Abstract
Members of the tetracycline class are frequently classified as bacteriostatic. However, recent findings have demonstrated an improved antibacterial killing profile, often achieving ≥3 log10 bacterial count reduction, when such antibiotics have been given for periods longer than 24 h. We aimed to study this effect with eravacycline, a novel fluorocycline, given in an immunocompetent murine thigh infection model over 72 h against two methicillin-resistant Staphylococcus aureus (MRSA) isolates (eravacycline MICs = 0.03 and 0.25 μg/ml) and three Enterobacteriaceae isolates (eravacycline MICs = 0.125 to 0.25 μg/ml). A humanized eravacycline regimen, 2.5 mg/kg of body weight given intravenously (i.v.) every 12 h (q12h), demonstrated progressively enhanced activity over the 72-h study period. A cumulative dose response in which bacterial density was reduced by more than 3 log10 CFU at 72 h was noted over the study period in the two Gram-positive isolates, and eravacycline performed similarly to comparator antibiotics (tigecycline, linezolid, and vancomycin). A cumulative dose response with eravacycline and comparators (tigecycline and meropenem) over the study period was also observed in the Gram-negative isolates, although more variability in bacterial killing was observed for all antibacterial agents. Overall, a bacterial count reduction of ≥3 log was achieved in one of the three isolates with both eravacycline and tigecycline, while meropenem achieved a similar endpoint against two of the three isolates. Bactericidal activity is typically defined in vitro over 24 h; however, extended regimen studies in vivo may demonstrate an improved correlation with clinical outcomes by better identification of antimicrobial effects.
INTRODUCTION
Antimicrobial resistance is an emerging health concern, warranting the development of new antimicrobial agents (1, 2). Eravacycline (TP-434) is a novel fluorocycline that belongs to the tetracycline class of antimicrobials and is currently being developed as an intravenous (i.v.) and oral medication for the treatment of serious infections caused by antibiotic-resistant bacteria (3). As with other members of the tetracycline antibiotic class, eravacycline is a potent, mechanism-based inhibitor of the bacterial ribosome; however, it is minimally affected by tetracycline-specific efflux and ribosome protection and inactivation, potentially making it a prospective agent for treatment of infections by multidrug-resistant (MDR) organisms (4). In vitro microbiological studies of eravacycline have demonstrated broad-spectrum Gram-positive and Gram-negative antibacterial activity, including strains with resistance mechanisms (3). These strains include Enterobacteriaceae isolates expressing resistance genes from multiple classes of extended-spectrum β-lactamases (ESBLs) and carbapenem-resistant mechanisms, carbapenem- and multidrug-resistant Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) (3).
In addition to understanding an agent's spectrum of activity, an assessment of the antibacterial profile (i.e., rate and extent of killing) of eravacycline is important to help understand its potential role in clinical practice. For instance, when considering antimicrobial therapies for infections that require hospitalization, agents possessing bactericidal activity have generally been advocated (5–8). Historically, bactericidal activity has been defined as a 3-log reduction in bacterial density over a 24-h period in assessments using static in vitro experiments (9). While this definition is widely applied to agents such as fluoroquinolones and aminoglycosides, it has not been utilized for the tetracyclines, which have slower activity with respect to their in vitro killing profiles (10). However, with respect to their efficacy over a course of treatment, these slowly occurring bactericidal effects result in substantial reductions in bacterial density. For instance, recent in vivo murine models with typically defined bacteriostatic agents, such as linezolid and tigecycline, achieved ≥3-log reductions in bacterial burden over a 72-h treatment period against Staphylococcus aureus and Enterobacteriaceae, respectively (11, 12).
As a result of these observations, the goal of the current study was to elucidate the bacterial killing profile of eravacycline using an immunocompetent murine thigh infection model over a multiple-day treatment period that may more closely approximate the clinical utilization of this new therapy. Additionally, we compared this profile to those of conventional antibacterial agents.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, female ICR (CD-1) mice weighing 20 to 22 g were obtained from Envigo RMS, Inc. (Indianapolis, IN). The animals were allowed to acclimate for a minimum of 48 h before commencement of experimentation and were provided food and water ad libitum. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT.
Antimicrobial test agent.
Eravacycline (Tetraphase Pharmaceuticals, Inc., Watertown, MA; lot B110342), provided in 52.5-mg vials, was used throughout these experiments. The vials were stored frozen at between −15 and −25°C and protected from light during storage until required for dosing solution preparation. Eravacycline vials were reconstituted to a 5 mg/ml (free base) concentration with 10 ml of sterile water for injection (Hospira, Inc., Lake Forest, IL) to yield a final volume of 10.5 ml. All subsequent dilutions were prepared in sterile 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL). Eravacycline was administered as intravenous (i.v.) injections of 0.2 ml, which is one of the intended routes for use in humans. The prepared dosing solution was kept on ice and protected from light. The eravacycline regimen of 2.5 mg/kg of body weight given every 12 h (q12h) was selected since it yielded a value corresponding to the area under the concentration-time curve for the free, unbound fraction of the drug from 0 to 12 h (fAUC0–12) of 0.82 μg · h/ml, which translates to an fAUC0–24 value of 1.64 μg · h/ml, which best mimics the human exposure from a 1 mg/kg q12h intravenous dose (23).
Humanized doses of tigecycline (Wyeth Pharmaceuticals, Inc., Dallas, TX; lot AJP512), linezolid (Pfizer Inc., Groton, CT; lot 1210600015), vancomycin (Hospira, Inc., Lake Forest, IL; lot 511048E03), and meropenem (Fresenius Kabi USA, LLC, Lake Zurich, IL; lot 0004D45) were prepared to produce exposures consistent with previously published data for each agent (11–14).
Bacterial isolates.
Two MRSA isolates (S. aureus 156 and S. aureus 426), as well as two isolates of Escherichia coli (EC 373 and EC C3-14) and one of Citrobacter freundii (CF 26), were selected for this study from the Center for Anti-infective Research and Development (CAIRD) culture collection. These bacterial isolates were selected as they had previously demonstrated growth in the immunocompetent thigh model (11).
MICs for the antibacterial agents were determined in triplicate by broth microdilution according to the 2015 Clinical and Laboratory Standards Institute (CLSI) guidelines, and the modal MIC was reported (15). All isolates were maintained within the Caird research facility. Isolates were stored in skim milk (BD BioSciences, Sparks, MD) at −80°C and were subcultured twice onto Trypticase soy agar with 5% sheep blood (TSA II; BD BioSciences, Sparks, MD) within 48 h prior to use for MIC studies and for thigh inoculation.
Immunocompetent thigh infection model.
Isolates were transferred twice on TSA II plates (BD BioSciences, Sparks, MD) and incubated at 37°C. After an 18-to-24-h incubation of the second bacterial transfer, a bacterial suspension of approximately 108 CFU/ml was made for inoculation. Final inoculum concentrations were confirmed by serial dilution and plating techniques. Thigh infection was produced by intramuscular injection of 0.1 ml of the inoculum into each thigh (n = 2) of the mouse 2 h prior to the initiation of antimicrobial therapy. Two hours postinfection, eravacycline was administered via the i.v. route. For meropenem-treated animals, a single 5 mg/kg dose of uranyl nitrate was given intraperitoneally 3 days prior to thigh infection; this treatment results in a predictable degree of renal impairment that helps simplify the humanized regimen (14). The predetermined regimens of eravacycline and the comparator antimicrobials simulating human exposures were studied in groups of 3 mice (n = 6 thighs) over the 72-h treatment period. Two additional mice per isolate were incorporated into the Gram-negative portion of the study, as more animal deaths were expected to occur. Additionally, the experiments involving the Gram-negative control and eravacycline treatment animals were run in duplicate to provide a more robust data set. Vancomycin, linezolid, and tigecycline were used as comparators with the Gram-positive isolates. Meropenem and tigecycline were used as comparators with the Gram-negative isolates. All comparators were administered subcutaneously.
Control animals received 0.2 ml of 0.9% normal saline solution subcutaneously at a frequency identical to that used for the most frequently dosed drug regimen. For each isolate tested, 3 untreated mice (n = 6 thighs) were used as the 0-h control group (sacrificed just prior to antibiotic treatment initiation) and 3 additional groups of mice (receiving 0.9% normal saline solution) were utilized as 24-, 48-, and 72-h controls. At 24, 48, and 72 h postinitiation of antimicrobial therapy, groups of three animals from each treatment arm, as well as control groups, was euthanized by CO2 exposure followed by cervical dislocation. After sacrifice, the thighs were removed and individually homogenized in 0.9% normal saline solution via the use of a Mini-BeadBeater (Biospec Products, Inc., Bartlesville, OK). Serial dilutions were plated on TSA II plates for CFU determination. The thighs of the mice that expired before the prespecified sacrifice time were harvested, and the CFU thigh determinations were included in the next harvest time point.
Statistical analysis.
The values corresponding to the log10 change in CFU at 24, 48, and 72 h were compared via one-way analysis of variance (ANOVA) testing (Holm-Sidak method) among all treatment regimens and control groups using SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA). A P value of ≤0.05 was considered significant.
RESULTS
In vitro susceptibility.
Eravacycline, tigecycline, linezolid, vancomycin, and meropenem MICs for the isolates evaluated for in vivo studies are shown in Table 1. These isolates had various phenotypic profiles, with eravacycline MICs ranging from 0.03 to 0.25 μg/ml. All comparators were susceptible to the isolates in their respective portions of the study, with the exception of meropenem against E. coli C3-14 (MIC = 4 μg/ml).
TABLE 1.
In vitro potency of eravacycline and comparator antibiotics against each isolatea
| Isolate | ERV modal MIC | Comparator MIC (μg/ml) |
Genotype/phenotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TGC | LZD | VAN | MEM | ETP | FEP | TZP | CIP | TOB | |||
| SA 156 | 0.03 | 0.125 | 4 | 1 | NA | NA | NA | NA | NA | NA | CA-MRSA |
| SA 426 | 0.25 | 0.5 | 2 | 1–2 | NA | NA | NA | NA | NA | NA | HA-MRSA |
| EC 373 | 0.25 | 0.25 | NA | NA | ≤0.063 | ≤0.016 | 0.25 | 128 | 16 | 2 | Non-ESBL |
| CF 26 | 0.125 | 0.25 | NA | NA | ≤0.063 | ≤0.016 | ≤0.063 | 1 | ≤0.016 | 1 | Inducible AmpC |
| EC C3-14 | 0.25 | 0.125 | NA | NA | 4 | 16 | >64 | >256 | 4 | 64 | Non-ESBL |
SA, S. aureus; EC, E. coli; CF, C. freundii; CA-MRSA, community-acquired methicillin-resistant S. aureus; HA-MRSA, hospital-acquired methicillin-resistant S. aureus; ESBL, extended-spectrum beta-lactamase; NA, not applicable; ERV, eravacycline; TGC, tigecycline; LZD, linezolid; VAN, vancomycin; MEM, meropenem; ETP, ertapenem; FEP, cefepime; TZP, piperacillin-tazobactam; CIP, ciprofloxacin; TOB, tobramycin.
Antibacterial efficacy of eravacycline.
The results of determinations of the antibacterial efficacy of eravacycline, linezolid, tigecycline, and vancomycin against S. aureus 156 and S. aureus 426 are illustrated in Fig. 1 and 2, respectively. For both MRSA isolates, all control and eravacycline-treated animals survived until the planned collection time. In vehicle control animals, both isolates maintained CFU levels above the input for 3 days. Compared to the input, the bacterial density of S. aureus 156 increased by 0.79, 0.73, and 0.39 log10 CFU in the untreated control animals at 24, 48, and 72 h. For S. aureus 426, bacterial density increased by 0.77, 0.72, and 0.58 log10 CFU at 24, 48, and 72 h, respectively.
FIG 1.
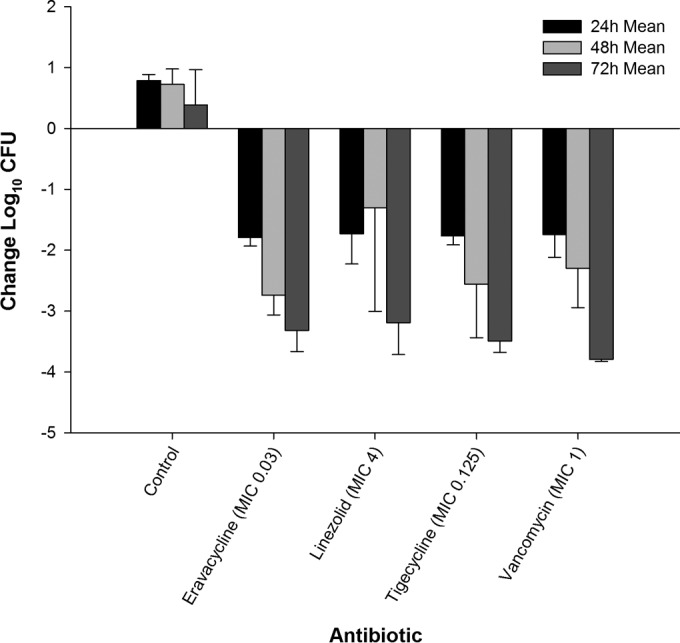
Efficacy of eravacycline, linezolid, tigecycline, and vancomycin at doses simulating human exposures against methicillin-resistant S. aureus 156 (MIC = 0.03 μg/ml) in an immunocompetent murine thigh infection model.
FIG 2.
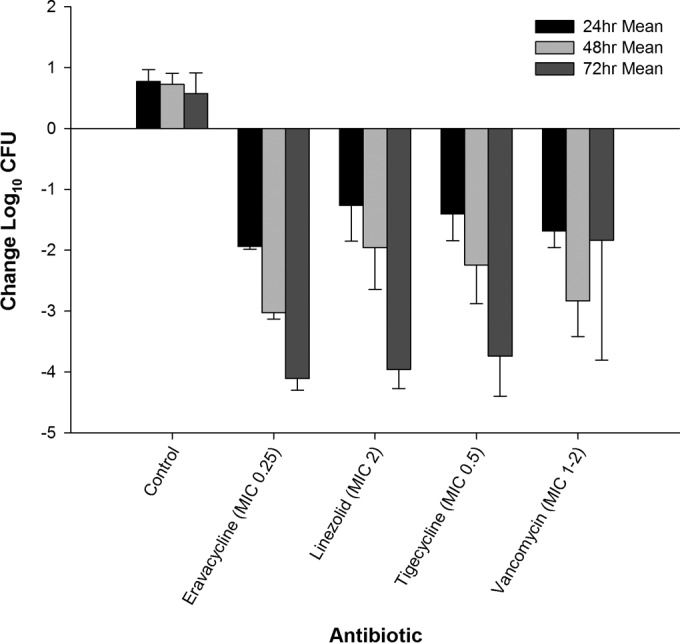
Efficacy of eravacycline, linezolid, tigecycline, and vancomycin at doses simulating human exposures against methicillin-resistant S. aureus 426 (MIC = 0.25 μg/ml) in an immunocompetent murine thigh infection model.
Eravacycline was more effective than the control against the two Gram-positive isolates throughout the study period (P < 0.001). Against S. aureus 156, the eravacycline regimen produced cumulative log10 CFU reductions of 1.79, 2.74, and 3.32 at 24, 48, and 72 h, respectively. Against S. aureus 426, eravacycline treatment resulted in cumulative log10 CFU reductions of 1.94, 3.03, and 4.11 log10 CFU at 24, 48, and 72 h, respectively.
The comparator antibiotics (linezolid, vancomycin, and tigecycline) demonstrated antibacterial activity against both S. aureus 156 (Fig. 1) and S. aureus 426 (Fig. 2). Of note, vancomycin did not achieve this endpoint until at least 48 h of therapy against both isolates. For S. aureus 156, eravacycline treatment was as effective as humanized regimens of the comparators at every time point. Additionally, there were no differences between the regimens at 24 h against S. aureus 426. The humanized eravacycline regimen was more effective than the linezolid regimen at 48 h (P = 0.03) but was similar in effectiveness to the tigecycline and vancomycin regimens (P = 0.22 and 0.92, respectively). The killing produced by vancomycin was not as robust as that produced by eravacycline (P = 0.001), linezolid (P = 0.002), and tigecycline (P = 0.005) at 72 h. The results of all other treatment regimens were not significantly different at 72 h.
The reductions in the bacterial burdens of E. coli 373, C. freundii 26, and E. coli C3-14 in the thigh model after administration of eravacycline, meropenem, and tigecycline are illustrated in Fig. 3, 4, and 5. As with the findings obtained with MRSA, the magnitude of the antibacterial killing observed with eravacycline, tigecycline, and meropenem was enhanced with cumulative exposures over the 3-day study period.
FIG 3.
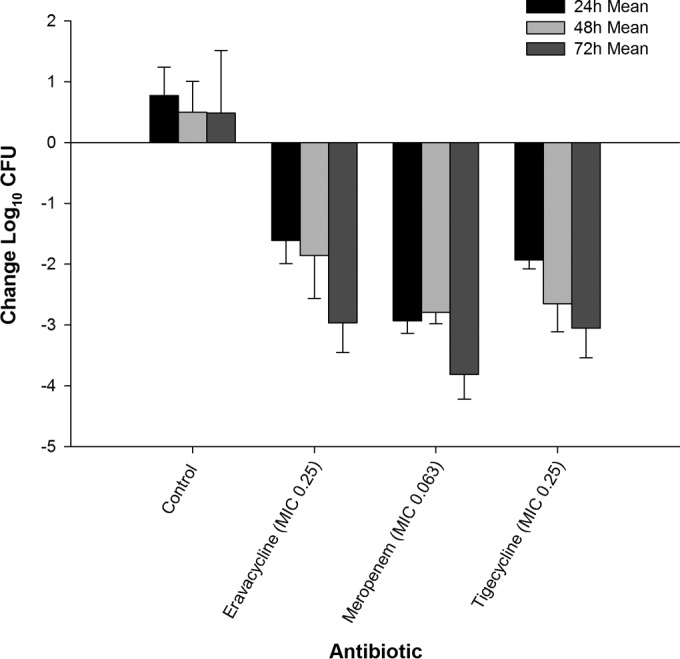
Efficacy of eravacycline, meropenem, and tigecycline at doses simulating human exposures against E. coli 373 (MIC = 0.25 μg/ml) in an immunocompetent murine thigh infection model.
FIG 4.
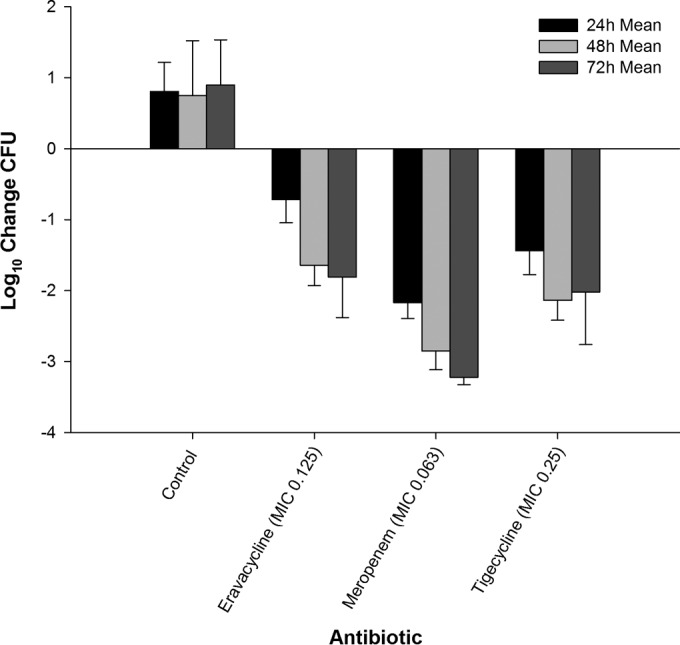
Efficacy of eravacycline, meropenem, and tigecycline at doses simulating human exposures against C. freundii 26 (MIC = 0.125 μg/ml) in an immunocompetent murine thigh infection model.
FIG 5.
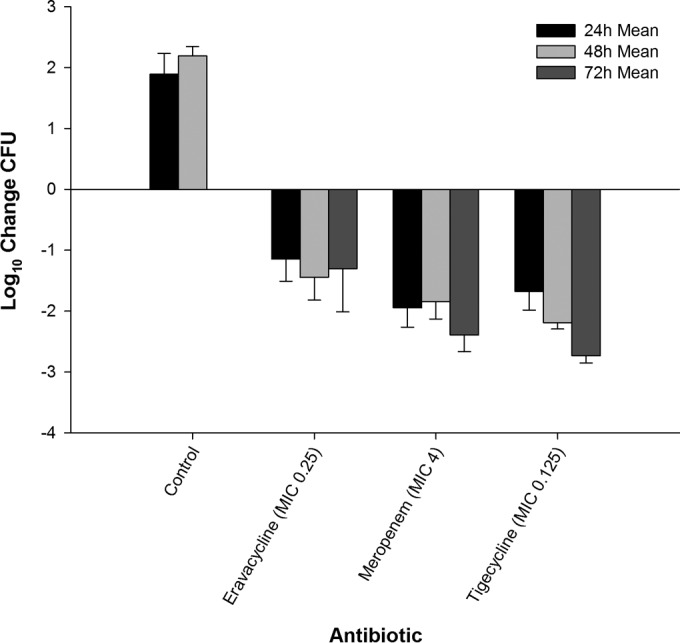
Efficacy of eravacycline, meropenem, and tigecycline at doses simulating human exposures against E. coli C3-14 (MIC = 0.25 μg/ml) in an immunocompetent murine thigh infection model.
Twenty-four hours after infection, the bacterial density of E. coli 373 increased by 0.77 log10 CFU in the untreated control animals, with a final 72-h increase from the 0-h control of 0.49 log10 CFU. At 24, 48, and 72 h, eravacycline treatment resulted in cumulative log10 CFU reductions of 1.61, 1.85, and 2.96, respectively (Fig. 3). C. freundii 26 maintained growth in the control animals, with log10 CFU increases over the 72-h time period of 0.81, 0.75, and 0.90 at 24, 48, and 72 h, respectively. In contrast, eravacycline treatment resulted in cumulative log10 CFU reductions of 0.71, 1.64, and 1.81 at 24, 48, and 72 h, respectively (Fig. 4). The bacterial density of E. coli C3-14 increased by 1.89 and 2.19 log10 CFU in the untreated control animals over 24 and 48 h, respectively. All of the control animals randomized to the 48- and 72-h control groups perished by 48 h. Therefore, CFU data from these animals were combined into the data for the 48-h control group (Fig. 5). At 24, 48, and 72 h of treatment, the eravacycline regimen produced cumulative reductions in log10 CFU of 1.14, 1.44, and 1.31, respectively.
The comparator antibiotics, meropenem and tigecycline, demonstrated cumulative antibacterial activity against all three Enterobacteriaceae isolates; however, meropenem was not bactericidal against E. coli C3-14, likely due to the elevated meropenem MIC of 4 μg/ml. Additionally, no ≥3-log reduction in log10 CFU was observed for either agent until 72 h of treatment. Moreover, E. coli C3-14 grew approximately 1-log more than the other two Gram-negative isolates, which is suggestive of increased fitness. This result was not unexpected, as different organisms have intrinsically different growth profiles; however, the killing profiles resemble those observed with the other organisms both among therapies and within time intervals. Eravacycline was more effective than the control against the three Gram-negative isolates throughout the study duration (P < 0.05). While differences were noted over the initial 48 h, the overall levels of bacterial killing at 72 h were not different between eravacycline and tigecycline against E. coli 373 (P = 0.79) and C. freundii 26 (P = 0.43). Meropenem produced greater antibacterial activity than eravacycline and tigecycline against the two isolates. Against E. coli C3-14, eravacycline was not different from meropenem (P = 0.9) or tigecycline (P = 0.9) at 24 h. At 48 and 72 h, both tigecycline and meropenem demonstrated a significantly greater reduction in log10 CFU than eravacycline (P < 0.003 for all comparisons).
DISCUSSION
To our knowledge, this was the first study assessing the bacterial killing profile of eravacycline against both Gram-positive and Gram-negative organisms with various phenotypic profiles over 72 h. As a novel antibiotic, assessing eravacycline's degree of antibacterial activity is important in determining its place in clinical practice. At 24 h, exposures of eravacycline equivalent to 1 g q12h in humans demonstrated antibacterial activity against MRSA and Enterobacteriaceae isolates. These findings are similar to those of previous 24-h murine infection models, where i.v. eravacycline total daily doses of 5.4 mg/kg and 5.3 mg/kg achieved 1 to 2 log10 reductions in CFU compared with untreated control animal results in infections by S. aureus and Streptococcus pyogenes, respectively (16). However, our study demonstrated that the efficacy of eravacycline was improved on each subsequent day of dosing after 72 h of treatment, achieving a ≥3-log reduction in bacterial counts in three of the five studied isolates.
Typically, antimicrobials with ribosome-mediated mechanisms of action (tetracyclines, macrolides, and oxazolidinones) are considered bacteriostatic, whereas agents acting on the bacterial cell wall (β-lactams, including meropenem, and often vancomycin) are typically classified as bactericidal (17–19). Notably, these definitions are not absolute, since the killing effect of the drug varies with the species being tested, as demonstrated in our study (20). For instance, vancomycin and meropenem did not achieve ≥3 log killing against the Gram-positive and Gram-negative isolates until the 72-h time point. Eravacycline, tigecycline, and linezolid achieved ≥3-log killing against both MRSA isolates at 72 h. Against S. aureus 426, eravacycline was the first agent to achieve ≥3-log killing at 48 h, which would be unexpected in applying the in vitro definitions.
Eravacycline performed similarly to tigecycline, with differences in CFU reductions between the two agents within 0.5 log10 at 72 h, with the exception of E. coli C3-14. With regard to in vitro MIC studies, eravacycline has demonstrated MIC90 values against Enterobacteriaceae that are 1-fold to 4-fold lower than those seen with tigecycline; however, tigecycline's MIC against E. coli C3-14 was 1 dilution lower than that of eravacycline, potentially resulting in the discordance in bacterial kill results (3, 21). Similarly to eravacycline, tigecycline was not bactericidal in two of the three Enterobacteriaceae isolates, but both antimicrobials achieved ≥3-log killing against the MRSA isolates.
Eravacycline has shown strain- and species-specific bactericidal ability in vitro (22). These studies demonstrated that administration of the compound results in a cumulative dose response and may achieve a ≥3-log reduction in bacterial colony counts in vivo after 72 h of treatment against MRSA and Enterobacteriaceae. Therefore, an analysis of the pharmacodynamic profile beyond 24 h should be considered when assessing the clinical efficacy of this novel agent.
ACKNOWLEDGMENTS
This project has been funded in part by Tetraphase Pharmaceuticals, Inc., and in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract no. HHSO100201200002C. D.P.N. is a member of the scientific board of Tetraphase Pharmaceuticals, Inc., and has received research support from Tetraphase Pharmaceuticals, Inc. The other authors have no conflicts of interest to disclose.
We thank Jared Crandon, Lucinda Lamb, Jennifer Tabor-Rennie, Sara Robinson, Debora Santini, Christina Sutherland, Kimelyn Greenwood, Yukihiro Hamada, Islam Ghazi, Wonhee So, and Jaime Verastegui from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance with the conduct of the study.
Funding Statement
This project has been funded in part by Tetraphase Pharmaceuticals, Inc., and in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract no. HHSO100201200002C.
REFERENCES
- 1.Boyle DP, Zembower TR. 2015. Epidemiology and management of emerging drug-resistant Gram-negative bacteria: extended-spectrum β-lactamases and beyond. Urol Clin North Am 42:493–505. doi: 10.1016/j.ucl.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. US Department of Health and Human Services, CDC, Atlanta, GA. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/. [Google Scholar]
- 3.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman TH, Starosta AL, Fyfe C, O'Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 56:2559–2564. doi: 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove SE, Fowler VG Jr. 2008. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46(Suppl 5):S386–S393. doi: 10.1086/533595. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society; Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 7.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. 2010. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DL, Bisno AL, Chambers HF, Dellinger PE, Goldstein EJC, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:147–159. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 9.Woods GL, Washington JA. 1995. The clinician and the microbiology laboratory, p 169–199. In Mandell G, Bennett J, Dolin R (ed), Mandell, Douglas and Bennett's principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, PA. [Google Scholar]
- 10.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 11.Keel RA, Tessier PR, Crandon JL, Nicolau DP. 2012. Comparative efficacies of human simulated exposures of tedizolid and linezolid against Staphylococcus aureus in the murine thigh infection model. Antimicrob Agents Chemother 56:4403–4407. doi: 10.1128/AAC.00122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tessier PR, Nicolau DP. 2013. Tigecycline displays in vivo bactericidal activity against extended-spectrum-β-lactamase-producing Enterobacteriaceae after 72-hour exposure period. Antimicrob Agents Chemother 57:640–642. doi: 10.1128/AAC.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crandon JL, Kuti JL, Nicolau DP. 2010. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother 54:5115–5119. doi: 10.1128/AAC.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeRyke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 51:1481–1486. doi: 10.1128/AAC.00752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing: 24th informational supplement—document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Grossman TH, Murphy TM, Slee AM, Lofland D, Sutcliffe JA. 2015. Eravacycline (TP-434) is efficacious in animal models of infection. Antimicrob Agents Chemother 59:2567–2571. doi: 10.1128/AAC.04354-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathwani D. 2005. Tigecycline: clinical evidence and formulary positioning. Int J Antimicrob Agents 25:185–192. doi: 10.1016/j.ijantimicag.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Schaper KJ, Schubert S, Dalhoff A. 2005. Kinetics and quantification of antibacterial effects of beta-lactams, macrolides, and quinolones against gram-positive and gram-negative RTI pathogens. Infection 33(Suppl 2):3–14. doi: 10.1007/s15010-005-8202-2. [DOI] [PubMed] [Google Scholar]
- 19.Rybak MJ, Hershberger E, Moldovan T, Grucz RG. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob Agents Chemother 44:1062–1066. doi: 10.1128/AAC.44.4.1062-1066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French GL. 2006. Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J Antimicrob Chemother 58:1107–1117. doi: 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- 21.Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother 59:1802–1805. doi: 10.1128/AAC.04809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman TH, O'Brien W, Fyfe C, Sutcliffe JA. 2014. Eravacycline is potent against third generation cephalosporin- and carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Acinetobacter baumannii, and has isolate-specific bactericidal activity, abstr C-1374. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, San Diego, CA. [Google Scholar]
- 23.Thabit AK, Monogue ML, Nicolau DP. 2016. Eravacycline pharmacokinetics and challenges in defining humanized exposure in vivo. Antimicrob Agents Chemother 60:5072–5075. doi: 10.1128/AAC.00240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


