Abstract
Dihydropteroate synthase (DHPS) is a known sulfa drug target in malaria treatment, existing as a bifunctional enzyme together with hydroxymethyldihydropterin pyrophosphokinase (HPPK). Polymorphisms in key residues of Plasmodium falciparum DHPS (PfDHPS) have been characterized and linked to sulfa drug resistance in malaria. Genetic sequencing of P. vivax dhps (Pvdhps) from clinical isolates has shown several polymorphisms at the positions equivalent to those in the Pfdhps genes conferring sulfa drug resistance, suggesting a mechanism for sulfa drug resistance in P. vivax similar to that seen in P. falciparum. To characterize the role of polymorphisms in the PvDHPS in sulfa drug resistance, various mutants of recombinant PvHPPK-DHPS enzymes were expressed and characterized. Moreover, due to the lack of a continuous in vitro culture system for P. vivax parasites, a surrogate P. berghei model expressing Pvhppk-dhps genes was established to demonstrate the relationship between sequence polymorphisms and sulfa drug susceptibility and to test the activities of PvDHPS inhibitors on the transgenic parasites. Both enzyme activity and transgenic parasite growth were sensitive to sulfadoxine to different degrees, depending on the number of mutations that accumulated in DHPS. Ki values and 50% effective doses were higher for mutant PvDHPS enzymes than the wild-type enzymes. Altogether, the study provides the first evidence of sulfa drug resistance at the molecular level in P. vivax. Furthermore, the enzyme inhibition assay and the in vivo screening system can be useful tools for screening new compounds for their activities against PvDHPS.
INTRODUCTION
Plasmodium vivax accounted for approximately 15.8 million cases of malaria worldwide in 2013 (1). Although P. vivax infection is usually nonfatal, severe complications, including renal failure, severe anemia, and cerebral malaria, have been reported (2–11). Because infections with P. vivax parasites can recrudesce months or years after the initial infection, the parasites are also more difficult to eliminate from the community. Drug-resistant malaria has emerged to become a major public health problem. Research for the discovery of drugs with activity against P. vivax remains challenging due to the lack of a stable continuous in vitro culture system, and an in vivo primate model is inaccessible for general laboratories (12–15).
Folate metabolism is important for malaria parasite survival, and several enzymes in the pathway have been well characterized to be the targets for several classes of antimalarial drugs. A combination of pyrimethamine and sulfadoxine (Fansidar) was widely used to treat malaria until resistance emerged 10 years after its introduction (16, 17). Previous studies revealed that point mutations in the dihydrofolate reductase (DHFR) of Plasmodium falciparum (PfDHFR) and the dihydropteroate synthase (DHPS) of P. falciparum (PfDHPS) contributed to antifolate and sulfa drug resistance, respectively (18–21). Due to the conserved nature of the enzymes in the folate metabolic pathway, similar polymorphisms in the P. vivax DHFR (PvDHFR) and P. vivax DHPS (PvDHPS) have also been suggested to reduce the efficacy of antifolates and sulfa drug treatment in P. vivax infection (22–25). Although the combination of pyrimethamine and sulfadoxine (Fansidar) is not normally used as a treatment for P. vivax infection, the regimen has been used to treat patients coinfected with P. falciparum and P. vivax, a practice that would have exposed P. vivax to drug pressure and led to key mutations for the resistant phenotypes reported in P. falciparum infection (26).
The malaria parasite DHPS forms a bifunctional enzyme with hydroxymethyldihydropterin pyrophosphokinase (HPPK) and locates at the C terminus of the bifunctional polypeptide chain. Four point mutations of PvDHPS at positions S382F/A/C, A383G, K512E/M/T, and A553G have been identified from P. vivax clinical isolates from Southeast Asia; these are equivalent to mutations of PfDHPS at positions S436F/A, A437G, K540E, and A581G, respectively (22, 26–29). Among these mutations, the A383G single mutation, A383G A553G double mutation, and S382A A383G A553G triple mutation were found in 90% of mutants in many areas where malaria is endemic (29). In addition to these conserved polymorphic residues, a V residue is found at position 585 (V585) of wild-type PvDHPS, while the equivalent position in PfDHPS carries A613 for the wild-type enzyme and A613S for the mutant enzyme. It was speculated that P. vivax may have innate resistance to sulfadoxine, as the bulky side chain of V585 may cause steric hindrance to sulfadoxine binding (22).
In this study, recombinant P. vivax HPPK-DHPS (PvHPPK-DHPS) enzymes were heterologously expressed to characterize the sulfa drug resistance mechanism in P. vivax. In addition to the enzyme target inhibition study, transgenic P. berghei parasites in which the native P. berghei hppk and dhps (Pbhppk-dhps) alleles were replaced with various Pvhppk-dhps alleles similar to those in clinical P. vivax isolates were generated to evaluate sulfa drug susceptibility in vivo. The results showed that mutations at PvDHPS affect the enzyme kinetic properties and susceptibility to sulfa drugs and that the accumulation of mutations is associated with reduced sensitivity to sulfa drugs. We also demonstrated that the surrogate DHPS in vivo mouse model can potentially be used as a system to screen for new compounds with activities against P. vivax.
MATERIALS AND METHODS
Plasmid construction for expression of recombinant protein in Escherichia coli.
Initially, total RNA of P. vivax-infected red blood cells of a Thai isolate (ms2002) was extracted by use of the TRIzol reagent (Life Technologies) by the protocol described by the manufacturer. Total RNA was then treated with RNase-free DNase I (New England BioLabs) to remove contaminating DNA. The cDNA was synthesized using oligo(dT) and Moloney murine leukemia virus reverse transcriptase (New England BioLabs). Pvhppk-dhps was amplified from P. vivax cDNA using Pfu DNA polymerase (Promega) with primers NdeI_PvHPPK_F and BamHI_PvDHPS_+2bp_R. The amplified PCR product was then cloned into pET22b and the sequence was verified by DNA sequencing (1st Base, Singapore). This plasmid was called pET22b Pvhppk-dhps-ms2002. The primers used in this study are listed in Table 1.
TABLE 1.
Primers used for construction and molecular characterization of transgenic P. berghei carrying Pvhppk-dhps
| Primer name | Sequence |
|---|---|
| NdeI_PvHPPK_F | 5′-GGGGCATATGGAGGATTCAAACACGGG-3′ |
| BamHI_PvDHPS_+2bp_R | 5′-GGGGGGATCCCTAGGTTGATGTATCCTTGTGAG-3′ |
| PvHPPK_V179L_F | 5′-AGACCCCCTCGCCATGCTCGTAATTTTAAAGTACATTGAGCA-3′ |
| PvHPPK_V179L_R | 5′-TGCTCAATGTACTTTAAAATTACGAGCATGGCGAGGGGGTCT-3′ |
| PvHPPK_M205I_F | 5′-GAAATATTTCAAAATCGCATAATAGACATTGACATTTTATTTTTTAAC-3′ |
| PvHPPK_M205I_R | 5′-GTTAAAAAATAAAATGTCAATGTCTATTATGCGATTTTGAAATATTTC-3′ |
| PvDHPS_A383G_F | 5′-CGGGGGGGAATCGTCCGCCCCTTATGTGGTCCCCAATC-3′ |
| PvDHPS_A383G_R | 5′-GATTGGGGACCACATAAGGGGCGGACGATTCCCCCCCG-′3 |
| PvDHPS_A553G_F | 5′-GATGTCGGCC TGGGGTTTGCCAAAAAGCACGACCAGTCTATTAAG-3′ |
| PvDHPS_A553G_R | 5′-CTTAATAGACTGGTCGTGCTTTTTGGCAAACCCCAGGCCGACATC-3′ |
| ApaI_5′UTR_PbDHPS_F | 5′-GGGGGGCCCGTTACACAAATTAGTAGTGTGTC-3′ |
| SalI_3′UTR_PbDHPS_R | 5′-GGGGTCGACCAGTTTCTATTAGTTCTTTGATATG-3′ |
| XmaI_3′UTR_PbDHPS_F | 5′-GGGCCCGGGCTTCAATGGATAATGTATAGTGG-3′ |
| KasI_3′UTR_PbDHPS_R | 5′-GGGGGCGCCG CTATATCTCTCTGTGCTTAC-3′ |
| SalI_Pv_PKDS_F | 5′-GGGGTCGACATGGAGGATTCAAACACGGG-3′ |
| SacII_Pv_PKDS_R | 5′-GGGCCGCGGCTAAGGTTGATGTATCCTTGTG-3′ |
| PbDHPS_F (a) | 5′-ATGGATATTATAGAAGAATCTAATAAATG-3′ |
| 3′int_PbDHPS_R (b) | 5′-CTGAGACATCAACGTGCCCTC-3′ |
| pL35_before_HindIII_F (c) | 5′-GTCTCTTCAATGATTCATAAATAG-3′ |
| PvHPPK_exon1_F (d) | 5′-CTCCGTCAGATAGAGCGCCG-3′ |
| pL35_before_Acc65I_F (e) | 5′-GACGGTCACAGCTTGTCTGT-3′ |
| pL35_after_KasI_F (f) | 5′-CTCATTAGGCACCCCAGGCT-3′ |
| PvDHPS_1767nt_F (g) | 5′-CACCCCCGGGGGGAAGGGTGGCGCGGCCATC-3′ |
| Pbshmt_α-tubulin-2_F | 5′-GCATGCTGGGAGCTATTTTG-3′ |
| Pbshmt_α-tubulin-2_R | 5′-GCTGGTTCAAATGCTGAGTTTG-3′ |
The DNA sequencing results revealed four nonsynonymous polymorphisms in Pvhppk-dhps, V179L, M205I, A383G, and A553G, compared to the sequence of P. vivax laboratory-adapted Sal-1 strain (PlasmoDB, www.plasmodb.org). PCR site-directed mutagenesis to convert these polymorphisms so that they were similar to those reported for P. vivax Sal-1 were performed using primer pairs PvHPPK_V179L_F and PvHPPK_V179L_R, PvHPPK_M205I_F and PvHPPK_M205I_R, PvDHPS_A383G_F and PvDHPS_A383G_R, and PvDHPS_A553G_F and PvDHPS_A553G_R. The resulting plasmid was named pET22b Pvhppk-dhps. The recombinant plasmids harboring different Pvhppk-dhps variants were used for protein expression.
Recombinant P. vivax HPPK-DHPS expression and purification.
E. coli Rosetta(DE3)pLysS harboring individual pET22b Pvhppk-dhps variants was cultured in LB supplemented with 100 μg/ml ampicillin and 34 μg/ml chloramphenicol until the cells had grown to an optical density at 600 nm of 1.0. The expression of recombinant PvHPPK-DHPS was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to the recombinant bacterial culture at a final concentration of 0.4 mM, and then the culture was maintained at 16°C overnight (∼16 to 20 h). Bacterial cells were harvested by centrifugation at 3,000 × g for 10 min and stored at −20°C.
For purification of recombinant PvHPPK-DHPS, the harvested bacterial pellet (30 g) was resuspended in 150 ml of lysis buffer (100 mM Tris-HCl, pH 7.5, 100 mM NaCl, 20% glycerol, 40 mM imidazole) and disrupted by use of a French pressure cell at 1,500 lb/in2. The total cell lysate was centrifuged twice at 27,000 × g for 30 min to remove the cell debris. The supernatant was loaded onto a 25-ml Ni–immobilized-metal affinity chromatography (IMAC) Sepharose column (GE Healthcare Life Sciences). The column was washed with 700 ml of washing buffer (100 mM Tris-HCl, pH 7.5, 300 mM NaCl, 20% glycerol, 40 mM imidazole). The bound proteins were then eluted with a linear gradient of elution buffer (100 mM Tris-HCl, pH 7.5, 100 mM NaCl, 20% glycerol, 40 to 250 mM imidazole). The purified PvHPPK-DHPS was eluted with imidazole at concentrations ranging from 70 to 130 mM. Fractions containing purified PvHPPK-DHPS were pooled and concentrated by use of an Amicon filter (Millipore, Billerica, MA, USA) at a 50-kDa-molecular-mass cutting size with exchange buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.5 mM EDTA, 20% glycerol, 10 mM dithiothreitol). Protein purity and the subunit molecular mass were analyzed using 12% SDS-PAGE. The native molecular mass of the recombinant PvHPPK-DHPS was determined by Superdex 200 HR 10/300 (GE Healthcare Life Sciences) gel filtration chromatography on a column equilibrated with 50 mM phosphate buffer (pH 7.0) in the presence of 150 mM NaCl. Reference proteins were thyroglobulin (669 kDa), ferritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), RNase A (13.7 kDa), and aprotinin (6.5 kDa). The native molecular mass of PvHPPK-DHPS was calculated on the basis of the calibration curve of the gel-phase distribution coefficient (Kav) versus the log molecular mass, for which Kav = (Ve − Vo)/(Vc − Vo), where Ve, Vo, and Vc are the elution volume, column void volume, and geometric column volume, respectively.
Enzyme kinetics and inhibition studies.
The activity of PvHPPK-DHPS was determined on the basis of incorporation of radioactive (14C-labeled) para-aminobenzoic acid ([14C]pABA) to form [14C]dihydropteroate ([14C]DHP), and the radiolabeled substrate and product were separated by paper chromatography as previously described (30, 31). The assay reaction mixture (50 μl) was composed of 100 mM Tris-HCl, pH 9.0, 10 mM MgCl2, 100 mM β-mercaptoethanol, 200 μM hydroxymethyldihydropterin (HMDHP), 10 mM ATP, 100 μg/ml bovine serum albumin, 5 μM [14C]pABA, and 0.05 to 0.1 μM PvHPPK-DHPS. The reaction was started by adding enzyme, and the mixture was incubated at 37°C for 10 min. Then, the reaction was stopped by boiling for 2 min. The supernatant (40 μl) was spotted on Whatman no. 3 paper (Whatman, GE Healthcare Life Sciences), and chromatography was performed in a chamber preequilibrated with 0.1 M potassium phosphate buffer, pH 7.0. The origin spot (2 cm by 2 cm) was cut and put into a scintillation vial containing 6 ml OptiPhase-HiSafe II liquid scintillation cocktail (PerkinElmer), and the radioactivity of [14C]DHP was measured with an LS 6500 multipurpose scintillation counter (Beckman Coulter).
The apparent Km for pABA was determined by the use of various concentrations of [14C]pABA (0.025 to 20 μM). Inhibition (Ki) of PvDHPS by sulfa drugs was determined as described above, except that the sulfa drugs were included at various concentrations (1 to 50,000 μM). The kinetic parameters were determined with Kaleidagraph (version 4.03) software (Synergy Software) using a nonlinear least-squares fit of the data to the Michaelis-Menten equation.
Plasmid construction for generation of transgenic P. berghei.
The plasmids used for the allelic replacement of Pbhppk-dhps (PBANKA_142670) by Pvhppk-dhps (PVX_123230) were constructed on the basis of pL0035 (The Malaria Research and Reference Reagent Resource Center [MR4]) containing human dihydrofolate reductase fused with the Saccharomyces cerevisiae yeast cytosine deaminase and uridyl phosphoribosyl transferase (hdhfr/yfcu) expression cassette, which served as positive and negative selection markers, respectively. Pyrimethamine and 5-fluorocytosine (5FC) were used to select transfected parasites and marker-free transgenic parasites, respectively. Three PCR amplicons, corresponding to the 5′ and 3′ untranslated regions (UTRs) of Pbhppk-dhps and to the coding sequence of Pvhppk-dhps, respectively, were individually amplified. The two fragments corresponding to the 5′ and 3′ UTRs (∼1 kb) were amplified from P. berghei genomic DNA (gDNA) using primer pair ApaI_5′UTR_PbDHPS_F and SalI_3′UTR_PbDHPS_R and primer pair XmaI_3′UTR_PbDHPS_F and KasI_3′UTR_PbDHPS_R, respectively. Different variants of the Pvhppk-dhps wild type and the Pvhppk-dhps A383G single mutant, A383G A553G double mutant, and S382A A383G A553G triple mutant were amplified from pET22b expression plasmids constructed as described above using primers SalI_Pv_PKDS_F and SacII_Pv_PKDS_R. The 3′ UTR fragment was primarily inserted into pL0035 at XmaI and KasI sites, and the acquired plasmid was named pL0035 3′UTR Pbhppk-dhps. The fragments of the 5′ UTR of Pbhppk-dhps and the coding sequence of Pvhppk-dhps were digested with SalI and then ligated together. The ligated fragment of the 5′ UTR of Pbhppk-dhps/Pvhppk-dhps was PCR amplified with primers ApaI_5′UTR_PbDHPS_F and SacII_Pv_PKDS_R. The resulting amplicons of the 5′ UTR of Pbhppk-dhps/Pvhppk-dhps was then inserted into plasmid pL0035 3′UTR Pbhppk-dhps at the ApaI and SacII sites. The transfection plasmid was named pL0035ΔPb(Pv)hppk-dhps. The DNA sequences of the pL0035ΔPb(Pv)hppk-dhps wild type and variants were confirmed by DNA sequencing (1st Base).
P. berghei parasite transfection.
All animal experiments were performed according to international and national guidelines for the ethical conduct for the care and humane use of animals with the approval of the Institutional Animal Care and Use Committee, National Center for Genetic Engineering and Biotechnology (Biotec), Thailand.
Transfection of plasmids carrying the Pvhppk-dhps wild type and A383G, A383G A553G, and S382A A383G A553G mutant sequences into P. berghei was performed as described previously (32). Initially, mouse strain ICR was intraperitoneally infected with P. berghei (1 × 106 infected red blood cells [iRBC]), and blood from the tail vein was collected for determination of the level of parasitemia. Approximately 10 μg of a transfection plasmid was linearized by ApaI and KasI enzymes, and the linearized DNA was transfected into P. berghei (ANKA strain) by use of a Basic Parasite Nucleofector kit 2 (Lonza AG) and Nucleofector device (Amaxa GmbH) using preset program U033. Transfected parasites were intravenously injected into the tail vein of the mice. Pyrimethamine (70 μg/ml) in drinking water was given to mice a day postinjection to select transgenic parasites. On approximately day 7 postinjection, a Giemsa-stained thin blood smear was used to determine the parasite density.
To obtain a clonal line of integrant transgenic parasites, the limiting dilution method was performed. In order to obtain marker-free parasites, cloned transgenic P. berghei parasites harboring Pvhppk-dhps were treated with 5FC by mixing the drug with drinking water (0.5 mg/ml) (33, 34). Transfectant parasites whose hdhfr/yfcu cassette was deleted were selected and cloned by the limiting dilution method.
Molecular characterization of transgenic P. berghei parasites with Pvhppk-dhps.
Once the parasitemia reached 5 to 10%, mouse blood was collected by heart puncture. White blood cells were removed by passage through a syringe packed with cellulose powder (Sigma-Aldrich). The gDNA of the transgenic parasites was extracted using phenol-chloroform extraction and ethanol precipitation and subjected to PCR amplification to monitor the disruption of the Pbhppk-dhps locus following specific integration of the targeting plasmid into the Pbhppk-dhps locus.
For Southern blot hybridization, gDNA (30 μg) from either P. berghei ANKA or cloned transgenic parasites was digested with PvuII and SpeI. The digested DNA was separated by 1% agarose gel electrophoresis at 50 V for 5 h. DNA fragments were denatured on agarose, neutralized, and then transferred to a positively charged nylon membrane (Merck Millipore) by capillary force. The transferred DNA on the membrane was fixed by UV cross-linking. PCR amplicons of the 5′ UTR and 3′ UTR of Pbdhps were used as the templates to generate digoxigenin (DIG)-labeled DNA probes. Hybridization was performed as described by the protocol of the manufacturer of the DIG High Prime DNA labeling and detection kit II (Invitrogen).
Parasite growth study.
Three BALB/c mice per group were injected intravenously in the tail vein with either P. berghei ANKA or transgenic P. berghei parasites carrying different Pvdhps genes (1 × 106 iRBC per mouse). The growth study was performed in triplicate. Parasite numbers were counted every day under a light microscope using Giemsa-stained blood smears.
Sulfa drug susceptibility of transgenic P. berghei parasites carrying different Pvhppk-dhps alleles.
The responses of the transgenic parasites to sulfadoxine and dapsone were monitored according to a 4-day suppressive drug test (35). Three mice per group were used, and the experiment was performed in triplicate with various drug concentrations. Briefly, 1 × 106 iRBC of transgenic parasites were injected into the tail vein of the BALB/c mice. Different concentrations of drugs mixed with the hydroxypropylmethyl cellulose (HPMC) suspension vehicle were administered to mice by oral gavage at the same time every day from day 1 to day 4. Giemsa-stained smears of blood (from tail vein) were prepared at the same time on day 5. Parasite numbers were counted under a light microscope. The dose-response curve was generated to determine the 50% effective doses (ED50s) of these drugs.
RESULTS
Heterologous expression of recombinant P. vivax HPPK-DHPS.
The open reading frame of Pvhppk-dhps was amplified from the cDNA of strain ms2002 (kindly provided by Jetsumon Sattabongkot Prachumsri, Mahidol University), a field isolate from Thailand, and cloned into pET22b. Four nonsynonymous polymorphisms compared with the sequence of the P. vivax Sal-1 strain (PlasmoDB, www.plasmodb.org) were observed; 2 of these were located in the hppk gene (V179L, GTA → TTA; M205I, ATG → ATA), and the other two were found in the dhps gene (A383G, GCC → GGC; A553G, GCC → GGC). While M205 is not conserved, V179, A383, and A553 are conserved among Plasmodium spp. and equivalent to V182, A437, and A581 in P. falciparum, respectively (Fig. 1). Mutations at A437 and A581 in the P. falciparum homolog have been reported to compromise sulfa drug susceptibility (19, 21). To explore the contribution of these polymorphisms in Pvhppk-dhps to sulfa drug sensitivity, expression constructs with single (A383G), double (A383G A553G; similar to the ms2002 sequence), and triple (S382A A383G A553G) mutations were prepared, as they are the prevalent mutations observed in nature (27, 29, 36, 37). In addition, a V585A mutant was included to determine whether V585 plays role in innate sulfa drug resistance, as previously postulated on the basis of structural modeling of the enzyme (22).
FIG 1.
Multiple-amino-acid-sequence alignment of HPPK-DHPS. Only a partial sequence is shown. The proteins from P. vivax (Sal-I), P. falciparum, P. berghei, and Francisella tularensis are bifunctional HPPK-DHPS, while those from E. coli, Bacillus anthracis, Staphylococcus aureus, and Mycobacterium tuberculosis are monofunctional proteins of HPPK and DHPS. Amino acids reported to show polymorphisms (V179, M205, S382, A383, K512, and A553) and V585 are emphasized in bold with underlining.
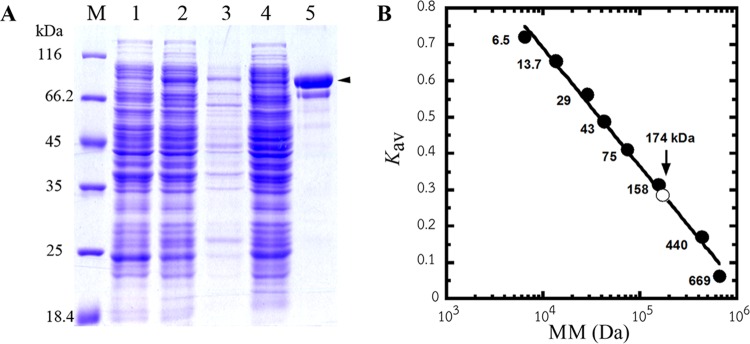
In general, PvHPPK-DHPS proteins in this study were expressed as soluble protein at a very low level (Fig. 2A). Various conditions, such as different E. coli strains and medium formulations, were used, without success, in an attempt to improve the yield (data not shown). The recombinant PvHPPK-DHPS enzymes of the wild type and the variants were purified using Ni-IMAC Sepharose. The subunit molecular mass of the recombinant PvHPPK-DHPS enzymes was estimated to be 85 kDa on the basis of migration on SDS-polyacrylamide gels (Fig. 2A), and this molecular mass is in agreement with the calculated molecular mass. The native molecular mass of PvHPPK-DHPS was determined to be 174 kDa, suggesting that PvHPPK-DHPS is a homodimeric protein (Fig. 2B). The recovery yield obtained was in the range of 0.13 to 0.3 mg/liter culture.
FIG 2.
Recombinant PvHPPK-DHPS expression in E. coli Rosetta(DE3)pLysS and its molecular mass. (A) SDS-PAGE analysis of recombinant PvHPPK-DHPS at different purification steps. Lanes: M, protein molecular mass marker (Fermentas); 1, uninduced cell lysate; 2, induced cell lysate; 3, inclusion body; 4, soluble fraction of cell lysate; 5, purified PvHPPK-DHPS. Arrowhead, a band of purified PvHPPK-DHPS with a subunit molecular mass of 85 kDa. (B) A plot of the gel-phase distribution coefficient (Kav) versus the protein molecular mass (MM; in log scale) to determine the native molecular mass of PvHPPK-DHPS (∼174 kDa), which is indicated as an open circle on the calibration curve.
P. vivax HPPK-DHPS enzyme kinetic and inhibition studies.
The apparent kcat and Km values of pABA for various polymorphic forms of PvHPPK-DHPS were determined and compared to those for wild-type PvDHPS; they appeared to show 6- and 3-fold maximum differences for kcat (range, 0.0024 to 0.0134 s−1) and Km (range, 0.15 to 0.48 μM), respectively (Table 2). The results suggest that these mutations have subtle effects on enzyme catalysis and pABA binding. Interestingly, the kcat and Km values for wild-type PvHPPK-DHPS were dissimilar to those for PfHPPK-DHPS (0.0024 and 0.03 s−1, respectively, for kcat and 0.15 and 1.25 μM, respectively, for Km) (38), yet the catalytic efficiency (kcat/Km) appeared to be of a similar magnitude (1.6 × 104 and 2.4 × 104 M−1 s−1, respectively). The catalytic efficiency for the mutant PvDHPS enzymes tested also demonstrated similar trends.
TABLE 2.
Kinetic parameters of recombinant PvHPPK-DHPS
| PvHPPK-DHPS | kcat (s−1) | Km pABA (μM) |
Ki (μM) |
||
|---|---|---|---|---|---|
| Sulfadoxine | Sulfathiazole | Dapsone | |||
| Wild type | 0.0024 ± 0.0003 | 0.15 ± 0.02 | 1.50 ± 0.11 | 0.15 ± 0.05 | 0.11 ± 0.01 |
| A383G mutant | 0.0055 ± 0.0005 | 0.33 ± 0.02 | 48.10 ± 5.45 | 2.95 ± 0.11 | 3.67 ± 0.60 |
| A383G A553G mutant | 0.0134 ± 0.0005 | 0.48 ± 0.05 | 176.19 ± 8.70 | 15.53 ± 1.37 | 4.54 ± 0.51 |
| S382A A383G A553G mutant | 0.0060 ± 0.0008 | 0.26 ± 0.01 | 266.83 ± 16.31 | 19.00 ± 1.74 | 17.73 ± 1.14 |
| V585A mutant | 0.0029 ± 0.0002 | 0.19 ± 0.01 | 3.01 ± 0.36 | 0.19 ± 0.02 | 0.53 ± 0.06 |
To characterize the roles of these PvDHPS mutations on sulfa drug sensitivity, three sulfa drugs, two with different sulfonamide substituents (sulfadoxine and sulfathiazole) and one with a sulfone moiety (dapsone), were chosen for the enzyme inhibition study. Sulfadoxine and dapsone are drugs known to be used for malaria treatment, while sulfathiazole is a good inhibitor of DHPS. The results from the enzyme inhibition study are shown in Table 2. The sensitivity to sulfadoxine varied depending on the variant, and more mutations led to higher Ki values. The sulfadoxine resistance of the mutants with single, double, and triple PvDHPS mutations significantly increased 30-, 120-, and 180-fold, respectively, compared to the sensitivity of the wild-type enzyme. The V585A variant revealed a subtle difference in sensitivity from that of the wild type. The results clearly indicate that the S382A, A383G, and A553G mutations contributed to sulfadoxine sensitivity and that the combination of these mutations increased sulfadoxine resistance in P. vivax. Likewise, the efficacies of dapsone and sulfathiazole were reduced in the PvDHPS mutants. Sulfathiazole and dapsone appear to be more effective inhibitors of both wild-type and mutant PvDHPS enzymes, similar to the results observed with PfDHPS enzymes (21). These results suggest that the mechanism in which sulfa drugs inhibit the malaria parasite DHPS and the mechanism by which the parasite evolved to resist sulfa drugs are similar across species.
Generation of transgenic P. berghei parasites with Pvhppk-dhps.
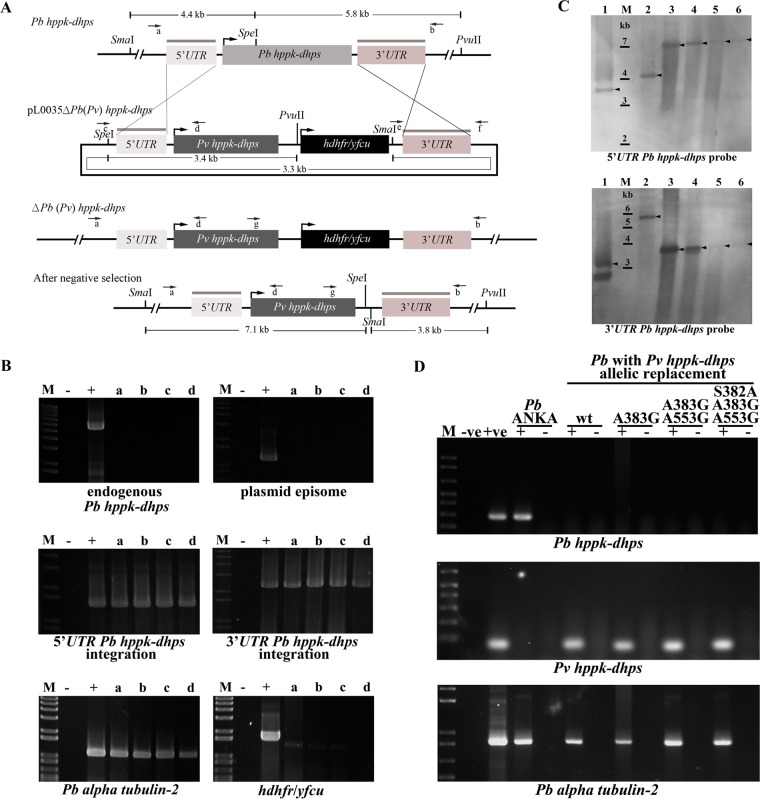
Since a system for the continuous in vitro culture of P. vivax is not routinely available, a surrogate model based on transgenic P. berghei, an in vivo model used for the testing of antimalarials, was established to study the effects of polymorphisms on sulfa drug sensitivity and to test the effects of anti-DHPS drugs on the P. vivax parasites according to a previously published protocol (32, 33, 39). Four transgenic P. berghei parasite lines carrying variants of Pvhppk-dhps were generated following double homologous recombination of linearized constructs carrying the coding sequences of Pvhppk-dhps of the wild type or the A383G single mutant, A383G A553G double mutant, or S382A A383G A553G triple mutant variant (Fig. 3A). Negative selection was conducted to remove the selection markers from the transfected parasite population, which was followed by a limiting dilution protocol to obtain clonal transgenic parasite lines (34).
FIG 3.
Molecular characterization of a transgenic P. berghei (Pb) parasite harboring Pvhppk-dhps. (A) Schematic diagram depicting the genomic organization before and after integration of Pvhppk-dhps at the Pbhppk-dhps locus. Arrows indicate the primers used for the molecular characterization of transgenic parasites (primers c and d or primers e and f for the episomal status of the transfected plasmid; primers a and b for the presence of endogenous Pbhppk-dhps; primers a and d and primers g and b for the 5′ UTR and the 3′ UTR of Pvhppk-dhps integration, respectively). Enzyme restriction sites along with fragment sizes for Southern blot hybridization are indicated. (B) PCR results for molecular characterization of transgenic P. berghei with Pvhppk-dhps. Lanes: M, 1-kb plus DNA marker (Invitrogen); −, sample with no template; +, positive control; a to d, transgenic P. berghei in which the hppk-dhps allele was replaced by wild-type Pvhppk-dhps and Pvhppk-dhps with the A383G, A383G A553G, and S382A A383G A553G mutations, respectively. The P. berghei alpha tubulin-2 gene was used for DNA quality control. (C) Southern blot hybridization of Pvhppk-dhps allelic replacement at the Pbhppk-dhps locus. A Southern blot was hybridized with the 3′ or 5′ UTR Pbhppk-dhps probe to confirm Pvhppk-dhps allelic replacement at the Pbhppk-dhps locus. The 3′ or 5′ UTR Pbhppk-dhps probe binding sites are indicated by gray lines in panel A. DNA was digested with PvuII and SpeI. Lanes: M, 1-kb plus ladder (Invitrogen); 1, transfection plasmid; 2, gDNA of P. berghei; 3 to 6, gDNA of transgenic P. berghei parasites in which the hppk-dhps allele was replaced by wild-type Pvhppk-dhps and Pvhppk-dhps with the A383G, A383G A553G, and S382A A383G A553G mutations, respectively. Arrowheads, hybridized bands. (D) RT-PCR for detection of Pbhppk-dhps, Pvhppk-dhps, and P. berghei alpha tubulin-2 transcripts. Lanes: M, 1-kb plus ladder; −ve, negative control (water); +ve, positive control; +, product of PCR with the indicated cDNA; −, product of control PCR without reverse transcriptase. wt, wild type.
The DHPS locus of the recovered transgenic parasite lines was characterized by PCR using specific primer pairs to demonstrate the replacement of the Pbhppk-dhps allele with the Pvhppk-dhps allele. The absence of a 5.4-kb PCR product when the sequence was amplified with primer pair a and b indicated the absence of the native Pbhppk-dhps gene. The presence of a 1.4-kb fragment when the sequence was amplified with primer pair a and d and the presence of a 2.1-kb fragment when the sequence was amplified with primer pair g and b indicated the replacement of the native hppk-dhps gene with Pvhppk-dhps in transgenic parasites (Fig. 3A and B). The 1.2-kb product was not observed when the sequence was amplified with primer pair c and d, suggesting that these transgenic parasite lines did not maintain the episomal plasmid form (Fig. 3A and B). Furthermore, a DNA band corresponding to the hdhfr/yfcu gene was not observed when the sequence was amplified with primers flanking this region, indicating successful removal of a negative selectable marker (Fig. 3B). The Southern blot hybridization confirmed the PCR results and showed band patterns corresponding to the absence of endogenous Pbhppk-dhps, the correct integration of the target plasmid in the native hppk-dhps locus of the transgenic P. berghei lines, and the absence of the hdhfr/yfcu marker (Fig. 3C). Reverse transcription-PCR (RT-PCR) demonstrated the expression of Pvhppk-dhps transcripts, but the endogenous Pbhppk-dhps transcripts were not detected in transgenic parasites (Fig. 3D).
Study of growth of transgenic P. berghei parasites with Pvhppk-dhps.
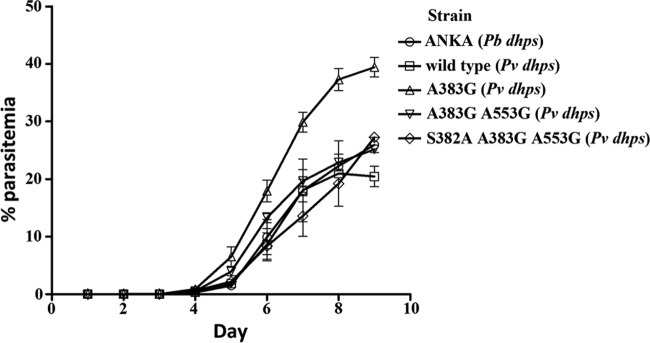
The growth profiles of transgenic P. berghei parasites were compared with the growth profile of the P. berghei ANKA strain (Fig. 4). With the exception of P. berghei transgenic parasites carrying A383G, the growth rates of the other transgenic lines were comparable to the growth rate of the reference P. berghei ANKA strain. The enzyme characteristics of the mutant with a single mutation (A383G) did not show that it had activity improved over that of the mutant enzymes with double and triple mutations as a reason for the improved fitness and growth observed in transgenic P. berghei parasites. On the basis of these results, it can be concluded that Pvhppk-dhps is a functional equivalent of Pbhppk-dhps.
FIG 4.
Growth of various transgenic P. berghei parasites in comparison to that of the parental strain, P. berghei ANKA. Thin blood smears were performed and the levels of parasitemia were determined every day postinfection for 9 days.
Susceptibility to sulfa drugs of transgenic P. berghei parasites carrying different Pvhppk-dhps mutations.
Transgenic P. berghei parasite lines and the control P. berghei ANKA strain were investigated for their susceptibility to sulfa drugs on the basis of a 4-day suppressive test described previously (35). Three different sulfa drugs were tested: sulfadoxine, dapsone, and sulfathiazole. Similar to the observation made during the enzyme assays, the results from the testing of the activities of the sulfa drugs against the control and transgenic parasites confirmed the association of increasing sulfa drug resistance with the accumulation of mutations in PvDHPS.
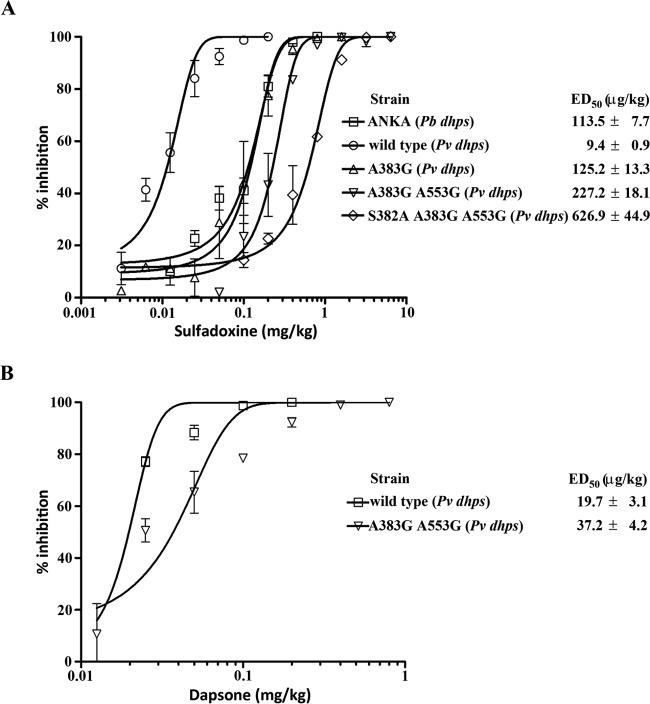
The ED50 value of sulfadoxine for transgenic P. berghei carrying the PvDHPS wild type (9.4 ± 0.9 μg/kg) was significantly lower than that for the P. berghei ANKA strain (113.5 ± 7.7 μg/kg) (Fig. 5A). It should be noted that position 591 in PbDHPS, which is a serine, is equivalent to position 613 in PfDHPS, where A613S has been shown to be associated with sulfa drug resistance.
FIG 5.
Four-day suppressive test with sulfadoxine (A) and dapsone (B) for various transgenic P. berghei parasites. The curve fit was done by using the nonlinear regression function for the sigmoidal dose-response to calculate the ED50.
When the ED50 values of sulfadoxine for the transgenic parasites were compared, the ED50 of transgenic P. berghei carrying the PvDHPS wild type (9.4 ± 0.9 μg/kg) was 13-, 24-, and 66-fold less than the ED50s of transgenic parasites carrying the A383G single mutation (ED50 = 125.2 ± 13.3 μg/kg), the A383G A553G double mutation (ED50 = 227.2 ± 18.1 μg/kg), and the S382A A383G A553G triple mutation (ED50 = 626.9 ± 44.9 μg/kg), respectively (Fig. 5A). The results presented here indicate that mutations in the PvDHPS gene affect sulfa drug sensitivity and that there is a correlation between the numbers of mutation that accumulate in PvDHPS and the degree of sulfadoxine susceptibility.
Dapsone is usually used in combination with chlorproguanil for the treatment of malaria (40, 41). Transgenic P. berghei parasites carrying the PvDHPS wild type (ED50 = 19.7 ± 3.1 μg/kg) showed less resistance to dapsone than transgenic P. berghei parasites carrying the A383G A553G double mutation (ED50 = 37.2 ± 4.2 μg/kg) (Fig. 5B).
In contrast, sulfathiazole did not affect parasite growth, even for transgenic parasites carrying wild-type Pvhppk-dhps (data not shown). The reason for this observation is not yet clear. The sulfa compounds possess different octanol-water partition coefficients, (logP)—0.7 for sulfadoxine, 0.97 for dapsone, and 0.05 for sulfathiazole (42–45)—suggesting that these compounds have different levels of cell permeation that may, consequently, affect the uptake or the efficacy of drugs.
DISCUSSION
This study demonstrates for the first time the molecular and biochemical basis of sulfa drug resistance in P. vivax, the routine cultivation of which remains difficult. In order to perform the enzyme inhibition study, various recombinant PvHPPK-DHPS proteins were produced. Unlike the previous efforts to produce a recombinant PfHPPK-DHPS enzyme (38), the yield obtained for PvHPPK-DHPS was very low (0.13 to 0.3 mg/liter). This is due to the low expression level and the fact that the majority of the protein was expressed as an insoluble form. Different approaches, including expression in a folP-knockout strain of E. coli, did not improve expression, as the E. coli C600 ΔfolP::Kanr strain carrying Pvhppk-dhps did not grow well (data not shown). The expression of recombinant PvHPPK-DHPS was possible following the use of E. coli Rosetta(DE3)pLysS, which was supplemented with the rare tRNAs for the AGG, AGA, AUA, CUA, CCC, and GGA codons. A total of 55 rare codons are present in PvHPPK-DHPS. Rosetta(DE3)pLysS cells were employed in combination with a lower induction temperature and affinity chromatography to obtain purified recombinant PvHPPK-DHPS proteins.
The effects of polymorphisms in the PvDHPS domain were investigated by comparing the kinetic parameters between the wild-type and variant enzymes. Comparable kcat/Km values for pABA were observed among the wild type and variant PvHPPK-DHPS enzymes (Table 2), indicating that mutations at these amino acids (S382A, A383G, and A553G) did not much influence the enzyme catalytic activity or that the mutated amino acids at these positions are functionally equivalent to wild-type amino acids. To date, known DHPS structures adopt a triosephosphate isomerase (TIM) barrel α/β structure that is connected via a cluster of amino acids, forming a loop structure (46, 47). In comparison with these available structures, PvDHPS S382 and A383 align with a structurally conserved region belonging to loop 2 in other DHPSs. The structure of loop 2 is highly flexible and has been proposed to stabilize pABA upon its binding to the enzyme (46, 47). Although these residues are conserved for Plasmodium enzymes, S382 is replaced by a related amino acid threonine, while the residue at A383 is replaced by various residues in others (Fig. 1). The A553 located on loop 6 is quite conserved among most organisms, including wild-type PvDHPS; however, glycine can be found in the equivalent position in E. coli and Francisella tularensis enzymes (Fig. 1).
Inhibition studies with sulfa drugs revealed differences in sulfa drug sensitivity between the wild-type and mutant enzymes, indicating that the polymorphisms are responsible for the sulfa drug resistance observed for P. vivax. Mutations which confer sulfa drug resistance have been reported in other enzymes, and most are located on loop 1 and loop 2 of the DHPS enzymes (46, 47). The A383G mutation is the allele most frequently found in areas of endemicity with a sulfa drug resistance problem (28, 29, 37, 48). It has been suggested that the PfDHPS A437G mutation is likely necessary for sulfa drug resistance, in part because the mutation is most commonly found in previously reported field isolates (21). For the same reason, it is possible that the equivalent residue in PvDHPS, A383G, is among the first mutations selected in sulfa drug-resistant P. vivax. Mutation at this position resulted in reduced susceptibilities by 30-fold for sulfadoxine (Ki of 48.10 ± 5.45 μM) as well as dapsone (3.67 ± 0.60 μM) and by 20-fold for sulfathiazole (2.95 ± 0.11 μM) compared to those of the wild-type enzyme (1.50 ± 0.11 μM for sulfadoxine, 0.15 ± 0.05 μM for sulfathiazole, and 0.11 ± 0.01 μM for dapsone). Accumulation of additional mutations appears to augment sulfa drug resistance. It should be mentioned that the mutations described in this study resulted in smaller amino acids (S382A, A383G, A553G). On the basis of the amino acid sequence alignment, these mutations are located on the flexible loop structures of the enzyme, and it is possible that these smaller amino acids may increase the loop dynamic that affects sulfa drug binding, such that the compound is not properly locked into place. Crystal structures of the Plasmodium enzyme in complex with substrates and inhibitors should shed more light on drug design and means of avoiding drug resistance.
Molecular modeling of PvHPPK-DHPS predicted that the steric hindrance of V585 would interfere with binding to sulfadoxine, suggesting innate resistance, but not the binding to sulfathiazole and dapsone (22). Contrary to the molecular modeling prediction, the mutant with the V585A mutation had a 2-fold increase in the level of resistance to sulfadoxine compared with that of the wild type carrying V585 (Ki of 3.01 ± 0.36 μM versus 1.5 ± 0.11 μM). Similarly, the V585A mutant was 4-fold more resistant to dapsone than the wild type (0.53 ± 0.06 μM versus 0.11 ± 0.01 μM). The data suggest that V585 may influence sulfa drug susceptibility but likely not through the steric hindrance of the valine side chain in V585.
In addition to the enzyme inhibition assay, in vivo inhibition by sulfa drugs was performed using transgenic P. berghei parasites in which the endogenous Pbhppk-dhps was replaced by Pvhppk-dhps. The approach used to generate transgenic P. berghei parasite lines carrying Pvhppk-dhps as an in vivo model for the screening of antimalarials is similar to that previously reported for P. berghei parasite lines carrying the P. vivax dihydrofolate reductase-thymidylate synthase (Pvdhfr-ts) gene (49). The generated transgenic parasites contain only 1 copy of the native hppk-dhps gene that was replaced by the Pvhppk-dhps allele. Moreover, these transgenic parasites are free of selection markers and can be stably maintained in a drug-free environment. This property is very useful for drug screening, since there is no interference from other compounds to complicate the results and the effect of the test drug can be directly addressed. The growth of these transgenic parasites is similar to that of the parental strain, P. berghei ANKA, for all mutant PvHPPK-DHPS transgenic lines except the line carrying the single mutation A383G. The mutant with this mutation demonstrated a 2-fold higher growth rate than the parental strain, but there was no striking difference between the enzyme activity of that mutant and the enzyme activities of the wild type and the other PvDHPS mutants. Nonetheless, the in vivo screening of sulfadoxine in transgenic parasites revealed a trend similar to that observed in the enzyme inhibition study: the A383G mutant became 13-fold more resistant to sulfadoxine than the wild type and mutations in addition to the A383G mutation resulted in a slight increase in resistance.
The data from the in vivo screening study (ED50s) are in agreement with those from the enzyme inhibition assay (Ki values). While the enzyme inhibition assay explains the susceptibility on the basis of the compound-target interaction, the in vivo screening system provides additional insight into the permeation of the compound as well as information on its metabolism in the host. Therefore, the two assays are complements for evaluation of the effects of inhibitors on P. vivax DHPS.
In summary, this study confirms the role of key mutations in DHPS in sulfa drug resistance in P. vivax. As in P. falciparum, the accumulation of key point mutations results in higher levels of resistance. The systems developed, the enzyme inhibition assay, and the growth inhibition assay in transgenic parasites can promote new antimalarial drug development, as the efficacy of compounds can now be experimentally assessed in conjunction with elucidation of the role of other mutations observed in PvDHPS. The system has the potential to provide an in vivo platform for the testing of compound efficacy and early insight into compound metabolism.
ACKNOWLEDGMENTS
The study was financially supported by the National Center for Genetic Engineering and Biotechnology (P-13-00629) and the Cluster Program and Management Office (P-10-11274 and P-13-00835), National Science and Technology Development Agency, Thailand.
We thank Jetsumon Sattabongkot Prachumsri at Mahidol University for the P. vivax ms2002 isolate.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.World Health Organization. 2014. World malaria report: 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Kaur D, Wasir V, Gulati S, Bagga A. 2007. Unusual presentation of Plasmodium vivax malaria with severe thrombocytopenia and acute renal failure. J Trop Pediatr 53:210–212. doi: 10.1093/tropej/fml092. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M. 2006. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr 52:49–51. doi: 10.1093/tropej/fmi069. [DOI] [PubMed] [Google Scholar]
- 4.Tanwar GS, Khatri PC, Sengar GS, Kochar A, Kochar SK, Middha S, Tanwar G, Khatri N, Pakalapati D, Garg S, Das A, Kochar DK. 2011. Clinical profiles of 13 children with Plasmodium vivax cerebral malaria. Ann Trop Paediatr 31:351–356. doi: 10.1179/1465328111Y.0000000040. [DOI] [PubMed] [Google Scholar]
- 5.Quispe AM, Pozo E, Guerrero E, Durand S, Baldeviano GC, Edgel KA, Graf PC, Lescano AG. 2014. Plasmodium vivax hospitalizations in a monoendemic malaria region: severe vivax malaria? Am J Trop Med Hyg 91:11–17. doi: 10.4269/ajtmh.12-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA Jr. 2002. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg 67:230–232. [DOI] [PubMed] [Google Scholar]
- 7.Tan LK, Yacoub S, Scott S, Bhagani S, Jacobs M. 2008. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis 8:449–454. doi: 10.1016/S1473-3099(08)70153-1. [DOI] [PubMed] [Google Scholar]
- 8.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. 2005. Plasmodium vivax malaria. Emerg Infect Dis 11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gougoutsi A, Karageorgopoulos DE, Dimitriadou A, Melas N, Kranidiotis G, Voutsinas D, Melidonis A. 2014. Severe Plasmodium vivax malaria complicated with acute respiratory distress syndrome: a case associated with focal autochthonous transmission in Greece. Vector Borne Zoonotic Dis 14:378–381. doi: 10.1089/vbz.2012.1192. [DOI] [PubMed] [Google Scholar]
- 10.Naing C, Whittaker MA, Nyunt Wai V, Mak JW. 2014. Is Plasmodium vivax malaria a severe malaria?: a systematic review and meta-analysis. PLoS Negl Trop Dis 8:e3071. doi: 10.1371/journal.pntd.0003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien AT, Ramirez JF, Martinez SP. 2014. A descriptive study of 16 severe Plasmodium vivax cases from three municipalities of Colombia between 2009 and 2013. Malar J 13:404. doi: 10.1186/1475-2875-13-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera S, Perlaza BL, Bonelo A, Arevalo-Herrera M. 2002. Aotus monkeys: their great value for anti-malaria vaccines and drug testing. Int J Parasitol 32:1625–1635. doi: 10.1016/S0020-7519(02)00191-1. [DOI] [PubMed] [Google Scholar]
- 13.Golenda CF, Li J, Rosenberg R. 1997. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc Natl Acad Sci U S A 94:6786–6791. doi: 10.1073/pnas.94.13.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Cui L, Udomsangpetch R. 2007. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol 37:1551–1557. doi: 10.1016/j.ijpara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Udomsangpetch R, Somsri S, Panichakul T, Chotivanich K, Sirichaisinthop J, Yang Z, Cui L, Sattabongkot J. 2007. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int 56:65–69. doi: 10.1016/j.parint.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Thaithong S, Chan SW, Songsomboon S, Wilairat P, Seesod N, Sueblinwong T, Goman M, Ridley R, Beale G. 1992. Pyrimethamine resistant mutations in Plasmodium falciparum. Mol Biochem Parasitol 52:149–157. doi: 10.1016/0166-6851(92)90047-N. [DOI] [PubMed] [Google Scholar]
- 17.Pinichpongse S, Doberstyn EB, Cullen JR, Yisunsri L, Thongsombun Y, Thimasarn K. 1982. An evaluation of five regimens for the outpatient therapy of falciparum malaria in Thailand 1980-81. Bull World Health Organ 60:907–912. [PMC free article] [PubMed] [Google Scholar]
- 18.Cowman AF, Morry MJ, Biggs BA, Cross GA, Foote SJ. 1988. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A 85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Read M, Sims PF, Hyde JE. 1997. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol Microbiol 23:979–986. doi: 10.1046/j.1365-2958.1997.2821646.x. [DOI] [PubMed] [Google Scholar]
- 20.Brooks DR, Wang P, Read M, Watkins WM, Sims PF, Hyde JE. 1994. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem 224:397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- 21.Triglia T, Menting JG, Wilson C, Cowman AF. 1997. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A 94:13944–13949. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korsinczky M, Fischer K, Chen N, Baker J, Rieckmann K, Cheng Q. 2004. Sulfadoxine resistance in Plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob Agents Chemother 48:2214–2222. doi: 10.1128/AAC.48.6.2214-2222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imwong M, Pukrittakayamee S, Looareesuwan S, Pasvol G, Poirreiz J, White NJ, Snounou G. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob Agents Chemother 45:3122–3127. doi: 10.1128/AAC.45.11.3122-3127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leartsakulpanich U, Imwong M, Pukrittayakamee S, White NJ, Snounou G, Sirawaraporn W, Yuthavong Y. 2002. Molecular characterization of dihydrofolate reductase in relation to antifolate resistance in Plasmodium vivax. Mol Biochem Parasitol 119:63–73. doi: 10.1016/S0166-6851(01)00402-9. [DOI] [PubMed] [Google Scholar]
- 25.Hastings MD, Porter KM, Maguire JD, Susanti I, Kania W, Bangs MJ, Sibley CH, Baird JK. 2004. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J Infect Dis 189:744–750. doi: 10.1086/381397. [DOI] [PubMed] [Google Scholar]
- 26.Snounou G, White NJ. 2004. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol 20:333–339. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Auliff A, Wilson DW, Russell B, Gao Q, Chen N, Anh LN, Maguire J, Bell D, O'Neil MT, Cheng Q. 2006. Amino acid mutations in Plasmodium vivax DHFR and DHPS from several geographical regions and susceptibility to antifolate drugs. Am J Trop Med Hyg 75:617–621. [PubMed] [Google Scholar]
- 28.Hawkins VN, Suzuki SM, Rungsihirunrat K, Hapuarachchi HC, Maestre A, Na-Bangchang K, Sibley CH. 2009. Assessment of the origins and spread of putative resistance-conferring mutations in Plasmodium vivax dihydropteroate synthase. Am J Trop Med Hyg 81:348–355. [PubMed] [Google Scholar]
- 29.Rungsihirunrat K, Sibley CH, Mungthin M, Na-Bangchang K. 2008. Geographical distribution of amino acid mutations in Plasmodium vivax DHFR and DHPS from malaria endemic areas of Thailand. Am J Trop Med Hyg 78:462–467. [PubMed] [Google Scholar]
- 30.Okinaka O, Iwai K. 1969. A radioassay for dihydropteroate-synthesizing enzyme activity. Anal Biochem 31:174–182. doi: 10.1016/0003-2697(69)90255-3. [DOI] [PubMed] [Google Scholar]
- 31.Nopponpunth V, Sirawaraporn W, Greene PJ, Santi DV. 1999. Cloning and expression of Mycobacterium tuberculosis and Mycobacterium leprae dihydropteroate synthase in Escherichia coli. J Bacteriol 181:6814–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janse CJ, Ramesar J, Waters AP. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 33.Braks JA, Franke-Fayard B, Kroeze H, Janse CJ, Waters AP. 2006. Development and application of a positive-negative selectable marker system for use in reverse genetics in Plasmodium. Nucleic Acids Res 34:e39. doi: 10.1093/nar/gnj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr RY, Philip N, Waters AP. 2012. Improved negative selection protocol for Plasmodium berghei in the rodent malarial model. Malar J 11:103. doi: 10.1186/1475-2875-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters W. 1975. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 69:155–171. [PubMed] [Google Scholar]
- 36.Lu F, Lim CS, Nam DH, Kim K, Lin K, Kim TS, Lee HW, Chen JH, Wang Y, Sattabongkot J, Han ET. 2010. Mutations in the antifolate-resistance-associated genes dihydrofolate reductase and dihydropteroate synthase in Plasmodium vivax isolates from malaria-endemic countries. Am J Trop Med Hyg 83:474–479. doi: 10.4269/ajtmh.2010.10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thongdee P, Kuesap J, Rungsihirunrat K, Tippawangkosol P, Mungthin M, Na-Bangchang K. 2013. Distribution of dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant alleles in Plasmodium vivax isolates from Thailand. Acta Trop 128:137–143. doi: 10.1016/j.actatropica.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Kasekarn W, Sirawaraporn R, Chahomchuen T, Cowman AF, Sirawaraporn W. 2004. Molecular characterization of bifunctional hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase from Plasmodium falciparum. Mol Biochem Parasitol 137:43–53. doi: 10.1016/j.molbiopara.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Gardiner DL, Skinner-Adams TS, Spielmann T, Trenholme KR. 2003. Malaria transfection and transfection vectors. Trends Parasitol 19:381–383. doi: 10.1016/S1471-4922(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 40.Croft AM. 2007. Malaria: prevention in travellers. BMJ Clin Evid 2007:0903. [PMC free article] [PubMed] [Google Scholar]
- 41.Amukoye E, Winstanley PA, Watkins WM, Snow RW, Hatcher J, Mosobo M, Ngumbao E, Lowe B, Ton M, Minyiri G, Marsh K. 1997. Chlorproguanil-dapsone: effective treatment for uncomplicated falciparum malaria. Antimicrob Agents Chemother 41:2261–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V, Tang A, Gabriel G, Ly C, Adamjee S, Dame ZT, Han B, Zhou Y, Wishart DS. 2014. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res 42:D1091–D1097. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS. 2011. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res 39:D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. 2008. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res 36:D901–D906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. 2006. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baca AM, Sirawaraporn R, Turley S, Sirawaraporn W, Hol WG. 2000. Crystal structure of Mycobacterium tuberculosis 7,8-dihydropteroate synthase in complex with pterin monophosphate: new insight into the enzymatic mechanism and sulfa-drug action. J Mol Biol 302:1193–1212. doi: 10.1006/jmbi.2000.4094. [DOI] [PubMed] [Google Scholar]
- 47.Yun MK, Wu Y, Li Z, Zhao Y, Waddell MB, Ferreira AM, Lee RE, Bashford D, White SW. 2012. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science 335:1110–1114. doi: 10.1126/science.1214641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuesap J, Rungsrihirunrat K, Thongdee P, Ruangweerayut R, Na-Bangchang K. 2011. Change in mutation patterns of Plasmodium vivax dihydrofolate reductase (Pvdhfr) and dihydropteroate synthase (Pvdhps) in P. vivax isolates from malaria endemic areas of Thailand. Mem Inst Oswaldo Cruz 106(Suppl 1):S130–S133. [DOI] [PubMed] [Google Scholar]
- 49.Somsak V, Uthaipibull C, Prommana P, Srichairatanakool S, Yuthavong Y, Kamchonwongpaisan S. 2011. Transgenic Plasmodium parasites stably expressing Plasmodium vivax dihydrofolate reductase-thymidylate synthase as in vitro and in vivo models for antifolate screening. Malar J 10:291. doi: 10.1186/1475-2875-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]