | KPC-2, CTX-M-15, MOX-2, SHV-OSBL, TEM-OSBL | |
| 1 | KPC-2, CTX-M-27, DHA-1, SHV-OSBL | ||
| KPC + ESBL ± OSBL | E. coli | 1 | KPC-2, CTX-M-15 |
| 1 | KPC-2, CTX-M-15, TEM-OSBL | ||
| K. oxytocab | 4 | KPC-2 | |
| 2 | KPC-2, TEM-OSBL | ||
| 1 | KPC-2, SHV-5, TEM-OSBL | ||
| 1 | KPC-2, SHV-12 | ||
| 1 | KPC-2, CTX-M-8, TEM-OSBL | ||
| 1 | KPC-2, CTX-M-15, TEM-OSBL | ||
| 2 | KPC-3, TEM-OSBL | ||
| K. pneumoniae | 1 | KPC-2, SHV-5, TEM-OSBL | |
| 29 | KPC-2, SHV-12 | ||
| 64 | KPC-2, SHV-12, TEM-OSBL | ||
| 1 | KPC-2, SHV-12, CTX-M-14, TEM-OSBL | ||
| 2 | KPC-2, SHV-12, CTX-M-65 | ||
| 1 | KPC-2, SHV-12, CTX-M-65, TEM-OSBL | ||
| 1 | KPC-2, SHV-28, CTX-M-15, TEM-OSBL | ||
| 3 | KPC-2, CTX-M-2, SHV-OSBL | ||
| 1 | KPC-2, CTX-M-2, TEM-OSBL | ||
| 9 | KPC-2, CTX-M-2, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, CTX-M-2, CTX-M-15, SHV-OSBL, TEM-OSBL | ||
| 2 | KPC-2, CTX-M-3, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, CTX-M-12 | ||
| 2 | KPC-2, CTX-M-12, SHV-OSBL | ||
| 1 | KPC-2, CTX-M-14, TEM-OSBL | ||
| 9 | KPC-2, CTX-M-14, SHV-OSBL, TEM-OSBL | ||
| 10 | KPC-2, CTX-M-15, SHV-OSBL | ||
| 14 | KPC-2, CTX-M-15, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, CTX-M-24, SHV-OSBL | ||
| 2 | KPC-2, CTX-M-65, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, CTX-M-67, SHV-OSBL | ||
| 1 | KPC-2, CTX-M-90, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, GES-6, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, VEB-1, SHV-OSBL | ||
| 16 | KPC-2, VEB-1, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-3, SHV-12 | ||
| 12 | KPC-3, SHV-12, TEM-OSBL | ||
| 1 | KPC-3, SHV-12, CTX-M-12, TEM-OSBL | ||
| 1 | KPC-3, SHV-28, CTX-M-15, TEM-OSBL | ||
| 1 | KPC-3, CTX-M-2, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-3, CTX-M-15, SHV-OSBL | ||
| 9 | KPC-3, CTX-M-15, SHV-OSBL, TEM-OSBL | ||
| 2 | KPC-9, VEB-1, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-12, SHV-2A | ||
| R. ornithinolytica | 1 | KPC-3, SHV-5, TEM-OSBL | |
| KPC + AmpC ± OSBL | C. amalonaticusc | 1 | KPC-2, TEM-OSBL |
| C. farmeric | 1 | KPC-3 | |
| C. freundiic | 4 | KPC-2 | |
| C. koseric | 1 | KPC-2 | |
| E. aerogenesc | 1 | KPC-2 | |
| 1 | KPC-2, TEM-OSBL | ||
| E. asburiaec | 1 | KPC-2 | |
| E. cloacaec | 1 | KPC-2 | |
| 1 | KPC-2, SHV-OSBL | ||
| 3 | KPC-2, TEM-OSBL | ||
| E. coli | 1 | KPC-2, CMY-2 | |
| 1 | KPC-2, CMY-2, TEM-OSBL | ||
| K. pneumoniae | 1 | KPC-2, ACT-type, SHV-OSBL | |
| 1 | KPC-2, CMY-2, SHV-OSBL | ||
| 1 | KPC-2, CMY-2, SHV-OSBL, TEM-OSBL | ||
| 1 | KPC-2, CMY-4, SHV-OSBL | ||
| 1 | KPC-2, DHA-1, SHV-OSBL | ||
| M. morganiic | 1 | KPC-2, TEM-OSBL | |
| S. marcescensc | 2 | KPC-2 | |
| P. aeruginosac | 28 | KPC-2 | |
| KPC ± OSBL | E. coli | 5 | KPC-2 |
| 6 | KPC-2, TEM-OSBL | ||
| 2 | KPC-3 | ||
| 5 | KPC-3, TEM-OSBL | ||
| 2 | KPC-18, TEM-OSBL | ||
| K. pneumoniae | 10 | KPC-2 | |
| 53 | KPC-2, SHV-OSBL | ||
| 3 | KPC-2, TEM-OSBL | ||
| 70 | KPC-2, SHV-OSBL, TEM-OSBL | ||
| 2 | KPC-3 | ||
| 31 | KPC-3, SHV-OSBL | ||
| 4 | KPC-3, TEM-OSBL | ||
| 98 | KPC-3, SHV-OSBL, TEM-OSBL |
MBL, metallo-β-lactamase; ESBL, extended-spectrum β-lactamase; OSBL, original spectrum β-lactamase (includes TEM-1, TEM-2, SHV-1, and SHV-11).
Presumed to also carry the intrinsic chromosomally encoded ESBL common to this species.
Presumed to also carry the intrinsic chromosomally encoded AmpC β-lactamase common to this species.
The in vitro activities of β-lactam agents and comparators against the overall collection of Enterobacteriaceae, P. aeruginosa, and subsets of KPC-positive isolates coproducing additional β-lactamases from Ambler class A, B, C, and D were determined (Table 4). As expected, the activities of β-lactams, including aztreonam, ceftazidime, cefepime, meropenem, imipenem, and piperacillin-tazobactam, were greatly reduced against the overall subset of KPC-producing Enterobacteriaceae, with <5% of isolates susceptible to any of these agents. Combination of avibactam, a non-β-lactam β-lactamase inhibitor, with aztreonam or ceftazidime enhanced the activities of these β-lactams against KPC-positive isolates of Enterobacteriaceae at least 64-fold. Aztreonam-avibactam resulted in MIC90 values of 0.5 to 1 μg/ml against all KPC-positive subsets, compared to MIC90 values of >128 μg/ml for aztreonam. The MIC90 values for ceftazidime-avibactam and ceftazidime were 2 to 4 μg/ml and >128 μg/ml, respectively, against KPC-positive isolates that did not coproduce a metallo-β-lactamase (MBL). The activities of agents from other drug classes against KPC-positive subsets were affected to different degrees; for example, the susceptibility to amikacin ranged from 42.5 to 78.6%, depending on the combination of coproduced β-lactamases, and the susceptibility to tigecycline ranged from 71.4 to 92.2%. The activity of colistin against subsets carrying different combinations of β-lactamases also varied, with susceptibilities ranging from 75.8 to 100%. Two isolates producing KPC and OXA-163 (OXA-48-like) showed low MIC values of ceftazidime-avibactam, aztreonam-avibactam, tigecycline, and colistin, but only aztreonam-avibactam and tigecycline were active in vitro against all six isolates carrying KPC and an MBL (Table 4).
TABLE 4.
In vitro activities of antimicrobial agents tested against carbapenem-nonsusceptible KPC-producing isolates collected in 2012 to 2014
| Organism subset (n) and agenta | MIC (μg/ml) |
% susceptibleb | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| All Enterobacteriaceae (38,266) | ||||
| Ceftazidime | ≤0.015 to >128 | 0.25 | 64 | 76.9 |
| Ceftazidime-avibactamc | ≤0.015 to >128 | 0.12 | 0.5 | 99.5 |
| Aztreonam | ≤0.015 to >128 | 0.12 | 64 | 75.7 |
| Aztreonam-avibactamc | ≤0.015 to >128 | 0.03 | 0.12 | NA |
| Cefepime | ≤0.12 to >16 | ≤0.12 | >16 | 78.8 |
| Meropenem | ≤0.004 to >8 | 0.03 | 0.12 | 97.3 |
| Imipenem | ≤0.03 to >8 | 0.25 | 2 | 85.3 |
| Piperacillin-tazobactam | ≤0.25 to >128 | 2 | 64 | 84.7 |
| Amikacin | ≤0.25 to >32 | 2 | 8 | 96.6 |
| Tigecycline | ≤0.015 to >8 | 0.5 | 2 | 92.9 |
| Colistind | ≤0.12 to >4 | ≤0.12 | >4 | 83.2 |
| KPC-positive Enterobacteriaceae | ||||
| All (557) | ||||
| Ceftazidime | 0.12 to >128 | >128 | >128 | 3.9 |
| Ceftazidime-avibactam | ≤0.015 to >128 | 1 | 4 | 97.5 |
| Aztreonam | 0.06 to >128 | >128 | >128 | 1.3 |
| Aztreonam-avibactam | ≤0.015 to 8 | 0.25 | 0.5 | NA |
| Cefepime | ≤0.12 to >16 | >16 | >16 | 4.8 |
| Meropenem | 0.06 to >8 | >8 | >8 | 3.1 |
| Imipenem | 0.5 to >8 | >8 | >8 | 0.5 |
| Piperacillin-tazobactam | 0.5 to >128 | >128 | >128 | 0.9 |
| Amikacin | ≤0.25 to >32 | 32 | >32 | 48.3 |
| Tigecycline | 0.06 to 8 | 1 | 2 | 91.6 |
| Colistin | ≤0.12 to >4 | 0.03 | >4 | 83.3 |
| KPC ± OSBL (291) | ||||
| Ceftazidime | 1 to >128 | 128 | >128 | 3.8 |
| Ceftazidime-avibactam | ≤0.015 to 128 | 1 | 4 | 98.6 |
| Aztreonam | 4 to >128 | >128 | >128 | 1.0 |
| Aztreonam-avibactam | ≤0.015 to 8 | 0.12 | 0.5 | NA |
| Cefepime | ≤0.12 to >16 | >16 | >16 | 5.5 |
| Meropenem | 0.25 to >8 | >8 | >8 | 3.1 |
| Imipenem | 2 to >8 | >8 | >8 | 0.0 |
| Piperacillin-tazobactam | 2 to >128 | >128 | >128 | 0.7 |
| Amikacin | 0.5 to >32 | 32 | 32 | 49.1 |
| Tigecycline | 0.06 to 8 | 1 | 2 | 91.8 |
| Colistin | ≤0.12 to >4 | 0.03 | >4 | 89.0 |
| KPC + ESBL ± OSBLe (219) | ||||
| Ceftazidime | 1 to >128 | >128 | >128 | 1.4 |
| Ceftazidime-avibactam | ≤0.015 to 16 | 1 | 4 | 99.1 |
| Aztreonam | 2 to >128 | >128 | >128 | 1.4 |
| Aztreonam-avibactam | ≤0.015 to 4 | 0.25 | 0.5 | NA |
| Cefepime | ≤0.12 to >16 | >16 | >16 | 2.3 |
| Meropenem | 0.5 to >8 | >8 | >8 | 1.4 |
| Imipenem | 0.5 to >8 | >8 | >8 | 0.9 |
| Piperacillin-tazobactam | 8 to >128 | >128 | >128 | 0.9 |
| Amikacin | ≤0.25 to >32 | 32 | >32 | 42.5 |
| Tigecycline | 0.06 to 8 | 1 | 2 | 92.2 |
| Colistin | ≤0.12 to >4 | 0.03 | >4 | 75.8 |
| KPC + AmpC ± OSBLf (25) | ||||
| Ceftazidime | 0.12 to >128 | 32 | >128 | 24.0 |
| Ceftazidime-avibactam | 0.03 to 2 | 0.5 | 2 | 100 |
| Aztreonam | 0.06 to >128 | >128 | >128 | 4.0 |
| Aztreonam-avibactam | ≤0.015 to 4 | 0.12 | 1 | NA |
| Cefepime | ≤0.12 to >16 | 16 | >16 | 16.0 |
| Meropenem | 0.06 to >8 | 8 | >8 | 16.0 |
| Imipenem | 2 to >8 | 8 | >8 | 0.0 |
| Piperacillin-tazobactam | 0.5 to >128 | >128 | >128 | 4.0 |
| Amikacin | 0.5 to >32 | 4 | >32 | 76.0 |
| Tigecycline | 0.06 to 4 | 1 | 2 | 92.0 |
| Colistin | ≤0.12 to >4 | ≤0.12 | >4 | 80.0 |
| KPC + ESBL + AmpC ± OSBLf (14) | ||||
| Ceftazidime | 2 to >128 | 64 | >128 | 14.3 |
| Ceftazidime-avibactam | 0.25 to 4 | 1 | 2 | 100 |
| Aztreonam | 16 to >128 | >128 | >128 | 0.0 |
| Aztreonam-avibactam | 0.03 to 1 | 0.25 | 0.5 | NA |
| Cefepime | ≤0.12 to >16 | >16 | >16 | 14.3 |
| Meropenem | 0.5 to >8 | 4 | >8 | 7.1 |
| Imipenem | 1 to >8 | 8 | >8 | 7.1 |
| Piperacillin-tazobactam | 64 to >128 | >128 | >128 | 0.0 |
| Amikacin | ≤0.25 to >32 | 4 | 32 | 78.6 |
| Tigecycline | 0.25 to 8 | 1 | 4 | 71.4 |
| Colistin | ≤0.12 to 0.06 | ≤0.12 | 0.06 | 100 |
| KPC + OXA-48-like + OSBL ± ESBL (2) | ||||
| Ceftazidime | 128 to >128 | 0.0 | ||
| Ceftazidime-avibactam | 1 to 2 | 100 | ||
| Aztreonam | 64 to >128 | 0.0 | ||
| Aztreonam-avibactam | 0.25 to 0.5 | NA | ||
| Cefepime | >16 to >16 | 0.0 | ||
| Meropenem | 2 to >8 | 0.0 | ||
| Imipenem | 4 to 8 | 0.0 | ||
| Piperacillin-tazobactam | >128 to >128 | 0.0 | ||
| Amikacin | 16 to >32 | 50.0 | ||
| Tigecycline | 2 −2 | 100 | ||
| Colistin | ≤0.12 to ≤0.12 | 100 | ||
| KPC + MBL ± ESBL ± AmpC ± OSBL (6) | ||||
| Ceftazidime | >128 to >128 | 0.0 | ||
| Ceftazidime-avibactam | >128 to >128 | 0.0 | ||
| Aztreonam | >128 to >128 | 0.0 | ||
| Aztreonam-avibactam | 0.5 to 1 | NA | ||
| Cefepime | >16 to >16 | 0.0 | ||
| Meropenem | >8 to >8 | 0.0 | ||
| Imipenem | >8 to >8 | 0.0 | ||
| Piperacillin-tazobactam | >128 to >128 | 0.0 | ||
| Amikacin | 16 to >32 | 16.7 | ||
| Tigecycline | 0.5 to 2 | 100 | ||
| Colistin | ≤0.12 to >4 | 50.0 | ||
| All P. aeruginosa (8,010) | ||||
| Ceftazidime | 0.06 to >128 | 2 | 64 | 77.4 |
| Ceftazidime-avibactam | 0.06 to >128 | 2 | 8 | 92.4 |
| Aztreonam | ≤0.015 to >128 | 8 | 32 | 61.4 |
| Aztreonam-avibactam | ≤0.015 to >128 | 8 | 32 | NA |
| Cefepime | ≤0.12 to >16 | 4 | 16 | 78.6 |
| Meropenem | ≤0.06 to >8 | 0.5 | >8 | 73.3 |
| Imipenem | ≤0.03 to >8 | 2 | >8 | 61.7 |
| Piperacillin-tazobactam | ≤0.25 to >128 | 8 | >128 | 69.1 |
| Amikacin | ≤0.25 to >32 | 4 | 16 | 90.2 |
| Colistin | ≤0.12 to >8 | 0.5 | 1 | 99.5 |
| KPC-positive P. aeruginosa | ||||
| All (29) | ||||
| Ceftazidime | 64 to >128 | 64 | >128 | 0.0 |
| Ceftazidime-avibactam | 4 to 64 | 8 | 32 | 75.9 |
| Aztreonam | >128 to >128 | >128 | >128 | 0.0 |
| Aztreonam-avibactam | 8 to >128 | 32 | 64 | NA |
| Cefepime | 0.5 to >16 | >16 | >16 | 3.4 |
| Meropenem | >8 to >8 | >8 | >8 | 0.0 |
| Imipenem | >8 to >8 | >8 | >8 | 0.0 |
| Piperacillin-tazobactam | >128 to >128 | >128 | >128 | 0.0 |
| Amikacin | 1 to >32 | 8 | >32 | 75.9 |
| Colistin | ≤0.06 to >8 | 0.5 | 2 | 96.6 |
| KPC + AmpCf (28) | ||||
| Ceftazidime | 64 to >128 | 64 | >128 | 0.0 |
| Ceftazidime-avibactam | 4 to 64 | 8 | 32 | 78.6 |
| Aztreonam | >128 to >128 | >128 | >128 | 0.0 |
| Aztreonam-avibactam | 8 to >128 | 32 | 64 | NA |
| Cefepime | 0.5 to >16 | >16 | >16 | 3.6 |
| Meropenem | >8 to >8 | >8 | >8 | 0.0 |
| Imipenem | >8 to >8 | >8 | >8 | 0.0 |
| Piperacillin-tazobactam | >128 to >128 | >128 | >128 | 0.0 |
| Amikacin | 1 to >32 | 8 | >32 | 78.6 |
| Colistin | ≤0.06 to >8 | 0.5 | 2 | 96.4 |
| KPC + AmpC + MBLf (1) | ||||
| Ceftazidime | 64 | 0.0 | ||
| Ceftazidime-avibactam | 64 | 0.0 | ||
| Aztreonam | >128 | 0.0 | ||
| Aztreonam-avibactam | 16 | NA | ||
| Cefepime | >16 | 0.0 | ||
| Meropenem | >8 | 0.0 | ||
| Imipenem | >8 | 0.0 | ||
| Piperacillin-tazobactam | >128 | 0.0 | ||
| Amikacin | >32 | 0.0 | ||
| Colistin | 2 | 100 | ||
MBL, metallo-β-lactamase; ESBL, extended-spectrum β-lactamase; OSBL, original-spectrum β-lactamase (includes TEM-1, TEM-2, SHV-1, and SHV-11).
Susceptibility percentages were determined using CLSI interpretive criteria. FDA breakpoints were applied for ceftazidime-avibactam (≤8 μg/ml, susceptible; ≥16 μg/ml, resistant) and tigecycline (≤2 μg/ml, susceptible; 4 μg/ml, intermediate; ≥8 μg/ml, resistant). EUCAST breakpoints were applied for colistin tested against Enterobacteriaceae (≤2 μg/ml, susceptible; ≥4 μg/ml, resistant).
Aztreonam-avibactam and ceftazidime-avibactam were tested at a fixed concentration of 4 μg/ml avibactam.
Colistin was tested with 0.002% polysorbate 80.
Includes the presumed chromosomally encoded ESBL common to K. oxytoca.
Includes plasmid-encoded and presumed chromosomally encoded AmpC β-lactamases common to Enterobacter spp., Citrobacter spp., M. morganii, S. marcescens, and P. aeruginosa.
The majority of antimicrobial agents tested were inactive against P. aeruginosa isolates producing KPC (susceptibilities of <4%), but 76% of the overall subset were susceptible to ceftazidime-avibactam or amikacin, and 96.6% were susceptible to colistin. However, only colistin remained active against the one P. aeruginosa isolate that coproduced KPC and an MBL (MIC, 2 μg/ml) (Table 4).
DISCUSSION
KPC carbapenemases hydrolyze penicillins, oxyimino-cephalosporins, cephamycins, monobactams, and carbapenems as well as the commercially available β-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam (12, 24). Carbapenem MIC values against KPC-producing bacteria can range from susceptible to fully resistant, with elevated KPC production due to increased blaKPC copy number and/or deletions in the upstream promoter region associated with higher MIC values in some isolates (12, 25, 26). Production of KPC is often accompanied by loss of either or both of the OmpK35 and OmpK36 porins, which further decreases susceptibility to carbapenems (25, 27–29). Four isolates (one K. pneumoniae and two E. coli collected from the same medical center in Colombia and one K. pneumoniae collected in the United States) that were susceptible to all tested carbapenems were identified, and they were presumed not to express KPC at significant levels.
blaKPC has disseminated from K. pneumoniae to P. aeruginosa and multiple species of Enterobacteriaceae, including E. coli, K. oxytoca, Enterobacter spp., Citrobacter spp., S. marcescens, M. morganii, and R. ornithinolytica, as described in this study and by others (12, 30). blaKPC has also been reported in Acinetobacter baumannii, Proteus mirabilis, Providencia stuartii, Pantoea agglomerans, Leclercia adecarboxylata, Kluyvera spp., Pseudomonas putida, and Salmonella spp. (12, 30–33). Intra- and interspecies spread of blaKPC is attributed to transposition of Tn4401, an active transposon with no target site specificity, to a variety of broad- and narrow-host-range plasmids capable of conjugation (34). Mathers et al. described three blaKPC-bearing plasmids identified during a hospital outbreak; one highly mobile plasmid was found in 11 isolates comprised of 9 unique strains (3 K. pneumoniae, 4 E. cloacae, and 1 each E. asburiae and C. freundii) collected from patients in various hospital units during an 8-month period, whereas the other two plasmids were found in 2 K. oxytoca isolates and 1 E. coli isolate, respectively. It should be noted that only approximately one-third of the affected patients had received treatment with a carbapenem, and one patient harbored two isolates (K. pneumoniae and E. asburiae) carrying the same KPC-encoding plasmid (14). In another study, three different KPC-producing species were sequentially collected from a patient over a 5-month period. Molecular analyses indicated that blaKPC was first transferred between plasmids carried by K. pneumoniae and E. coli via a Tn4401-mediated event, followed by conjugation of the blaKPC-bearing plasmid from E. coli into S. marcescens (35). The rapid and global spread of blaKPC has also been facilitated by carriage by K. pneumoniae strains belonging to clonal complex 258 (CC258), most frequently ST258 (36, 37). CC258 isolates tend to be multidrug resistant (MDR). In addition to blaKPC-bearing plasmids conferring resistance to β-lactams, members of CC258 often carry additional plasmids encoding resistance to aminoglycosides, trimethoprim, sulfonamides, and macrolides (16, 36, 38, 39). CC258 isolates also possess chromosomal mutations in gyrA and parC conferring fluoroquinolone resistance, and colistin-resistant ST258 isolates have been reported (36, 37, 39).
Treatment options available for managing patients infected with carbapenem-resistant or MDR pathogens have not kept pace with the emergence of resistance mechanisms in the patient population. Patients hospitalized in long-term and acute-care facilities are at significant risk for acquiring isolates producing KPC (40, 41). KPC-producing MDR isolates often remain susceptible only to tigecycline, polymyxins, and some aminoglycosides (e.g., gentamicin or amikacin); however, monotherapy with tigecycline or colistin is frequently associated with high treatment failure rates (4, 8–10, 42). Ceftazidime-avibactam was recently used in combination with ertapenem to successfully treat a patient infected with a KPC-producing K. pneumoniae isolate that had become resistant to tigecycline and colistin after treatment for successive nosocomial infections (43). However, one KPC-producing isolate that was resistant to ceftazidime-avibactam via an unknown mechanism has also been reported (44). In this study, avibactam restored the in vitro activity of both ceftazidime and aztreonam against KPC-producing isolates of Enterobacteriaceae, including, in the case of aztreonam-avibactam, activity against isolates that coproduced MBLs.
Reports of the emergence of colistin-resistant KPC-producing K. pneumoniae potentially further limit the number of therapeutic options available to treat infections caused by these challenging pathogens. Ceftazidime-avibactam and aztreonam-avibactam demonstrate potent in vitro activity against KPC-producing Enterobacteriaceae and may be powerful additions to the existing armamentarium of antimicrobial agents.
Supplementary Material
ACKNOWLEDGMENTS
AstraZeneca Pharmaceuticals provided financial support for this investigation.
All authors generated data or provided data analysis and have read and approved the final manuscript.
K.M.K., D.J.B., M.H., S.R., S.K.B., and D.F.S. are employees of International Health Management Associates, Inc. (IHMA). None of the IHMA authors have personal financial interests in the sponsor of this paper (AstraZeneca Pharmaceuticals). B.L.M.D.J. and P.A.B. are employees and stock holders of AstraZeneca Pharmaceuticals LP.
We gratefully acknowledge the contributions of the clinical site investigators, laboratory personnel, and all members of the global surveillance program who contributed isolates and information for this study.
Funding Statement
This investigation was funded by AstraZeneca Pharmaceuticals as part of a global surveillance program. The sponsor approved the overall study design. All investigative sites were recruited and study supplies were provided by IHMA, Inc. Analysis of the final MIC and molecular data was performed by IHMA, Inc., and was independent of sponsor analysis for this study.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00107-16.
REFERENCES
- 1.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. 2014. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 3.Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 4.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordmann P. 2014. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect 44:51–56. doi: 10.1016/j.medmal.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva RM, Traebert J, Galato D. 2012. Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae: a review of epidemiological and clinical aspects. Exper Opin Biol Ther 12:663–671. doi: 10.1517/14712598.2012.681369. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee GC, Burgess DS. 2012. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob 11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systemic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 11.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 13.Pitout JDD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AKC, Carrol J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2(6):e00204-11. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naas T, Bonnin RA, Cuzon G, Villegas MV, Nordmann P. 2013. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J Antimicrob Chemother 68:1757–1762. doi: 10.1093/jac/dkt094. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. 2014. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol 22:686–696. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y-Y, Giu D-X, Cai J-C, Zhou H-W, Zhang R. 2015. Emergence of KPC-2-producing Pseudomonas aeruginosa sequence type 463 isolates in Hangzhou, China. Antimicrob Agents Chemother 59:2914–2917. doi: 10.1128/AAC.04903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Chavda KD, DeLeo FR, Bryant KA, Jacobs MR, Bonomo RA, Kreiswirth BN. 2015. Genome sequence of a Klebsiella pneumoniae sequence type 258 isolate with prophage-encoded K. pneumoniae carbapenemase. Genome Announc 3(3):e00659-15. doi: 10.1128/genomeA.00659-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa A, del Campo R, Perenguez M, Blanco VM, Rodriguez-Banos M, Perez F, Maya JJ, Rojas L, Canton R, Arias CA, Villegas MV. 2015. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob Agents Chemother 59:2421–2425. doi: 10.1128/AAC.03926-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA, USA. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA, USA. [Google Scholar]
- 22.Lob SH, Kazmierczak KM, Badal RE, Hackel MA, Bouchillon SK, Biedenbach DJ, Sahm DF. 2015. Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART program, 2009 to 2013. Antimicrob Agents Chemother 59:3606–3610. doi: 10.1128/AAC.05186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manageiro V, Ferreira E, Almeida J, Barbosa S, Simoes C, Antibiotic Resistance Surveillance Program in Portugal, Bonomo RA, Canica M. 2015. Predominance of KPC-3 in a survey for carbapenemase-producing Enterobacteriaceae in Portugal. Antimicrob Agents Chemother 59:3588–3592. doi: 10.1128/AAC.05065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papp-Wallace KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, Bonomo RA. 2010. Inhibitor resistance in the KPC-2 β-lactamase, a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother 54:890–897. doi: 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitchel B, Rasheed JK, Endimiani A, Hujer AM, Anderson KF, Bonomo RA, Patel JB. 2010. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 54:4201–4207. doi: 10.1128/AAC.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naas T, Cuzon G, Truong H-V, Nordmann P. 2012. Role of ISKpn7and deletions in blaKPC gene expression. Antimicrob Agents Chemother 56:4753–4759. doi: 10.1128/AAC.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodford N, Tierno PM Jr, Young K, Tysall L, Palepou MF, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 48:4793–4799. doi: 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landman D, Bratu S, Quale J. 2009. Contribution of OmpK36 to carbapenem susceptibility in KPC-producing Klebsiella pneumoniae. J Med Microbiol 58:1303–1308. doi: 10.1099/jmm.0.012575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams-Sapper S, Nolen S, Donzelli GF, Lal M, Chen K, Justo da Silva LH, Moreira BM, Riley LW. 2015. Rapid induction of high-level carbapenem resistance in heteroresistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 59:3281–3289. doi: 10.1128/AAC.05100-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavares CP, Pereira PS, Marques A, Faria C Jr, de Souza P, de Almeida R, Alves F, Asensi MD, Carvalho-Assef AP. 2015. Molecular epidemiology of KPC-2-producing Enterobacteriaceae (non-Klebsiella pneumoniae) isolated from Brazil. Diagn Microbiol Infect Dis 82:326–330. doi: 10.1016/j.diagmicrobio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Robledo IE, Aquino EE, Sante MI, Santana JL, Otero DM, Leon CF, Vazquez GJ. 2010. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother 54:1354–1357. doi: 10.1128/AAC.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geffen Y, Adler A, Paikin S, Khabra E, Gorenshtein S, Aronov R, Carmeli Y. 2013. Detection of the plasmid-mediated KPC-2 carbapenem-hydrolysing enzyme in three unusual species of the Enterobacteriaceae family in Israel. J Antimicrob Chemother 68:719–720. doi: 10.1093/jac/dks443. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez E, Bautista A, Barrero L. 2014. First report of a Salmonella enterica serovar Typhimurium isolate with carbapenemase (KPC-2) in Colombia. Antimicrob Agents Chemother 58:1263–1264. doi: 10.1128/AAC.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuzon G, Naas T, Nordmann P. 2011. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother 55:5370–5373. doi: 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, Doi H. 2009. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis 49:1736–1738. doi: 10.1086/648077. [DOI] [PubMed] [Google Scholar]
- 36.Bowers JR, Kitchel B, Driebe EM, MacCannell DR, Roe C, Lemmer D, de Man T, Rasheed JK, Engelthaler DM, Keim P, Limbago BM. 2015. Genomic analysis of the emergence and rapid global dissemination of the clonal group 258 Klebsiella pneumoniae pandemic. PLoS One 10:e0133727. doi: 10.1371/journal.pone.0133727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathers AJ, Peirano G, Pitout JDD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLeo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A 111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban C, Bradford PA, Tuckman M, Segal-Maurer S, Wehbeh W, Grenner L, Colon-Urban R, Mariano N, Rahal JJ. 2008. Carbapenem-resistant Escherichia coli harbouring Klebsiella pneumoniae carbapenemase β-lactamases associated with long-term care facilities. Clin Infect Dis 46:127–130. doi: 10.1086/588048. [DOI] [PubMed] [Google Scholar]
- 41.Lin MY, Lyles-Banks RD, Lolans K, Hines DW, Spear JB, Petrak R, Trick WE, Weinstein RA, Hayden MK, Centers for Disease Control and Prevention Epicenters Program. 2013. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 57:1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camargo JF, Simkins J, Beduschi T, Tekin A, Aragon L, Perez-Cardona A, Prado CE, Morris MI, Abbo LM, Canton R. 2015. Successful treatment of carbapenemase-producing pandrug-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 59:5903–5908. doi: 10.1128/AAC.00655-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. 2015. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 59:6605–6607. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.