Abstract
The objective of this study was to describe amikacin pharmacokinetics (PK) in critically ill patients receiving equal doses (30 ml/kg of body weight/h) of continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF). Patients receiving amikacin and undergoing CVVH or CVVHDF were eligible. Population pharmacokinetic analysis and Monte Carlo simulation were undertaken using the Pmetrics software package for R. Sixteen patients (9 undergoing CVVH, 11 undergoing CVVHDF) and 20 sampling intervals were analyzed. A two-compartment linear model best described the data. Patient weight was the only covariate that was associated with drug clearance. The mean ± standard deviation parameter estimates were 25.2 ± 17.3 liters for the central volume, 0.89 ± 1.17 h−1 for the rate constant for the drug distribution from the central to the peripheral compartment, 2.38 ± 6.60 h−1 for the rate constant for the drug distribution from the peripheral to the central compartment, 4.45 ± 2.35 liters/h for hemodiafiltration clearance, and 4.69 ± 2.42 liters/h for hemofiltration clearance. Dosing simulations for amikacin supported the use of high dosing regimens (≥25 mg/kg) and extended intervals (36 to 48 h) for most patients when considering PK/pharmacodynamic (PD) targets of a maximum concentration in plasma (Cmax)/MIC ratio of ≥8 and a minimal concentration of ≤2.5 mg/liter at the end of the dosing interval. The mean clearance of amikacin was 1.8 ± 1.3 liters/h by CVVHDF and 1.3 ± 1 liters/h by CVVH. On the basis of simulations, a strategy of an extended-interval high loading dose of amikacin (25 mg/kg every 48 h) associated with therapeutic drug monitoring (TDM) should be the preferred approach for aminoglycoside treatment in critically ill patients receiving continuous renal replacement therapy (CRRT). (This study is a substudy of a trial registered at ClinicalTrials.gov under number NCT01403220.)
INTRODUCTION
Aminoglycosides have been used for many years mostly in combination with other antibiotics to treat infections in critically ill patients. The optimal dosing regimen should achieve a pharmacodynamic (PD) target of a maximum concentration in plasma (Cmax)/MIC ratio of ≥8 to 10 and an area under the concentration-time curve (AUC) from time zero to 24 h (AUC0–24) of >70 to 90 and should have the least toxicity possible, depending on the AUC0–24 and trough (minimum) concentrations (Cmins) (1–5). In critically ill patients undergoing renal replacement therapy (RRT), optimization of the antibiotic dosing regimen is a crucial but difficult issue. Critically ill patients have severely altered pharmacokinetics (PK) (increased volume of distribution or altered drug clearance) due to hypoalbuminemia, increased capillary permeability, or organ dysfunctions (6, 7). In patients with acute kidney injury (AKI), the extracorporeal clearance of drugs during RRT could significantly change antibiotic pharmacokinetics due to the type of RRT mode, hemofilter, fluid replacement mode (pre- or postdilution), or RRT settings applied. The physicochemical properties of the drug also influence the amount of drug cleared by continuous renal replacement therapy (CRRT). As amikacin is a small hydrophilic molecule with a very low level of protein binding, it is likely that CRRT affects amikacin PK. Instead of intermittent hemodialysis, CRRT techniques are widely used in critically ill patients, as better hemodynamic stability and lower metabolic changes associated with possible better long-term outcomes have been reported (8, 9). These alterations could dramatically reduce the likelihood of achieving therapeutic exposures of these drugs.
In previous studies with intensive care unit (ICU) patients undergoing CRRT, amikacin clearance varied from 0.53 to 5.34 liters/h, accounting for 40 to 89% of the total body clearance (10–15). These studies also reported conflicting results on the correlation between drug clearance and CRRT settings (11, 14). As a general rule, it is expected that the efficiency of drug removal would be higher in continuous venovenous hemodiafiltration (CVVHDF; diffusive and convective technique) modalities than in continuous venovenous hemofiltration (CVVH; convective technique) modalities (16). However, the pharmacokinetics of amikacin between these two different CRRT modalities using equal weight-based doses (effluent rate = 30 ml/kg of body weight/h, regardless of the technique used) have never been compared. Indeed, a better understanding of the influence of different CRRT modalities on the PK/PD of aminoglycosides in critically ill patients may lead to optimized dosing strategies that could improve patient care.
The aim of the present study was to describe the pharmacokinetics of amikacin in ICU patients undergoing CVVH or CVVHDF with equal weight-based doses. Using population pharmacokinetic modeling and Monte Carlo simulations, we aimed to determine optimized amikacin dosing regimens for these patients.
MATERIALS AND METHODS
Setting.
This observational pharmacokinetic study was a substudy of a randomized trial comparing the efficacy of equal doses of CVVH (a 30-ml/kg/h ultrafiltrate flow rate) and CVVHDF (a 15-ml/kg/h dialysate flow rate plus a 15-ml/kg/h ultrafiltrate flow rate) in a 16-bed tertiary referral ICU in Nîmes University Hospital (ClinicalTrials.gov NCT01403220; completed but as yet unpublished). Ethics approval was obtained from the local ethics committee of Nîmes, France (Comité de Protection des Personnes Sud Mediterranée III, 5 February 2012). Written informed consent was obtained from either the patient or the patient's nominated substitute decision maker.
Study population.
Patient eligibility for this study was (i) AKI necessitating either CVVH or CVVHDF according to the randomly allocated mode in the primary study and (ii) a clinical indication for amikacin use.
Patients were randomized to CVVH or CVVHDF (according to the primary study protocol) when the need for RRT was first identified. If the patient subsequently had at least an 8-h break from RRT, then the protocol of the primary study required that the patient then receive the alternative RRT mode. When the patient was on antibiotic therapy across both RRT modes, the patient was sampled on two different occasions encompassing both CVVH and CVVHDF.
Patients were excluded if they had a (i) requirement for RRT for cardiogenic pulmonary edema, metabolic acidosis (pH <7.15, HCO3 concentration, <12 mmol/liter), or hyperkalemia (potassium concentration, >6.5 mmol/liter) or (ii) a history of allergy to amikacin or a contraindication to treatment with amikacin.
Renal replacement therapy settings.
Both techniques were performed using an Aquarius system (Nikkiso, Japan) and a polysulfone-type hemofilter with a surface of 1.9 m2 (Aquamax HF19).
The RRT settings were as follows. (i) A 30-ml/kg/h ultrafiltrate rate was used for CVVH and a 15-ml/kg/h ultrafiltrate rate plus a 15-ml/kg/h dialysate flow rate were used for CVVHDF. For both techniques, the replacement mode applied was the postdilution mode. (ii) The net fluid removal rate was set at the physician's discretion and was between 0 and 200 ml/h. (iii) The targeted blood flow rate was aimed at the provision of a filtration ratio of <20%. The filtration ratio was calculated as follows: filtration ratio = ultrafiltrate flow rate/blood flow rate. It is the fraction of plasma that is removed from blood during hemofiltration (17).
Study protocol.
A dosing regimen of 15 to 30 mg/kg amikacin was administered intravenously (i.v.) over 30 min as part of the antimicrobial therapy prescribed for the patient. The subsequent doses were administered at doses and frequencies (typically every 24 or 36 h) according to the therapeutic drug monitoring results. Prefilter blood samples to determine plasma amikacin concentrations were taken at the baseline (predose), at the end of infusion (0.5 h), 30 min after the end of infusion (1 h), and 1.5, 2, 4, 8, 12, and 24 h after the start of infusion. Postfilter blood samples were taken up to 2, 4, 8, 12, and 24 h after the start of infusion.
Sample handling, storage, and measurement.
Blood samples were immediately placed on ice and within 60 min were centrifuged at 3,000 rpm for 10 min and then stored at −80°C. Samples were transported by a commercial courier company to the Burns, Trauma, and Critical Care Research Centre, The University of Queensland, Brisbane, Queensland, Australia, for analysis. Amikacin concentrations ranging from 0.5 to 100 mg/liter in plasma were measured by a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with a hydrophilic interaction liquid chromatography (HILIC) column on a Shimadzu Nexera system coupled to a Shimadzu 8030+ triple-quadrupole mass spectrometer. Clinical samples were assayed alongside calibrators and quality controls and met the batch acceptance criteria of the U.S. Food and Drug Administration (U.S. FDA).
Sample preparation.
Plasma (100 μl) was spiked with the internal standard (vancomycin), and 15% trichloroacetic acid was added to precipitate the proteins. An aliquot of 0.2 μl of the supernatant extract was injected onto the LC-MS/MS.
Chromatography.
The stationary phase was an Agilent Poroshell 120 HILIC column (2.1 by 50 mm, 2.7 μm, 120 Å). The mobile phase used was a gradient with mobile phase A, consisting of 0.2% (vol/vol) formic acid, and mobile phase B, consisting of acetonitrile with 0.2% (vol/vol) formic acid. The flow rate was 0.3 ml/min.
Validation.
The assay method was validated for linearity, lower limit of quantitation, matrix effects, precision, accuracy, stock solution stability, long-term storage stability, and freeze-thaw stability using the U.S. FDA criteria for bioanalysis. Precision and accuracy were within 8.9%, 2.8%, and 1.5% at the three concentrations tested (1.5, 14, and 60 mg/liter), respectively. The limit of detection was 0.07 mg/liter.
Patient data collection.
Additional clinical and demographic data were collected, including the simplified acute physiology score II (SAPS II) (18) at ICU admission, the modified sequential organ failure assessment (SOFA) (19) score, comorbidities, and 28-day outcome. During the RRT treatment, CVVH or CVVHDF settings were also recorded. The type of infection, the antimicrobial agents administered, microbiological culture results, and Etest MICs (bioMérieux, Marcy l'Etoile, France) (if available) were collected.
Population pharmacokinetic modeling.
One- and two-compartment models were developed with the nonparametric adaptive grid (NPAG) algorithm within the freely available Pmetrics software package for R (Laboratory of Applied Pharmacokinetics, University of Southern California, Los Angeles, CA) (20, 21). Elimination from the central compartment and the intercompartmental distribution (two-compartment model) into the peripheral compartment were modeled as first-order processes.
Population pharmacokinetic covariate screening.
Actual body weight, serum creatinine concentration, serum albumin concentration, age, SOFA score, vasopressor use, residual renal function, filter age, and the difference in the patient's weight between the time of admission and the sampling day were evaluated as covariates. If inclusion of the covariate resulted in a statistically significant improvement in the log-likelihood values (P < 0.05) and/or improved the goodness-of-fit plots, then it was included.
Model diagnostics.
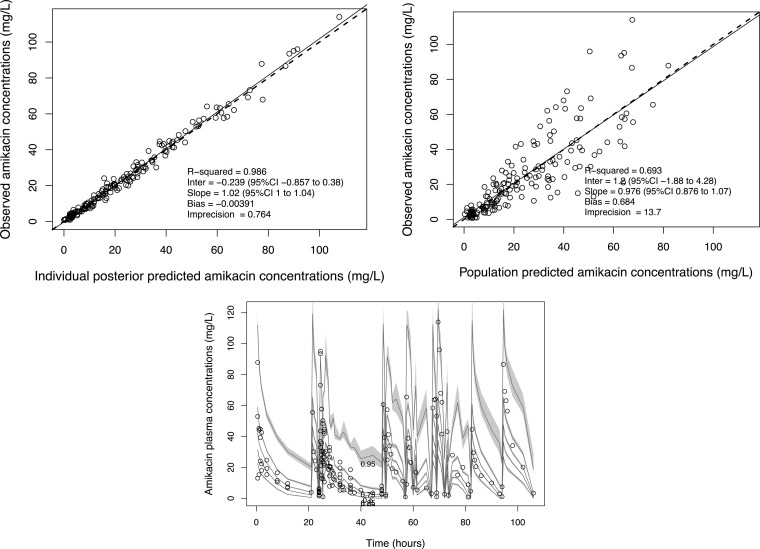
Goodness of fit was assessed by linear regression with an observed-predicted plot, coefficients of determination, and log-likelihood values. Predictive performance evaluation was based on the mean prediction error (bias) and the mean bias-adjusted squared prediction error (imprecision) for the population and individual prediction models. Using the final covariate model, a visual predictive check (VPC) was performed using the bootstrapping method by simulating 1,000 subjects to assess the predictive performance of the model.
PTA.
Monte Carlo simulations (n = 1,000) were employed using Pmetrics to determine the probability of target attainment (PTA) for the PK/PD targets, which were a Cmax/MIC ratio of ≥8 (2, 3, 5) or an AUC/MIC of ≥70 (3, 5), for various MICs (0.125 to 32 mg/liter) during the first 24 h of treatment for patients with total body weights of 60 kg, 80 kg, and 100 kg. Supratherapeutic exposures were described as a trough concentration (Cmin) of ≥2.5 mg/liter, in line with French national guidelines (22).
Fractional target attainment calculation.
MIC data for Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa from the EUCAST database (available at www.eucast.org; accessed 30 December 2015) were used to determine fractional target attainment. The fractional target attainment identifies the likely success of treatment by comparing the pharmacodynamic exposure (PTA) against an MIC distribution. A priori, a dosing regimen was considered successful if the fractional target attainment was ≥85% (34).
Estimation of the clearance of amikacin by CRRT.
The clearance of amikacin by CRRT was assessed as follows: amikacin clearance by CCRT = (Cpre − Ccorpost)/Ccorpost · BFR, where Cpre is the amikacin prehemofilter concentration, Ccorpost is the corrected amikacin posthemofilter concentration, and BFR is the blood flow rate. Posthemofilter concentrations were corrected for postdilution as follows: Ccorpost = Cpost · (BFR − PDR − FRR)/BFR, where Cpost is the measured amikacin posthemofilter concentration, BFR is the blood flow rate, PDR is the postdilution flow rate, and FRR is the fluid removal rate.
Statistical analysis.
Continuous data are presented as the mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical data are presented as counts (in percent). Comparisons used the Mann-Whitney U and chi-square tests as appropriate. Correlations were assessed by means of a scatter graph and the Pearson correlation coefficient (r). Differences in amikacin clearance by CVVH and CVVHDF were analyzed using a Student t test. A P value of <0.05 was considered statistically significant, and all analyses were performed using GraphPad Prism software (version 6.0; San Diego, CA, USA).
RESULTS
Demographic and clinical data.
Sixteen patients were recruited into the study per the study protocol, and data from 20 RRT sessions were analyzed. Demographic data are presented in Table 1. The patients included in the study required vasopressor support during 17 (85%) RRT sessions. The patients had pulmonary (n = 4), intra-abdominal (n = 7), urinary tract (n = 4), and vascular prosthesis (n = 1) infections. The samples studied microbiologically were positive for 7 (44%) patients. One Serratia marcescens isolate, one multidrug-resistant (MDR) K. pneumoniae isolate, three E. coli isolates, one Enterococcus faecium isolate, one Enterococcus faecalis isolate, one Morganella morganii isolate, one MDR Citrobacter braakii isolate, three staphylococci (including one methicillin-resistant Staphylococcus aureus isolate and one MDR Staphylococcus capitis isolate), and two streptococci were identified. The MICs for the Gram-negative bacilli ranged from 2 mg/liter to 16 mg/liter. The MICs for the Gram-positive cocci ranged from 4 to 64 mg/liter. Among the 16 patients included, the 28-day mortality rate was 38%. Four of the six deaths were related to the infectious episode for which amikacin was prescribed.
TABLE 1.
Descriptive data for the studied populationa
| Demographic data | Result |
|---|---|
| No. of females/no. of males | 4/12 |
| Median (IQR) age (yr) | 72 (65–75) |
| Median (IQR) wt (kg) at admission | 80 (73–89) |
| Median (IQR) ht (cm) | 167 (162–178) |
| Median (IQR) body mass index (kg/m2) | 27 (24–32) |
| No. of patients with the following comorbidity: | |
| Hypertension | 8 |
| Chronic heart failure | 2 |
| COPD | 2 |
| Cirrhosis | 1 |
| Nondialysis chronic renal disease | 2 |
| Median (IQR) severity score | |
| SAPS II at admission | 51 (41–59) |
| SOFA score at admission | 10 (8–12) |
Data are for 16 patients. COPD, chronic obstructive pulmonary disease; SAPS II, simplified acute physiology score II; SOFA, sequential organ failure assessment.
Replacement renal therapy.
Four patients underwent both CVVH and CVVHDF. Of the remaining patients, 5 received CVVH only and 7 received CVVHDF only. The RRT settings across both CVVH and CVVHDF treatments are shown in Table 2.
TABLE 2.
Clinical data and RRT parameters during RRT sessionsa
| RRT | Cmax (mg/liter) | Cmin (mg/liter) | Dosing interval (h) | 12-h urine output | MAP (mm Hg) | Heart rate (no. of beats/min) | Albumin concn (g/liter) | Creatinine concn (μmol/liter) | Urea concn (mmol/liter) | Blood flow rate (ml/min) | Dialysate flow rate (ml/kg/h) | Ultrafiltration rate (ml/kg/h) | Fraction filtration ratio (%) | RRT downtime (h) | Filter age (h) | Transmembrane pressure (mm Hg) | Fluid removal rate (ml/kg/h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CVVHDF (n = 11) | 52.9 (29.8–68.4) | 3.1 (1.6–3.5) | 24 (24–38) | 110 (98–338) | 77 (72–84) | 92 (83–102) | 27.6 (22–30.7) | 243 (191–300) | 20 (12–25) | 200 (180–215) | 15.4 (15.1–16.4) | 16.3 (14–16.9) | 11 (9–12) | 0.15 (0–1) | 15 (4–21) | 39 (20–52) | 17 (15–17) |
| CVVH (n = 9) | 60.6 (44.5–86.7) | 2.9 (2.5–3) | 24.5 (24–34) | 700 (30–725) | 70 (67–86) | 100 (70–106) | 27.9 (22.8–29.8) | 248 (211–280) | 12 (8.2–15.4) | 250 (200–250) | NA | 33.3 (31.2–36.8) | 20 (18–21) | 0.15 (0–0.75) | 15 (14–21) | 77 (72–86) | 21 (17–21) |
Data are expressed as the median (IQR). CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration; MAP, mean arterial pressure; RRT, renal replacement therapy; NA, not available.
Pharmacokinetic model building.
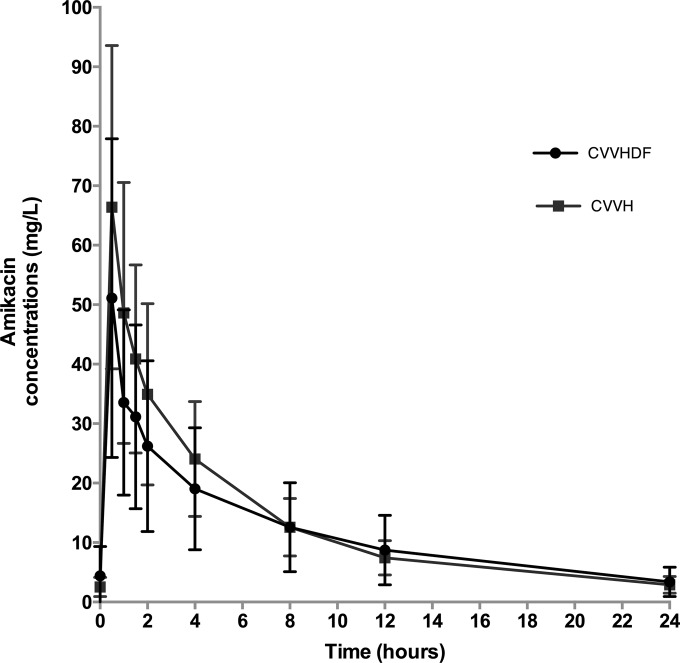
The mean observed concentration-time profile of amikacin is shown in Fig. 1. The blood samples were taken at a median time of 31 h (IQR, 16 to 69 h) after the start of amikacin therapy. A two-compartment linear model best described the time course of 261 total plasma concentrations of amikacin. This model included a zero-order input of drug into the central compartment. The only covariate that improved the fit of the model was, for amikacin clearance, weight normalized to 80 kg for CVVH and to 75 kg for CVVHDF (to the power of 0.75). After the inclusion of weight in the model, the −2 log-likelihood value was reduced from 801 to 786, although the log-likelihood values were not statistically significantly different (P = 0.55), and the goodness of fit improved. For these reasons, weight was retained in the final model.
FIG 1.
Observed mean concentration-time profiles for the amikacin dosing sampling interval in critically ill patients receiving CVVH (n = 9) or CVVHDF (n = 11). Error bars represent standard deviations.
The final model was described as follows: amikacin CL = CLhf · [(WT/80)0.75] + CLhdf · [(WT/75)0.75], where CL is clearance, WT is total body weight, CLhf is total amikacin clearance on hemofiltration, and CLhdf is total amikacin clearance on hemodiafiltration. CLhdf was 0 when hemodiafiltration was applied, and CLhf was 0 when hemofiltration was applied.
The mean population pharmacokinetic parameter estimates from the final covariate model were 25.2 ± 17.3 liters for central volume, 0.89 ± 1.17 liters/h for the rate constant for the drug distribution from the central to the peripheral compartment (kcp), 2.38 ± 6.60 liters/h for the rate constant for the drug distribution from the peripheral to the central compartment (kpc), 4.45 ± 2.35 liters/h for population mean total amikacin clearance on hemodiafiltration, and 4.69 ± 2.42 liters/h for population mean total amikacin clearance on hemofiltration. The mean individual empirical Bayesian clearance estimates of the patients undergoing CVVH and CVVHDF were estimated to be 5.19 ± 0.74 and 4.08 ± 0.50 liters/h (P = 0.21), respectively. The diagnostic plots to confirm the goodness of fit of the model were considered acceptable and are shown in Fig. 2. The final covariate model was then used for dosing simulations.
FIG 2.
Diagnostic plots for the final covariate model. Observed versus population predicted concentrations (top right) and individual predicted concentrations (top left) in plasma. (Bottom) Visual predictive check. CI, confidence interval. Inter, intercept.
Dosing simulations.
The Monte Carlo simulations and PTAs of a Cmax/MIC ratio of ≥8 and an AUC/MIC of ≥70 for various amikacin doses for patients that weighed 60 kg, 80 kg, or 100 kg are described in Table 3. The Monte Carlo simulations and PTAs of a Cmax/MIC ratio of ≥8 and a Cmin of ≥2.5 mg/liter for various amikacin dosing regimens for a patient that weighed 80 kg and for an isolate for which the MIC was 4 mg/liter are shown in Fig. 3 (CVVH) and Fig. 4 (CVVHDF). An MIC of 4 mg/liter was chosen to indicate that suboptimal achievement of therapeutic targets occurs at this MIC.
TABLE 3.
Monte Carlo simulations and PTA in plasma for various i.v. amikacin doses for patients with body weights of 60 kg, 80 kg, and 100 kg undergoing CVVH or CVVHDF for various MICsa
| RRT, body wt, and dosage | Achievement of the indicated PK/PD target for MIC of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤1 mg/liter |
2 mg/liter |
4 mg/liter |
8 mg/liter |
|||||
| Cmax/MIC ratio ≥ 8 | AUC/MIC ≥ 70 | Cmax/MIC ratio ≥ 8 | AUC/MIC ≥ 70 | Cmax/MIC ratio ≥ 8 | AUC/MIC ≥ 70 | Cmax/MIC ratio ≥ 8 | AUC/MIC ≥ 70 | |
| CVVH | ||||||||
| 60 kg | ||||||||
| 15q24 | + | + | − | − | − | − | − | − |
| 20q24 | + | + | + | + | − | − | − | − |
| 25q24 | + | + | + | + | − | − | − | − |
| 30q24 | + | + | + | + | + | + | − | − |
| 35q24 | + | + | + | + | + | + | − | − |
| 80 kg | ||||||||
| 15q24 | + | + | − | − | − | − | − | − |
| 20q24 | + | + | + | + | − | − | − | − |
| 25q24 | + | + | + | + | + | − | − | − |
| 30q24 | + | + | + | + | + | + | − | − |
| 35q24 | + | + | + | + | + | + | − | − |
| 100 kg | ||||||||
| 15q24 | + | + | − | − | − | − | − | − |
| 20q24 | + | + | + | + | + | − | − | − |
| 25q24 | + | + | + | + | + | + | − | − |
| 30q24 | + | + | + | + | + | + | − | − |
| 35q24 | + | + | + | + | + | + | + | − |
| CVVHDF | ||||||||
| 60 kg | ||||||||
| 15q24 | + | + | + | + | − | − | − | − |
| 20q24 | + | + | + | + | − | − | − | − |
| 25q24 | + | + | + | + | − | − | − | − |
| 30q24 | + | + | + | + | + | − | − | − |
| 35q24 | + | + | + | + | + | + | − | − |
| 80 kg | ||||||||
| 15q24 | + | + | + | + | − | − | − | − |
| 20q24 | + | + | + | + | − | − | − | − |
| 25q24 | + | + | + | + | + | − | − | − |
| 30q24 | + | + | + | + | + | + | − | − |
| 35q24 | + | + | + | + | + | + | − | − |
| 100 kg | ||||||||
| 15q24 | + | + | + | + | − | − | − | − |
| 20q24 | + | + | + | + | + | − | − | − |
| 25q24 | + | + | + | + | + | + | − | − |
| 30q24 | + | + | + | + | + | + | − | − |
| 35q24 | + | + | + | + | + | + | − | − |
The target was a Cmax/MIC ratio of ≥8 and AUC/MIC of ≥70. CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration; +, achievement of PK/PD target; −, nonachievement of PK/PD target; 15q24, 20q24, 25q24, 30q24, and 35q24, doses of 15, 20, 25, 30, and 35 mg/kg administered every 24 h, respectively.
FIG 3.
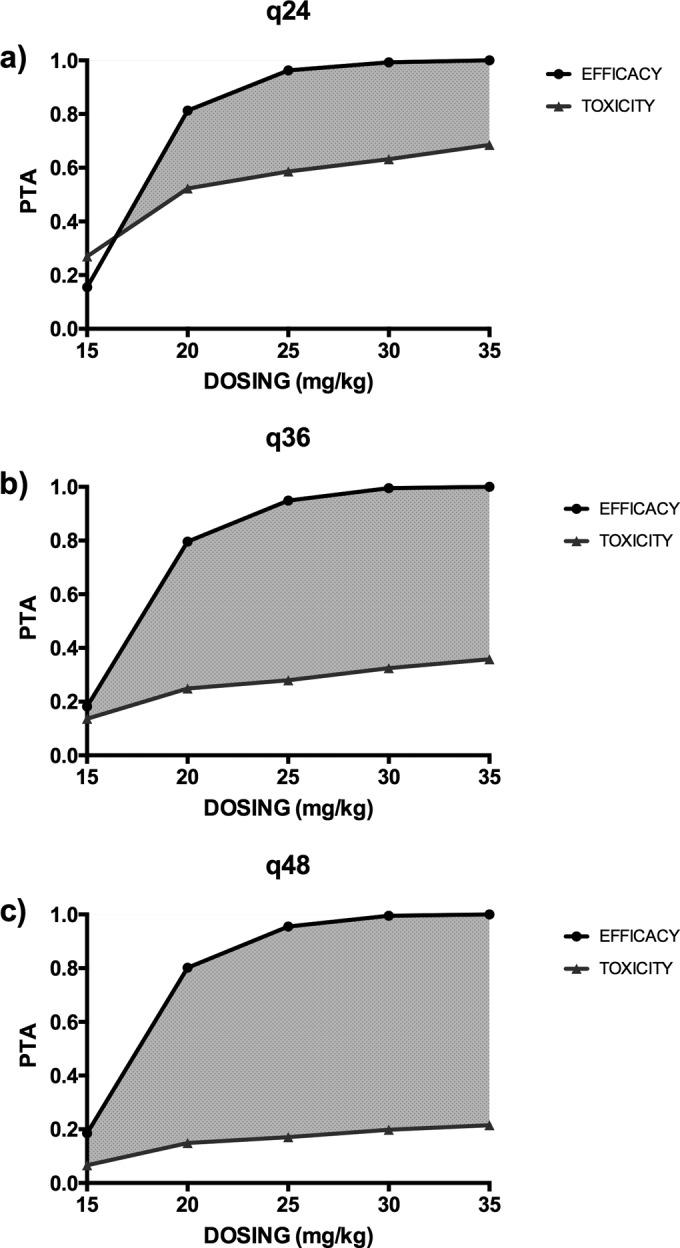
PTA of efficacy (Cmax/MIC ratio ≥ 8) and toxicity (Cmin ≥ 2.5 mg/liter at the end of the dosing interval) for a patient undergoing CVVH with a body weight of 80 kg with dosing every 24 h (q24) (a), 36 h (q36) (b), and 48 h (q48) (c) and targeting pathogens with an MIC of 4 mg/liter.
FIG 4.
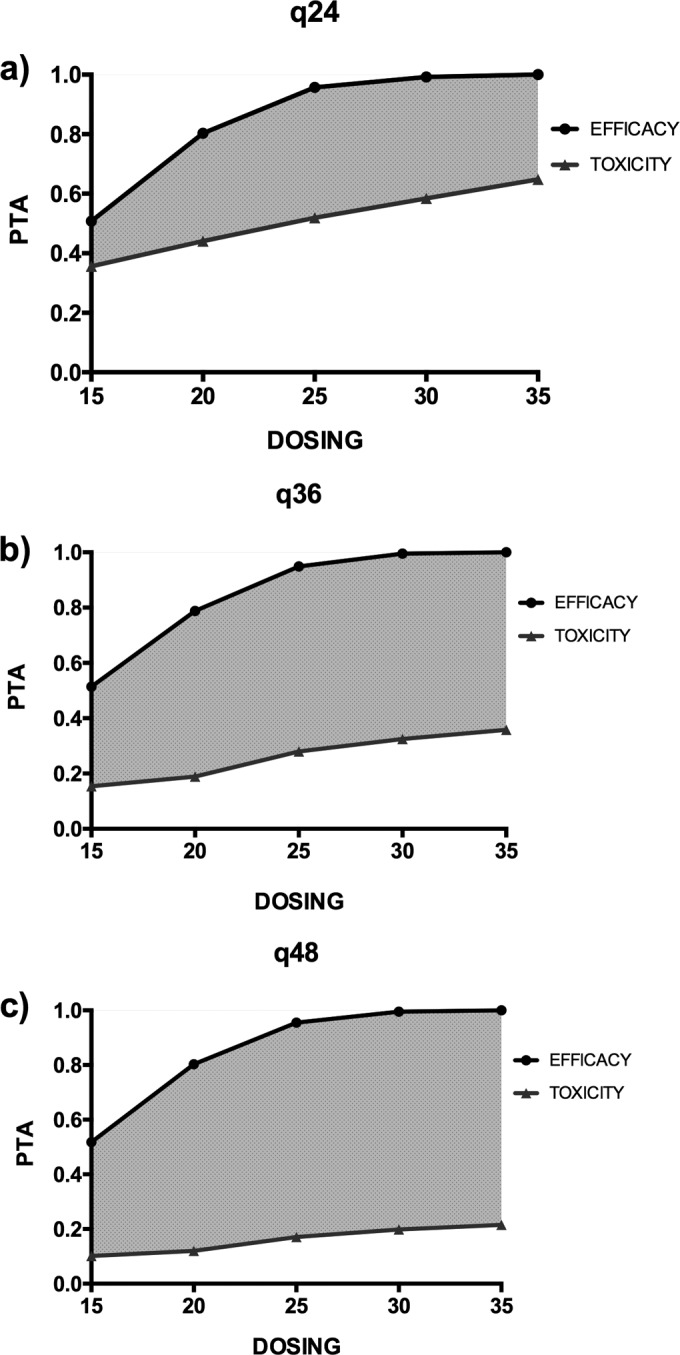
PTA of efficacy (Cmax/MIC ratio ≥ 8) and toxicity (Cmin ≥ 2.5 mg/liter at the end of the dosing interval) for a patient undergoing CVVHDF with a body weight of 80 kg with dosing every 24 h (a), 36 h (b), and 48 h (c) and targeting pathogens with an MIC of 4 mg/liter. Units for dosing are milligrams per kilogram.
Fractional target attainment.
The fractional target attainments for the simulated PTAs for a range of amikacin doses, dose intervals, and patient body weights against susceptible MIC distributions (MICs ≤ 8.0 mg/liter) for A. baumannii, K. pneumoniae, E. coli, and P. aeruginosa are shown in Table 4.
TABLE 4.
Fractional target attainment for the various amikacin doses every 24 h for patients with body weights of 60 kg, 80 kg, or 100 kg receiving CVVH or CVVHDF for susceptible MIC distributions for A. baumannii, K. pneumoniae, E. coli, and P. aeruginosaa
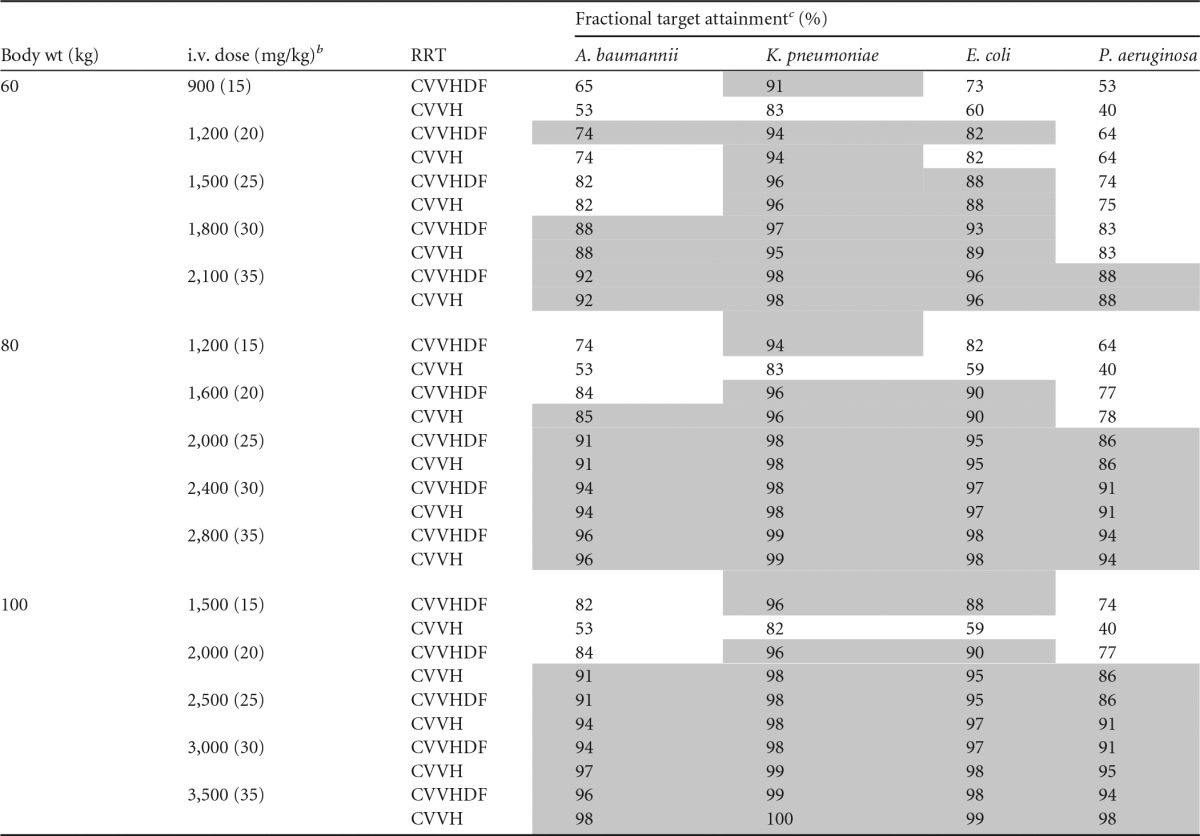
CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration.
b Weight-based dosing regimens (in milligrams per kilogram) are given in parentheses.
c Gray shading, achievement of the a priori PTA of a Cmax/MIC ratio of ≥8 against at least 85% of isolates.
Amikacin clearance across the hemofilter during CRRT.
The mean clearance of amikacin by CRRT was 1.8 ± 1.3 liters/h by CVVHDF and 1.3 ± 1.0 liters/h by CVVH.
DISCUSSION
In the present study, the extended-interval high amikacin dose of 25 mg/kg every 48 h is optimal in critically ill patients undergoing CVVH or CVVHDF to achieve the PK/PD targets and to limit toxicity when infections with difficult-to-treat pathogens are suspected. Indeed, this dose and dosing interval led to the optimal amikacin exposure, as this dosing regimen provides the largest difference between the efficacy and the toxicity probability (23). Several studies have reported that the pharmacokinetics of aminoglycosides are altered in critically ill patients, leading to a Cmax lower than the targeted Cmax in 30 to 40% of critically ill patients using standard dosing regimens (24–27). The increased volume of distribution observed in critically ill patients and the concentration-dependent killing activity of aminoglycosides support the use of regimens with higher doses of amikacin to achieve adequate Cmaxs. The use of dosing regimens of ≥25 mg/kg in critically ill patients has been suggested by several authors and recommended by some national guidelines (13, 24, 27, 28). However, some concerns remain about aminoglycoside-related nephrotoxicity using high aminoglycoside dosing regimens, especially in patients with AKI requiring RRT. Our results showed that the PK/PD targets (Cmax/MIC ratio ≥ 8, AUC/MIC ≥ 70) were achieved with a dosing regimen of 25 mg/kg or higher in critically ill patients undergoing CVVH or CVVHDF. These results are consistent with those reported by Taccone et al. (11). They reported that a 25-mg/kg loading dose of amikacin in patients undergoing CVVHDF at a dose of 30 ml/kg/h and a blood flow rate setting at 150 to 200 ml/min achieved the targeted Cmax of 64 mg/liter in 9 (69%) patients. The amikacin clearance reported in that study widely varied between 0.006 and 0.198 liter/kg/h, but the mean clearance of amikacin was lower than that reported in the present study (11). The authors did not find any correlation between RRT settings and amikacin clearance. However, some RRT parameters, such as pre- or postdilution mode or downtime, were not recorded. RRT filter downtime is also a factor that may influence RRT-related drug clearance but is rarely reported. In the present study, the filter downtime was very low during the dosing interval and likely did not affect amikacin clearance, and only the postdilution mode was used.
As CVVHDF combines convection and diffusion processes to clear molecules from blood, there is a general assumption that clearance by CVVHDF is higher than clearance by CVVH (16). In the present study, using the recommended 30-ml/kg/h effluent rate for both modes (a 30-ml/kg/h ultrafiltrate flow rate for CVVH and a 15-ml/kg/h dialysate flow rate associated with a 15-ml/kg/h ultrafiltrate flow rate for CVVHDF) (29), similar blood flow rates, and similar membranes during the RRT sessions, we did not show any significant differences in amikacin clearance. It could be hypothesized that when the recommended high doses of the effluent rate for continuous RRT are applied, the difference between convection and/or diffusion might not be significant for antibiotic pharmacokinetics (30).
Most importantly, none of the simulated dosing regimens achieved the PK/PD targets for the EUCAST MIC breakpoint of 8 mg/liter. These findings might encourage physicians to increase amikacin doses, but the increasing risk of renal toxicity should be considered. As the clearance decreases in the context of AKI, the drug half-life increases, in which case an extended dosing interval would be required to enable redosing at the recommended trough concentrations. In the present study, extended intervals (i.e., every 36 h up to every 48 h) achieved the lowest probability of toxicity without an apparent decrease in efficacy. Extending the dosing interval may allow a drug-free period and may reduce the risk of aminoglycoside accumulation in the renal cortex. In a previous study, we highlighted that the use of a regimen with a higher dose (30 mg/kg) in association with therapeutic drug monitoring led to extended dosing intervals in half of the critically ill patients studied (28). Taccone et al. reported that the median time to achieve a trough concentration below 5 mg/liter was 36 h (IQR, 14 to 76 h) in patients undergoing CVVHDF (11). The extended dosing intervals suggested by the results of the present study are consistent with recommendations for aminoglycoside dosing regimens in critically ill patients receiving continuous RRT (30–32). However, as highlighted by the results of the present study, regimens with doses higher than those recommended (e.g., a loading dose of 10 mg/kg and maintenance doses of 7.5 mg/kg every 24 to 48 h) should be used to ensure efficacy and to limit toxicity at the same time. Some authors have even suggested that a dosing regimen of 50 mg/kg of amikacin followed by continuous RRT be used to limit toxicity in a patient with a multidrug-resistant P. aeruginosa infection (33).
The present study has limitations to consider. First, we could not assess mechanistically if the concentrations observed were caused by RRT or non-RRT clearance because the clearance of amikacin into the RRT effluent was not available in this study, although this limitation does not alter our dosing recommendations. Moreover, no correlation between residual renal function and amikacin clearance was found in this model. Second, we used a targeted trough concentration of 2.5 mg/liter to define aminoglycoside-related renal toxicity, even though its relevance could be questioned. However, French guidelines recommend redosing only when aminoglycoside trough concentrations are below 2.5 mg/liter. Third, further prospective studies need to confirm that extended dosing intervals do not reduce the antimicrobial effects of aminoglycosides.
Conclusion.
In the present study, we observed a high degree of variability in the pharmacokinetics of amikacin in critically ill patients. Suboptimal achievement of therapeutic targets for the EUCAST breakpoint of 8 mg/liter occurs when standard dosing is used. We recommend a loading dose of 25 mg/kg every 48 h adjusted according to the findings of therapeutic drug monitoring as the preferred approach for aminoglycoside treatment in critically ill patients receiving continuous renal replacement therapy.
ACKNOWLEDGMENTS
We thank Loubna Elotmani, Audrey Ayral, Sophie Lloret, Jenny Ordonez, and all ICU nurses for their help in sampling, data collection, and assays.
This work was supported by an academic grant from the Nîmes University Hospital. J.A.R. is funded by a career development fellowship from the National Health and Medical Research Council of Australia (APP1048652). We acknowledge other funding to the Burns, Trauma, and Critical Care Research Centre from the National Health and Medical Research Council of Australia for a project grant (APP1044941) and the Centre for Research Excellence (APP1099452).
C.R. made substantial contributions to study conception, study design, acquisition and interpretation of the data, and drafting of the manuscript and approved the final version to be published. S.C.W. made substantial contributions to acquisition, analysis, and interpretation of the data, revised the manuscript for important intellectual content, and approved the final version to be published. L.M. made substantial contributions to study conception, study design, interpretation of the data, and drafting of the manuscript and approved the final version to be published. G.S. made substantial contributions to acquisition, analysis, and interpretation of the data, revised the manuscript for important intellectual content, and approved the final version to be published. J.L. made substantial contributions to study conception, study design, interpretation of the data, revised the manuscript for important intellectual content, and approved the final version to be published. J.-Y.L. made substantial contributions to study conception, study design, interpretation of the data, and drafting of the manuscript and approved the final version to be published. J.A.R. made substantial contributions to study conception, study design, analysis and interpretation of the data, and drafting of the manuscript, revised the manuscript for important intellectual content, and approved the final version to be published.
Funding Statement
This work was supported by an academic grant from the Nîmes University Hospital.
REFERENCES
- 1.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of Cmax concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Moore RD, Smith CR, Lietman PS. 1984. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med 77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 3.Craig WA, Redington J, Ebert SC. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother 27(Suppl C):C29–C40. [DOI] [PubMed] [Google Scholar]
- 4.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother 43:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. 2003. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother 52:668–674. doi: 10.1093/jac/dkg403. [DOI] [PubMed] [Google Scholar]
- 6.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 7.Pea F, Viale P, Furlanut M. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet 44:1009–1034. doi: 10.2165/00003088-200544100-00002. [DOI] [PubMed] [Google Scholar]
- 8.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney ( BEST Kidney) Investigators. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 9.Schneider AG, Bellomo R, Bagshaw SM, Glassford NJ, Lo S, Jun M, Cass A, Gallagher M. 2013. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med 39:987–997. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 10.Akers KS, Cota JM, Frei CR, Chung KK, Mende K, Murray CK. 2011. Once-daily amikacin dosing in burn patients treated with continuous venovenous hemofiltration. Antimicrob Agents Chemother 55:4639–4642. doi: 10.1128/AAC.00374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taccone FS, de Backer D, Laterre PF, Spapen H, Dugernier T, Delattre I, Wallemacq P, Vincent JL, Jacobs F. 2011. Pharmacokinetics of a loading dose of amikacin in septic patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents 37:531–535. doi: 10.1016/j.ijantimicag.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 12.D'Arcy DM, Casey E, Gowing CM, Donnelly MB, Corrigan OI. 2012. An open prospective study of amikacin pharmacokinetics in critically ill patients during treatment with continuous venovenous hemodiafiltration. BMC Pharmacol Toxicol 13:14. doi: 10.1186/2050-6511-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert R, Rochard E, Malin F, Bouquet S. 1991. Amikacin pharmacokinetics during continuous veno-venous hemofiltration. Crit Care Med 19:588–589. doi: 10.1097/00003246-199104000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Lam SW, Bauer SR. 2013. Amikacin pharmacokinetics during continuous veno-venous hemodialysis. Infect Dis Ther 2:217–226. doi: 10.1007/s40121-013-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinowski JM, de la Coussaye JE, Bressolle F, Fabre D, Saissi G, Bouvet O, Galtier M, Eledjam JJ. 1993. Multiple-dose pharmacokinetics of amikacin and ceftazidime in critically ill patients with septic multiple-organ failure during intermittent hemofiltration. Antimicrob Agents Chemother 37:464–473. doi: 10.1128/AAC.37.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pea F, Viale P, Pavan F, Furlanut M. 2007. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet 46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 17.Joannidis M, Oudemans-van Straaten HM. 2007. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care 11:218. doi: 10.1186/cc5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. 1993. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working Group on “Sepsis-Related Problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 20.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ANSM. Bon usage des aminosides administrés par voie injectable: gentamycine, tobramycine, netimicine, amikacine—mise au point. ANSM, Paris, France: http://ansm.sante.fr/Mediatheque/Publications/Recommandations-Medicaments. [Google Scholar]
- 23.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 24.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, Jacobs F. 2010. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 14:R53. doi: 10.1186/cc8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvez R, Luengo C, Cornejo R, Kosche J, Romero C, Tobar E, Illanes V, Llanos O, Castro J. 2011. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents 38:146–151. doi: 10.1016/j.ijantimicag.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Roger C, Nucci B, Molinari N, Bastide S, Saissi G, Pradel G, Barbar S, Aubert C, Lloret S, Elotmani L, Polge A, Lefrant JY, Roberts JA, Muller L. 2015. Standard dosing of amikacin and gentamicin in critically ill patients results in variable and subtherapeutic concentrations. Int J Antimicrob Agents 46:21–27. doi: 10.1016/j.ijantimicag.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 27.de Montmollin E, Bouadma L, Gault N, Mourvillier B, Mariotte E, Chemam S, Massias L, Papy E, Tubach F, Wolff M, Sonneville R. 2014. Predictors of insufficient amikacin Cmax concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med 40:998–1005. doi: 10.1007/s00134-014-3276-x. [DOI] [PubMed] [Google Scholar]
- 28.Roger C, Nucci B, Louart B, Friggeri A, Knani H, Evrard A, Lavigne JP, Allaouchiche B, Lefrant JY, Roberts JA, Muller L. 2016. Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother 71:208–212. doi: 10.1093/jac/dkv291. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012. KDIGO clinical practice guideline for acute kidney injury. Chapter 5.8: dose of renal replacement therapy in AKI. Kidney Int 2(Suppl):113–115. [Google Scholar]
- 30.Heintz BH, Matzke GR, Dager WE. 2009. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy 29:562–577. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- 31.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 41:1159–1166. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 32.Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med 37:2268–2282. doi: 10.1097/CCM.0b013e3181aab3d0. [DOI] [PubMed] [Google Scholar]
- 33.Brasseur A, Hites M, Roisin S, Cotton F, Vincent JL, De Backer D, Jacobs F, Taccone FS. 2016. A high-dose aminoglycoside regimen combined with renal replacement therapy for the treatment of MDR pathogens: a proof-of-concept study. J Antimicrob Chemother 71:1386–1394. doi: 10.1093/jac/dkv491. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JA, Udy AA, Jarrett P, Wallis SC, Hope WW, Sharma R, Kirkpatrick CM, Kruger PS, Roberts MS, Lipman J. 2015. Plasma and target-site subcutaneous tissue population pharmacokinetics and dosing simulations of cefazolin in post-trauma critically ill patients. J Antimicrob Chemother 70:1495–1502. doi: 10.1093/jac/dku564. [DOI] [PubMed] [Google Scholar]