Abstract
Recent observational studies have suggested possible reductions in mortality in patients receiving cefazolin versus antistaphylococcal penicillins. We examined 90-day mortality in patients receiving cefazolin compared to nafcillin for methicillin-susceptible Staphylococcus aureus (MSSA) bloodstream infection (BSI). We identified persons with MSSA BSI admitted to San Francisco General Hospital from January 2008 to July 2013 through a hospital-wide infection surveillance system and confirmed 90-day mortality using U.S. national vital registries. We included persons receiving cefazolin or nafcillin as the predominant intravenous antimicrobial agent; all participants received inpatient Infectious Diseases service consultation. We estimated the association between receipt of cefazolin and 90-day risk of death by multivariate logistic regression, including a propensity score for receiving cefazolin as the second predictor. Of 230 MSSA BSI cases, 30 received nafcillin and 70 received cefazolin as the predominant antimicrobial; 10 died within 90 days, 5 from each group. Unadjusted analysis showed substantial but not statistically significant reduced odds of death in those receiving cefazolin (odds ratio, 0.38; 95% confidence interval [CI], 0.10 to 1.44). Multivariate analysis with propensity scores found a similar adjusted odds ratio (0.40; 95% CI, 0.09 to 1.74; P = 0.22). We found a large reduction in 90-day mortality in those receiving cefazolin compared to nafcillin for MSSA BSI, but this finding was not statistically significant. The magnitude of effect seen in this and other studies justifies further study.
INTRODUCTION
Staphylococcus aureus is a leading cause of both community-onset and hospital-acquired bloodstream infection (BSI) (1, 2). S. aureus BSI (SABSI) has a considerable disease burden throughout the world, with an estimated incidence of 19.7 to 50 per 100,000 person-years (3, 4) and a contemporary 30-day mortality rate of 20%, although this estimate varies considerably by study (20 to 36%) (4, 5).
While methicillin-resistant S. aureus (MRSA) is often the focus of S. aureus surveillance (6–8), methicillin-sensitive S. aureus (MSSA) BSI still accounts for the majority of SABSI in many regions (9, 10). Some recent data suggest that, adjusting for host factors and other confounders, mortality outcomes in MSSA BSI are equivalent to those in MRSA BSI, although this is controversial (4, 11).
The preferred antimicrobial agents for MSSA BSI are generally either the antistaphylococcal penicillins (nafcillin, flucoxacillin, cloxacillin, or oxacillin, depending on local antibiotic availability) or cefazolin, a first-generation cephalosporin (12, 13). While both options are widely used in clinical practice and toxicities exist with each of these antimicrobials, cefazolin has possible advantages over antistaphylococcal penicillins for the treatment of MSSA BSI, including less frequent dosing and dialysis dosing regimens for those on hemodialysis with limited venous access (12, 13).
A concern with cefazolin is that some strains exhibit an inoculum effect in vitro such that at an inoculum of ∼107 CFU/ml, the MIC is severalfold greater than the MIC at the standard inoculum of ∼105 CFU/ml, and this could result in treatment failure (14). However, several observation studies suggest treatment failure is not more common with cefazolin than with antistaphylococcal penicillins (15–17), and a recent large Canadian observational study found a substantial mortality reduction in those receiving cefazolin compared to those receiving cloxacillin (18), although this hazard reduction was not statistically significant (18). Thus, further data on the comparative outcomes for MSSA BSI treated by cefazolin or antistaphylococcal penicillins are needed to clarify whether either of these treatment options is superior.
We examined comparative 90-day mortality in those receiving cefazolin compared to nafcillin for MSSA BSI in a San Francisco safety-net tertiary hospital.
(Elements of this study were presented as a scientific poster at ID Week 2015, San Diego, CA.)
MATERIALS AND METHODS
Study setting and population.
We identified persons with MSSA BSI presenting to or occurring in San Francisco General Hospital (SFGH) between 1 January 2008 and 1 July 2013 by a hospital-wide infection surveillance system; collected clinical, demographic, and microbiological data; and entered them into a database maintained by the hospital infection control program. A hospital policy mandates compulsory Infectious Diseases service consultation for all SABSI inpatient cases.
Inclusion and exclusion criteria.
Adults who were admitted to SFGH and had one or more blood cultures that were found to be positive for MSSA between 1 January 2008 and 1 July 2013 were eligible for inclusion in the study. The following participants were excluded from participation: those with dual MRSA/MSSA infections, those who received both cefazolin and nafcillin, those who received fewer than 5 days of cefazolin or nafcillin, those who initiated cefazolin or nafcillin more than 7 days after blood culture positivity, those who received a longer or equivalent duration of intravenous (i.v.) therapy with an antistaphylococcal antibiotic other than cefazolin or nafcillin, children (age of <18 years), and those found to have SABSI at other hospitals and transferred to SFGH.
Statistical analysis.
Receiving cefazolin (compared to receiving nafcillin) was the primary exposure, and all-cause mortality within 90 days of blood culture positivity was the primary outcome. In addition to the study predictor variable, we collected a wide number of sociodemographic, microbiological, clinical, and covariate data. We confirmed deaths by both the U.S. Social Security death master file and the U.S. National Death Index (National Center for Health Statistics, Hyattsville, MD). We estimated the unadjusted relationship between exposure and outcome using logistic regression. We considered hemodialysis and endocarditis to be a priori confounders to be included in subsequent multivariate analysis, as cefazolin may be more likely than nafcillin to be used in hemodialysis as it is administered only after hemodialysis, and renal disease is an independent risk factor for death in SABSI (13, 19, 20). In addition, infectious disease guidelines recommend nafcillin as the first-line agent for MSSA endocarditis (12, 21), and endocarditis is an independent risk factor for death in SABSI (22).
We considered a large number of additional potentially confounding covariates, including comorbidities (including diabetes and alcohol abuse), age, and other demographic factors, severity of presenting illness (including the presence of systemic inflammatory response syndrome [SIRS] and treatment in intensive care units [ICU]), receipt of other antistaphylococcal antimicrobials, and location/site of SABSI acquisition. We examined how each of these a priori and potential confounding covariates changed the association between receipt of cefazolin and death at 90 days in bivariate models (that is, models which contained the study predictor variable of receipt of cefazolin, the potentially confounding predictor covariate, and the outcome of death at 90 days).
Due to the large number of potential predictors relative to outcome events, we performed propensity score analysis and used the log odds of receipt of cefazolin in a 2-predictor model with receipt of cefazolin as the sole other predictor in the primary multivariate analysis. As the total number receiving nafcillin was limited, we restricted the propensity score model to covariates that substantially changed the association between receipt of cefazolin and death at 90 days in bivariate models.
To test the robustness of the adjusted associations estimated by propensity scores, to examine the assumption that hemodialysis and endocarditis are reasonable a priori covariates, and to minimize any residual confounding effect, we also fit comparative models: (i) a model containing covariates that changed the odds ratio (OR) by >15% in the bivariate models, (ii) a model yielding the highest adjusted OR and containing the two a priori predictors and a third potentially confounding predictor, and (iii) a model yielding the lowest adjusted OR and containing the two a priori predictors and a third potentially confounding predictor.
Finally, given the potential biases introduced by exclusion criteria, we performed several sensitivity analyses, including (i) those who received <5 days of nafcillin or cefazolin, (ii) those who received an equivalent or longer duration of i.v. therapy with another antistaphylococcal antimicrobial, (iii) those who received cefazolin or nafcillin >7 days after blood culture positivity, and (iv) those who received both cefazolin and nafcillin (with treatment group allocation based on the antimicrobial administered first).
Logistic regression models for death (with propensity score adjustment) underwent appropriate checking for linearity and collinearity, in addition to a Hosmer-Lemeshow test. All statistical analyses were performed using Stata version 13.1 (College Station, Texas).
Ethics.
This study was approved by the UCSF Committee for Human Research (CHR number 13-11790).
RESULTS
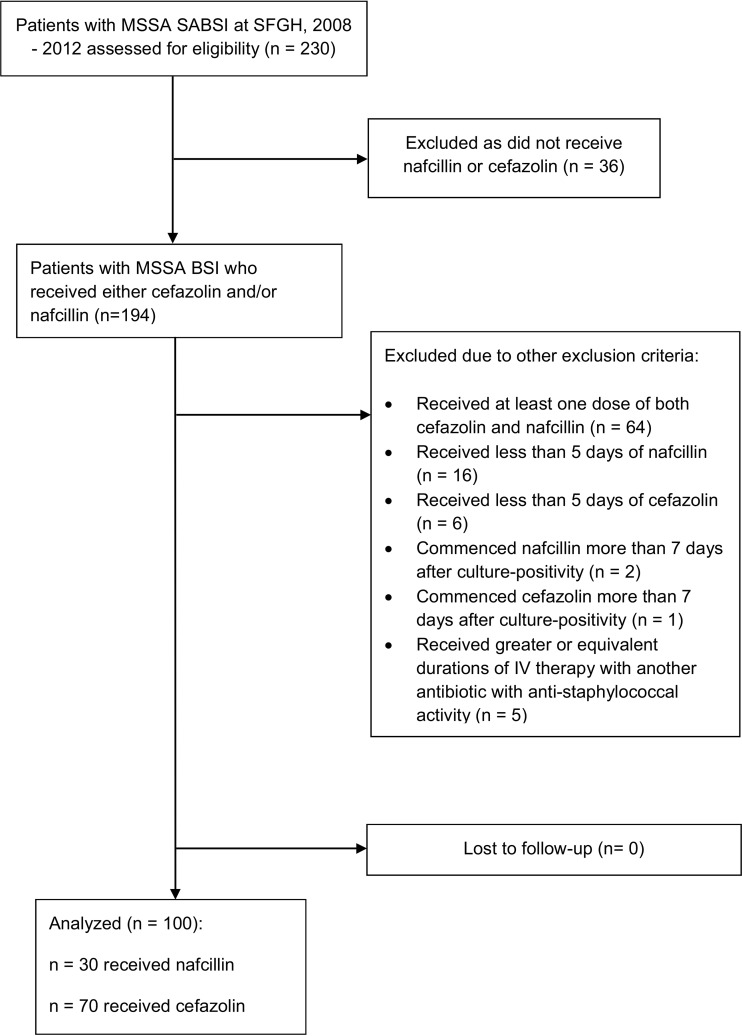
Figure 1 summarizes the enrollment of MSSA BSI cases into the prospective cohort and their exclusion from it. Of 230 patients diagnosed with MSSA BSI at SFGH between 1 January 2008 and 1 July 2013, 100 (43%) participants met all inclusion criteria: 30 had received nafcillin and 70 received cefazolin. Notably, those participants who had received both nafcillin and cefazolin mostly did so sequentially and not as combined cefazolin-nafcillin therapy. In most cases, participants received nafcillin before cefazolin. Table 1 presents baseline sociodemographic characteristics and past medical history of these two groups. Table 2 presents the participant characteristics related to the MSSA BSI, including cointerventions. There were 5 deaths by 90 days in each of the treatment groups.
FIG 1.
Inclusion and exclusion of methicillin-sensitive S. aureus bloodstream infection cases into the cohort study at San Francisco Hospital, January 2008 to July 2013.
TABLE 1.
Baseline sociodemographic characteristics, comorbidities, and medical history of MSSA BSI cases at San Francisco Hospital, January 2008 to July 2013
| Parameter or characteristic | Value(s) for patients receiving: |
|
|---|---|---|
| Nafcillin | Cefazolin | |
| Total (no. [%]) | 30 (100) | 70 (100) |
| Race (no. [%]) | ||
| White | 15 (50) | 22 (31) |
| African-American | 4 (13) | 11 (16) |
| Hispanic | 5 (17) | 14 (20) |
| Asian | 4 (13) | 19 (27) |
| Native American | 1 (3) | 1 (1) |
| Pacific Islander | 1 (2) | 3 (4) |
| Female (no. [%]) | 23 (77) | 48 (69) |
| Age, mean yr (SD) | 50.4 (12.5) | 53.0 (15.1) |
| Homeless (no. [%]) | 9 (30) | 11 (16) |
| Long-term-care facility residence (no. [%]) | 0 (0) | 8 (11) |
| Condition or treatment (no. [%]) | ||
| Hemodialysis | 16 (53) | 43 (61) |
| Diabetes mellitus | 4 (13) | 26 (37) |
| Cancer | 1 (3) | 1 (1) |
| Chemotherapy | 1 (3) | 0 (0) |
| Anti-tumor necrosis factor therapy | 0 (0) | 1 (1) |
| Steroids | 1 (3) | 4 (6) |
| Cirrhosis | 3 (10) | 5 (7) |
| IVDUa | 8 (27) | 12 (17) |
| Alcohol abuse | 11 (37) | 7 (10) |
| Recent trauma | 2 (7) | 2 (3) |
| Previous valvular disease | 1 (3) | 0 (0) |
| HIV | 6 (20) | 8 (11) |
| Charlson index, median score (IQRb) | 3 (1, 7) | 4 (2, 7) |
| i.v. access device (no. [%]) | 6 (20) | 19 (27) |
| Medical history (no. [%]) | ||
| Hospitalized within last 90 days | 1 (3) | 14 (20) |
| Hospitalized within last 12 mo | 11 (37) | 27 (39) |
| Recent surgery | 5 (17) | 11 (16) |
| S. aureus infection within last 90 days | 0 (0) | 1 (1) |
| S. aureus infection within last 12 mo | 2 (7) | 4 (6) |
IVDU, intravenous drug use.
IQR, interquartile range.
TABLE 2.
Clinical characteristics of MSSA BSI cases at San Francisco Hospital, January 2008 to July 2013
| Parameter or characteristic | No. (%) receiving: |
|
|---|---|---|
| Nafcillin | Cefazolin | |
| Total (no. [%]) | 30 (100) | 70 (100) |
| Admitted to ICU (no. [%]) | 8 (27) | 9 (13) |
| SIRS (no. [%]) | 20 (67) | 48 (69) |
| Hospital-onset MSSA BSI (no. [%]) | 7 (23) | 8 (11) |
| Length of stay, mean daysa (SD) | ||
| Baseline | 1.3 (2.6) | 3.7 (21.5) |
| Total | 18.0 (15.7) | 25.2 (51.0) |
| Documented presumed source of MSSA BSI (no. [%]) | ||
| Community-acquired pneumonia | 2 (7) | 0 (0) |
| Hospital-acquired pneumonia | 1 (3) | 1 (1) |
| Ventilator-associated pneumonia | 1 (3) | 0 (0) |
| Implanted prosthetic material | 1 (3) | 5 (7) |
| Intravascular catheter | 3 (10) | 13 (19) |
| Abscess | 2 (7) | 5 (7) |
| Cellulitis | 0 (0) | 3 (4) |
| Musculoskeletal, bone | 3 (10) | 3 (4) |
| Musculoskeletal, joint | 0 (0) | 2 (3) |
| Surgical-site infection | 0 (0) | 0 (0) |
| Urinary tract | 1 (3) | 2 (3) |
| Wound infection | 0 (0) | 5 (7) |
| Unknown | 16 (53) | 28 (40) |
| Other | 0 (0) | 3 (4) |
| Source risk for complicated MSSA BSI | ||
| Low | 4 (13) | 15 (21) |
| Moderate | 20 (67) | 47 (67) |
| High | 6 (20) | 8 (11) |
| Other i.v. antibiotics administered (no. [%]) | ||
| Vancomycin | 29 (97) | 67 (96) |
| Aminoglycoside | 5 (17) | 4 (6) |
| Carbapenem or 3rd/4th-generation cephalosporin | 23 (77) | 53 (76) |
| Aminopenicillin | 1 (3) | 3 (4) |
| Linezolid | 0 (0) | 0 (0) |
| Daptomycin | 0 (0) | 0 (0) |
| Treatment duration, median days (IQR) | ||
| Cefazolin or nafcillin | 12 (8, 29) | 20 (11, 29) |
| Vancomycin | 2 (2, 3) | 2 (1, 3) |
| Carbapenem or 3rd/4th-generation cephalosporin | 1 (1, 3) | 1 (1 ,2) |
| Duration of bacteremia, mean days (SD) | 1.7 (1.4) | 1.3 (0.8) |
| Complications of MSSA BSI (no. [%]) | ||
| Endocarditis | 5 (17) | 10 (14) |
| Vertebral osteomyelitis and/or epidural abscess | 2 (7) | 4 (6) |
Derived from hospital-onset cases only, admission date to date of blood culture positivity.
Unadjusted analysis showed a substantial, but nonstatistically significant, reduction in the odds of 90-day mortality in those receiving cefazolin (unadjusted OR, 0.38; 95% confidence interval [CI], 0.10 to 1.44; P = 0.16). Multivariate analysis was performed using a propensity score for receipt of cefazolin derived from the covariates hemodialysis, endocarditis, age, Charlson comorbidity index, alcohol abuse, hospital onset of MSSA BSI, homelessness, and admission to ICU. This model also showed a large but not statistically significant reduction in the adjusted odds ratio (aOR) of 90-day mortality in those receiving cefazolin (aOR, 0.40; 95% CI, 0.09 to 1.74; P = 0.22). The propensity score probabilities were not extreme (range, 0.22 to 0.92), and the log odds of receipt of cefazolin overlapped reasonably well between the cefazolin and nafcillin groups, with only one death in a nonoverlapping range. Repeat analysis with propensity scores restricted to the overlapping range estimated a similar effect (Table 3).
TABLE 3.
Analyses of cefazolin receipt on 90-day mortality by comparative models and sensitivity analysis for excluded participants
| Analysis type | OR (95% CI) | P value |
|---|---|---|
| Univariate analysis | 0.38 (0.10–1.44) | 0.16 |
| Smallest estimate by a 2-predictor modelj | 0.25 (0.06–1.10) | 0.067 |
| Largest estimate by a 2-predictor modelk | 0.46 (0.12–1.83) | 0.27 |
| Propensity score model 1 | 0.40 (0.09–1.74) | 0.22 |
| Propensity score model 2a | 0.36 (0.08–1.61) | 0.18 |
| 3-Predictor logistic regression model 1b | 0.21 (0.04–1.03) | 0.054 |
| 3-Predictor logistic regression model 2c | 0.50 (0.12–2.03) | 0.33 |
| 4-Predictor logistic regression modeld | 0.24 (0.04–1.29) | 0.096 |
| Sensitivity analysis 1ef | 0.32 (0.10–1.10) | 0.071 |
| Sensitivity analysis 2eg | 0.46 (0.11–1.86) | 0.28 |
| Sensitivity analysis 3eh | 0.31 (0.08–1.23) | 0.096 |
| Sensitivity analysis 4ei | 0.58 (0.14–2.40) | 0.46 |
Restricted to propensity scores overlapping the cefazolin and noncefazolin groups.
Adjusted for endocarditis, hemodialysis, and age, which produced the smallest OR.
Adjusted for endocarditis, hemodialysis, and hospital onset of MSSA BSI, which produced the largest OR.
Adjusted for age, Charlson index, alcohol abuse, and hospital onset of MSSA BSI.
Estimated using propensity score model 1.
Includes those who received <5 days of nafcillin or cefazolin.
Includes those who commenced nafcillin or cefazolin >7 days after blood culture positivity.
Includes those who received another i.v. antimicrobial of duration equal to or greater than that for cefazolin or nafcillin.
Includes those who received both cefazolin and nafcillin, with treatment group defined by first antibiotic received.
Adjusted for age.
Adjusted for hospital onset of MSSA BSI.
The effect measured when accounting for propensity score of cefazolin treatment was robust to comparative analysis, alternative direct fitted models, and sensitivity analyses (Table 3). The smallest and lowest OR estimated by a two-predictor model, including receipt of cefazolin and any single primary (a priori) or secondary potential confounder, are also presented in Table 3 and were broadly consistent with the other multivariate model results.
Given the considerable difference in antimicrobial duration between the nafcillin and cefazolin groups (Table 1), we further examined whether this difference was a confounder or mediator with a multivariate analysis including the propensity score and duration of antimicrobial (nafcillin or cefazolin). This repeat analysis still yielded a substantial reduction in the odds of death (aOR, 0.47; P = 0.35; 95% CI, 0.10 to 2.26), but this was not statistically significant.
DISCUSSION
Our findings by propensity score analysis found a 60% reduction in adjusted odds of 90-day mortality in those receiving cefazolin rather than nafcillin for MSSA BSI. The confidence interval leaves open the possibility of an effect in either direction (95% CI, 0.09 to 1.74). Comparative analyses showed similar effect sizes (estimates ranged from 50% to 79% reductions in odds of death), although they did not reach statistical significance either.
These findings agree with other observational studies that have examined outcomes of MSSA BSI treated with cefazolin against those treated with antistaphylococcal penicillins. A large multicenter study in Canada suggested a substantive reduction in the hazard of 90-day mortality in those who received cefazolin compared to cloxacillin, with borderline statistical significance (hazard ratio [HR], 0.58; 95% CI, 0.31 to 1.08; P = 0.08) (18).
With respect to other observational studies that have also examined this study question, Lee et al. examined the effect of cefazolin versus nafcillin on MSSA BSI treatment failure (16). They found little reduction in the odds of treatment failure (unadjusted OR, 0.7; 95% CI, 0.2 to 2.1; P = 0.50) in those receiving cefazolin but with very wide uncertainty. The adjusted estimate was in the opposite direction (aOR, 1.2; 95% CI 0.3 to 4.5; P = 0.76) but again with very wide uncertainty. A study by Li et al. (15) found a statistically significant difference in the comparison of the raw proportions of failures in cefazolin and oxacillin groups, 24% versus 47% (P = 0.04), but did not present a multivariate-adjusted effect size estimate or confidence interval, although choice of antimicrobial was not statistically significantly associated with treatment failure in a multivariate model (P = 0.36). Paul et al. also examined the association between receipt of cefazolin (compared to oxacillin) and death at 90 days (as measured by a national death registry) in MSSA BSI cases and found a reduction in the odds of death (aOR, 0.81) but a wide confidence interval (95% CI, 0.18 to 3.62) (17). Taken together, these comparative studies generally also suggest a beneficial effect of cefazolin over nafcillin for MSSA BSI, but none have provided strong evidence for this.
The mechanism behind the trend toward reduced mortality seen in our and some other studies is unclear, but it could be related to a higher proportion of adverse reactions and discontinuations in those receiving nafcillin compared to cefazolin, which was noted in a comparison of these two drugs when used in a North American outpatient setting (23). Indeed, the relatively high number of patients that had received both nafcillin and cefazolin (with nafcillin administered first in most cases) suggest a discontinuation of nafcillin for cefazolin after nafcillin toxicity, although this is speculative. While our study did not collect data on adverse drug reactions and reasons for drug discontinuation, this could be addressed in further observational studies examining this study question.
There are several limitations in the interpretation of the results of this study. First, there were limitations to models due to the relatively small sample size, small number of outcomes, and small number of participants receiving nafcillin alone. As such, residual confounding may have occurred. Reasons for the considerably smaller nafcillin group could include a high proportion of dialysis patients, a predominantly under- or uninsured population, and/or the overall dosing convenience of cefazolin over nafcillin. Nevertheless, our models appear relatively robust based on comparative analyses by propensity scores, examination of the effect of each individual confounder on estimates fitted by 2-predictor models, and selected models with more than 2 predictors. Second, selection biases may have been introduced by the exclusion criteria, although our findings remained similar in sensitivity analyses that varied these criteria. Third, while our study sample did include a proportion of complicated MSSA BSI similar to those of some of the comparable studies described, there was only a small number of patients with endocarditis and other deep-seated infections who may be particularly prone to inoculum effect-mediated cefazolin failure (1). The strengths of this study included a prospective design and a wide number of covariates measured, allowing extensive confounder assessment. The valid measurement of 90-day all-cause mortality by use of both a National Death Index and Social Security death master file was also crucial in making inferences about the choice of antimicrobial on mortality outcome and minimizing the risk of differential loss to follow-up.
While this study provides further support for the use of cefazolin as an option in MSSA BSI (and its interchangeability with nafcillin for this indication), there is insufficient evidence from this study to recommend cefazolin over nafcillin (or vice versa) for the treatment of MSSA BSI. A meta-analysis may be useful for pooling results to produce stronger evidence for cefazolin superiority and may at least inform randomized clinical trial design on this study question. Additionally, the possibility of improved patient outcomes with cefazolin compared to antistaphylococcal penicillins may be sufficient to justify randomized clinical trials to address this issue.
ACKNOWLEDGMENTS
S.M.B. is supported by the UCSF Traineeship in AIDS Prevention Studies (U.S. National Institutes of Health [NIH] T32 MH-19105). H.F.C. has received research funding from Cerexa, Cubist Pharmaceuticals, Astra-Zeneca, Pfizer, and Theravance. S.D. has received research funding from Merck, Genentech, Cerexa, and Cubist Pharmaceuticals.
REFERENCES
- 1.Naber CK. 2009. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 48(Suppl 4):S231–S237. doi: 10.1086/598189. [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. 2007. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect 135:1037–1042. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laupland KB. 2013. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect 19:492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 4.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhoj P. 2007. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2014. Active Bacterial Core surveillance (ABCs) list of pathogens. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/abcs/pathogens/pathogen-links.html. [Google Scholar]
- 7.Australian Government Department of Health. 2014. Staphylococcus aureus annual reports prepared by the Australian Group on Antimicrobial Resistance. Australian Government Department of Health, Canberra, Australia: http://www.health.gov.au/internet/main/publishing.nsf/content/cda-mrsa-anrep.htm. [Google Scholar]
- 8.Kock R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Navarro Torne A, Witte W, Friedrich AW. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15:19688. [DOI] [PubMed] [Google Scholar]
- 9.Coombs GW, Nimmo GR, Daly DA, Le TT, Pearson JC, Tan HL, Robinson JO, Collignon PJ, McLaws ML, Turnidge JD. 2014. Australian Staphylococcus aureus sepsis outcome programme annual report, 2013. Commun Dis Intell Q Rep 38:E309–E319. [DOI] [PubMed] [Google Scholar]
- 10.Naber CK, Baddour LM, Giamarellos-Bourboulis EJ, Gould IM, Herrmann M, Hoen B, Karchmer AW, Kobayashi Y, Kozlov RS, Lew D, Miro JM, Moellering RC Jr, Moreillon P, Peters G, Rubinstein E, Seifert H, Corey GR. 2009. Clinical consensus conference: survey on Gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin Infect Dis 48(Suppl 4):S260–S270. doi: 10.1086/598185. [DOI] [PubMed] [Google Scholar]
- 11.Yaw LK, Robinson JO, Ho KM. 2014. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis 14:967–975. doi: 10.1016/S1473-3099(14)70876-X. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert DN, Moellering RC Jr, Eliopoulos GM, Chambers HF, Saag MS (ed). 2012. The Sanford guide to antimicrobial therapy, 42nd ed Antimicrobial Therapy, Inc., Sperryville, VA. [Google Scholar]
- 13.Antibiotic Expert Groups. 2014. Therapeutic guidelines: antibiotic, version 15. Therapeutic Guidelines Limited, Melbourne, Australia. [Google Scholar]
- 14.Nannini EC, Singh KV, Arias CA, Murray BE. 24 June 2013. In vivo effect of cefazolin, daptomycin, and nafcillin in experimental endocarditis with a methicillin-susceptible Staphylococcus aureus strain showing an inoculum effect against cefazolin. Antimicrob Agents Chemother doi: 10.1128/aac.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS Jr. 2014. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 58:5117–5124. doi: 10.1128/AAC.02800-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 55:5122–5126. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z, Leibovici L, Bishara J. 2011. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 17:1581–1586. doi: 10.1111/j.1469-0691.2010.03425.x. [DOI] [PubMed] [Google Scholar]
- 18.Bai AD, Showler A, Burry L, Steinberg M, Ricciuto DR, Fernandes T, Chiu A, Raybardhan S, Science M, Fernando E, Tomlinson G, Bell CM, Morris AM. 2015. Comparative effectiveness of cefazolin versus cloxacillin as definitive antibiotic therapy for MSSA bacteraemia: results from a large multicentre cohort study. J Antimicrob Chemother 70:1539–1546. doi: 10.1093/jac/dku560. [DOI] [PubMed] [Google Scholar]
- 19.Renaud CJ, Lin X, Subramanian S, Fisher DA. 2011. High-dose cefazolin on consecutive hemodialysis in anuric patients with Staphylococcal bacteremia. Hemodial Int 15:63–68. doi: 10.1111/j.1542-4758.2010.00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, Hsueh PR, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van Pham H. 2010. Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect 61:299–306. doi: 10.1016/j.jinf.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JO, Pozzi-Langhi S, Phillips M, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ. 2012. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 31:2421–2428. doi: 10.1007/s10096-012-1585-y. [DOI] [PubMed] [Google Scholar]
- 23.Youngster I, Shenoy ES, Hooper DC, Nelson SB. 2014. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 59:369–375. doi: 10.1093/cid/ciu301. [DOI] [PMC free article] [PubMed] [Google Scholar]