Abstract
In vitro susceptibility of 933 Candida isolates, from 16 French hospitals, to micafungin was determined using the Etest in each center. All isolates were then sent to a single center for determination of MICs by the EUCAST reference method. Overall essential agreement between the two tests was 98.5% at ±2 log2 dilutions and 90.2% at ±1 log2 dilutions. Categorical agreement was 98.2%. The Etest is a valuable alternative to EUCAST for the routine determination of micafungin MICs in medical mycology laboratories.
TEXT
The echinocandin antifungal drug micafungin is highly effective in vitro against most Candida species (1–3). Micafungin is now widely used for prophylaxis and treatment of invasive candidiasis (IC) (4, 5). During the last decade, acquired resistance of various Candida species to echinocandins has emerged worldwide, including in France, and may become an important issue in the therapeutic management of IC (6–10).
In vitro antifungal susceptibility testing is currently recommended to detect resistance in Candida species and to guide antifungal treatment (6, 11). Microdilution broth methods such as those published by EUCAST and CLSI are the reference methods for antifungal susceptibility testing. Nevertheless, because these reference methods are labor-intensive and time-consuming, most clinical microbiology laboratories use commercial methods, such as the Etest, for routine determination of MICs. It is therefore essential to evaluate these commercial tests and to determine their ability to give MIC values that agree with those from the reference methods. With this aim, a prospective, multicenter French study was performed to compare the EUCAST and Etest methods for micafungin susceptibility testing of a large panel of clinical isolates of different Candida species.
(This study was presented in part at the 25th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Copenhagen, Denmark, 25 to 28 April 2015.)
Sixteen centers (6 in the Paris area and 10 elsewhere across France) participated in the study. Over a 2-month period, each center was asked to test 64 Candida isolates, from any clinical sample, of the following species: 10 isolates of each of the six most common pathogenic species (Candida albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. kefyr, and C. krusei) and four isolates belonging to other Candida species. Species identification was performed in each center according to the currently recommended phenotypic methods (12). Micafungin susceptibility testing was performed using the Etest (bioMérieux, Marcy l'Etoile, France), according to the manufacturer's instructions. Candida isolates were then sent to a single center for MIC determination by the EUCAST reference method (13). C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were included as quality control strains (14). For comparison purposes, Etest MICs were increased to the next higher corresponding EUCAST concentration (15). Resistance was based on EUCAST clinical breakpoints. When clinical breakpoints were not available (i.e., for C. krusei and C. tropicalis), epidemiological cutoff values (ECOFFs) were used to categorize isolates as non-wild-type isolates (16). The same ECOFFs (defined by EUCAST) were used for analyzing Etest results, as Etest-specific ECOFFs have not yet been determined. C. albicans, C. glabrata, and C. parapsilosis isolates were considered susceptible or resistant to micafungin when MICs were ≤0.016 or >0.016 μg/ml, ≤0.03 or >0.03 μg/ml, and ≤0.002 or >2 μg/ml, respectively. C. krusei and C. tropicalis isolates were considered wild-type isolates or non-wild-type isolates with respect to micafungin susceptibility when MICs were ≤0.25 or >0.25 μg/ml and ≤0.06 or >0.06 μg/ml, respectively. MIC results obtained by the two methods were considered to be in essential agreement when they were within ±2 log2 dilutions. Agreement at ±1 log2 dilutions was also calculated. Categorical agreement was defined as the percentage of isolates classified in the same category (i.e., as susceptible, intermediate, or resistant isolates and wild-type or non-wild-type isolates) by both techniques (15). Discrepancies (very major, major, and minor errors) were defined as described previously (15).
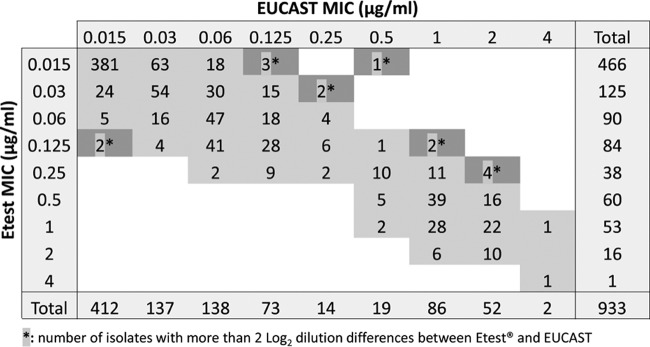
Results from antifungal susceptibility testing were available for 933 Candida isolates, including 878 isolates of the six most medically important Candida species and 55 other Candida species. Table 1 shows the micafungin MICs for the 933 isolates determined by the EUCAST reference method. Micafungin MICs for C. parapsilosis isolates (modal MIC of 1 μg/ml) were several dilutions higher than for the other common species (modal MIC of 0.015 μg/ml for C. albicans, C. tropicalis, and C. glabrata and 0.03 and 0.06 μg/ml for C. kefyr and C. krusei, respectively). MICs for rare species were similar to those of the common species except for C. colliculosa and some isolates of C. guilliermondii and C. famata. According to the current clinical breakpoints (16), the micafungin resistance rates were <2% for C. albicans and C. parapsilosis and 3.9% for C. glabrata. Based on ECOFFs, the rates for the non-wild-type isolates were 0.7% for C. tropicalis and 0% for C. krusei. The overall essential agreement between EUCAST and Etest results was high (98.5% at ±2 log2 dilutions and 90.2% at ±1 log2 dilutions) (Fig. 1), with minor differences between species (Table 2). The lowest essential agreement (96.7% at ±2 log dilutions) was observed for C. parapsilosis. An overall categorical agreement of 98.2% was observed for the 742 isolates belonging to the five species for which clinical breakpoints or ECOFFs were available (Table 3). The highest (100%) and lowest (96.7%) categorical agreements were found for C. krusei and C. glabrata, respectively. Major errors were observed in six cases (three C. albicans isolates, two C. tropicalis isolates, and one C. glabrata isolate) and very major errors in six cases (two C. albicans isolates and four C. glabrata isolates). These 12 discrepancies were observed for strains isolated and tested in eight different centers.
TABLE 1.
Distribution of micafungin MICs for different Candida species determined by the EUCAST broth microdilution method
| Species (no. of isolates) | No. of isolates with an MIC (μg/ml) of: |
% R/non-WTa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | ||
| C. albicans (159) | 157 | 1 | 1 | 1.3 | ||||||
| C. glabrata (152) | 137 | 9 | 4 | 1 | 1 | 3.9 | ||||
| C. parapsilosis (152) | 1 | 5 | 13 | 79 | 52 | 2 | 1.3 | |||
| C. tropicalis (152) | 97 | 48 | 6 | 1 | 0.7 | |||||
| C. kefyr (136) | 7 | 67 | 49 | 13 | NDb | |||||
| C. krusei (127) | 3 | 1 | 59 | 56 | 8 | 0 | ||||
| C. lusitaniae (23) | 5 | 16 | 2 | ND | ||||||
| Other Candida spp. (n = 32)c | 11 | 6 | 3 | 1 | 1 | 5 | 5 | ND | ||
| All isolates (n = 933) | 412 | 137 | 138 | 73 | 14 | 19 | 86 | 52 | 2 | |
Resistance (R) and non-wild-type susceptibility (WT) were defined based on EUCAST clinical breakpoints or ECOFFs when clinical breakpoints were not available.
ND, not determined.
Other Candida spp. included C. guilliermondii (9 isolates), C. norvegensis (5), C. inconspicua (5), C. famata (3), C. pelliculosa (2), C. lambica (2), C. sphaerica (1), C. ciferrii (1), C. catenulata (1), C. utilis (1), C. colliculosa (1), and C. nivariensis (1).
FIG 1.
Correlation between the EUCAST and Etest methods for in vitro testing of the susceptibility of 933 Candida isolates to micafungin.
TABLE 2.
In vitro susceptibilities of the 933 Candida isolates to micafungin as determined by the Etest method and EUCAST broth microdilution methoda
| Species (no. of isolates) | Etest MIC (μg/ml) |
EUCAST MIC (μg/ml) |
% essential agreement | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | GM | Range | MIC50 | MIC90 | GM | ||
| C. albicans (159) | ≤0.015–0.06 | 0.015 | 0.015 | 0.016 | ≤0.015–0.06 | 0.015 | 0.015 | 0.016 | 100 |
| C. glabrata (152) | ≤0.015–0.125 | 0.015 | 0.015 | 0.016 | ≤0.015–1 | 0.015 | 0.015 | 0.018 | 98.7 |
| C. parapsilosis (152) | 0.06–4 | 0.5 | 2 | 0.63 | ≤0.125–4 | 1 | 2 | 1.15 | 96.7 |
| C. tropicalis (152) | ≤0.015–0.5 | 0.015 | 0.03 | 0.019 | ≤0.015–1 | 0.015 | 0.03 | 0.021 | 99.3 |
| C. kefyr (136) | ≤0.015–0.25 | 0.03 | 0.125 | 0.036 | ≤0.015–0.125 | 0.03 | 0.06 | 0.044 | 97.8 |
| C. krusei (127) | ≤0.015–0.25 | 0.125 | 0.125 | 0.084 | ≤0.015–0.25 | 0.125 | 0.125 | 0.089 | 98.4 |
| Other Candida spp.b (55) | ≤0.015–1 | 0.03 | 0.25 | 0.057 | ≤0.015–1 | 0.06 | 0.5 | 0.068 | 98.2 |
| Total (933) | ≤0.015–4 | 0.03 | 0.5 | 0.046 | ≤0.015–4 | 0.03 | 1 | 0.054 | 98.5 |
GM, geometric mean. Percent essential agreement data represent ±2 log2 dilutions.
Other Candida spp. included C. lusitaniae (23 isolates), C. guilliermondii (9), C. norvegensis (5), C. inconspicua (5), C. famata (3), C. pelliculosa (2), C. lambica (2), C. sphaerica (1), C. ciferrii (1), C. catenulata (1), C. utilis (1), C. colliculosa (1), C. nivariensis (1).
TABLE 3.
Categorical agreement between the EUCAST and Etest methods for in vitro testing of susceptibility of the major pathogenic Candida species to micafungina
| Species (total no. of isolates) | Categorical agreement |
Minor error |
Major error |
Very major error |
||||
|---|---|---|---|---|---|---|---|---|
| No. of isolates | % of isolates | No. of isolates | % of isolates | No. of isolates | % of isolates | No. of isolates | % of isolates | |
| C. albicans (159) | 154 | 96.9 | 3 | 1.9 | 2 | 1.2 | ||
| C. glabrata (152) | 147 | 96.7 | 1 | 0.7 | 4 | 2.6 | ||
| C. parapsilosis (152) | 151 | 99.3 | 1 | 0.7 | 0 | 0 | 0 | 0 |
| C. tropicalis (152) | 150 | 98.7 | 2 | 1.3 | 0 | 0 | ||
| C. krusei (127) | 127 | 100 | 0 | 0 | 0 | 0 | ||
| All isolates (742) | 729 | 98.2 | 1 | 0.1 | 6 | 0.8 | 6 | 0.8 |
For both techniques, categorization of isolates as resistant or non-wild-type isolates was performed based on EUCAST endpoints (clinical breakpoints or ECOFFs when clinical breakpoints were not available).
The Etest has been used in several studies for micafungin susceptibility testing of Candida spp. (17–22), but only a few comparative studies with a reference method have been performed (17, 20–22). In one of those previous studies, Marcos-Zambrano et al. (21) tested 160 yeast isolates with both the Etest and EUCAST methods and reported an essential agreement of 90.3% at ±2 log2 dilutions (85.8% at ±1 log2 dilutions) and categorical agreement of >90%. Similarly, in another study, a comparison between Etest and CLSI methods showed an overall essential agreement of 94.7% and a categorical agreement of 97.2% (20). The ability of the Etest to detect micafungin resistance, for most of the species, has also been demonstrated previously by testing FKS mutant isolates (17, 21, 22). We enrolled 16 centers and demonstrated that the Etest gave micafungin susceptibility results that were very similar to those given by the EUCAST reference method under real-life conditions.
Taken together, our results show that the Etest is a valuable and reliable method for routine testing of the in vitro susceptibility of clinical Candida isolates to micafungin. In vitro micafungin resistance among the main Candida species isolated from clinical samples remains uncommon in France.
ACKNOWLEDGMENTS
We thank Mélanie Girard (Paris) and Nadine François (Lille) for their technical help.
This study was supported by a grant from AstellasPharma.
M.-E. Bougnoux received grants from Astellas, Gilead, and Merck and speaker's fees from Astellas and Merck. E. Dannaoui has received grants from Gilead, Ferrer, and Bio-Rad and payment for lectures from Gilead, MSD, and Schering and has been a consultant for Astellas and Innothera. I. Accoceberry received speaker's fees from Merck. A. Angoulvant received funds for speaking from Merck and for travel expenses from Astellas, Merck, and Pfizer. E. Bailly received grants from Merck. F. Botterel received grants from Astellas and speaker's fees from Merck. T. Chouaki received speaker's fees from Merck and Gilead. M. Cornet received travel grants from Gilead, Pfizer, and Merck and received remuneration for talks on behalf of Pfizer. F. Dalle received grants from Astellas, Pfizer, and Merck. A. Fekkar received funds for speaking from Merck, for consultancy from Pfizer, and for travel expenses from Astellas, Gilead, Merck, and Pfizer. J. P. Gangneux received speaker's fees from Astellas, MSD, Gilead, and Pfizer. C. Hennequin received travel grants from MSD, Astellas, Pfizer, and Gilead and speaker's fees from MSD and Astellas. Y. Le Govic received grant support from Merck. P. Le Pape is a consultant to Basilea and received grants from Astellas and Pfizer and speaker's fees from Merck and Gilead. D. Maubon received travel grants from Pfizer. B. Sendid received grant support from Astellas, Merck, and bioMérieux. J. Chandenier received grants from Astellas, Pfizer, and Merck and speaker's fees from Astellas and Pfizer. S. Chevrier, A. Datry, A. Dupuis, J. Guitard, S. Ranque, and M. Sautour declare that we have no conflict of interest.
REFERENCES
- 1.Dannaoui E, Lortholary O, Raoux D, Bougnoux ME, Galeazzi G, Lawrence C, Moissenet D, Poilane I, Hoinard D, Dromer F. 2008. Comparative in vitro activities of caspofungin and micafungin, determined using the method of the European Committee on Antimicrobial Susceptibility Testing, against yeast isolates obtained in France in 2005–2006. Antimicrob Agents Chemother 52:778–781. doi: 10.1128/AAC.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montagna MT, Lovero G, Coretti C, Martinelli D, De Giglio O, Iatta R, Balbino S, Rosato A, Caggiano G. 2015. Susceptibility to echinocandins of Candida spp. strains isolated in Italy assessed by European Committee for Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute broth microdilution methods. BMC Microbiol 15:106. doi: 10.1186/s12866-015-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Espinel-Ingroff A, Bustamante B, Canton E, Diekema DJ, Fothergill A, Fuller J, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Martin-Mazuelos E, Meis JF, Ostrosky-Zeichner L, Pelaez T, St-Germain G, Turnidge J. 2014. Multicenter study of anidulafungin and micafungin MIC distributions and epidemiological cutoff values for eight Candida species and the CLSI M27-A3 broth microdilution method. Antimicrob Agents Chemother 58:916–922. doi: 10.1128/AAC.02020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ; ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 5.Groll AH, Stergiopoulou T, Roilides E, Walsh TJ. 2005. Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin Investig Drugs 14:489–509. doi: 10.1517/13543784.14.4.489. [DOI] [PubMed] [Google Scholar]
- 6.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, Baixench MT, Bretagne S, Dromer F, Lortholary O. 2012. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis 18:86–90. doi: 10.3201/eid1801.110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fekkar A, Dannaoui E, Meyer I, Imbert S, Brossas JY, Uzunov M, Mellon G, Nguyen S, Guiller E, Caumes E, Leblond V, Mazier D, Fievet MH, Datry A. 2014. Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis 33:1489–1496. doi: 10.1007/s10096-014-2096-9. [DOI] [PubMed] [Google Scholar]
- 9.Fekkar A, Meyer I, Brossas JY, Dannaoui E, Palous M, Uzunov M, Nguyen S, Leblond V, Mazier D, Datry A. 2013. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother 57:2380–2382. doi: 10.1128/AAC.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlin DS. 2014. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs 74:1573–1585. doi: 10.1007/s40265-014-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Florl C, Richardson MD, Akova M, Bassetti M, Calandra T, Castagnola E, Cornely OA, Garbino J, Groll AH, Herbrecht R, Hope WW, Kullberg BJ, Lortholary O, Meersseman W, Petrikkos G, Roilides E, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 18(Suppl 7):9–18. doi: 10.1111/1469-0691.12038. [DOI] [PubMed] [Google Scholar]
- 12.Howell S, Hazen KC. 2011. Candida, Cryptococcus, and other yeasts of medical importance, p 1793–1821. In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 13.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuenca-Estrella M, Arendrup MC, Chryssanthou E, Dannaoui E, Lass-Florl C, Sandven P, Velegraki A, Rodriguez-Tudela JL. 2007. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect 13:1018–1022. doi: 10.1111/j.1469-0691.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 15.Dannaoui E, Paugam A, Develoux M, Chochillon C, Matheron J, Datry A, Bouges-Michel C, Bonnal C, Dromer F, Bretagne S. 2010. Comparison of antifungal MICs for yeasts obtained using the EUCAST method in a reference laboratory and the Etest in nine different hospital laboratories. Clin Microbiol Infect 16:863–869. doi: 10.1111/j.1469-0691.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 16.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW, European Committee on Antimicrobial Susceptibility Testing—Subcommittee on Antifungal Susceptibility Testing. 2014. EUCAST technical note on Candida and micafungin, anidulafungin and fluconazole. Mycoses 57:377–379. doi: 10.1111/myc.12170. [DOI] [PubMed] [Google Scholar]
- 17.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela JL, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and IsoSensitest media. Antimicrob Agents Chemother 54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axner-Elings M, Botero-Kleiven S, Jensen RH, Arendrup MC. 2011. Echinocandin susceptibility testing of Candida isolates collected during a 1-year period in Sweden. J Clin Microbiol 49:2516–2521. doi: 10.1128/JCM.00201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baixench MT, Aoun N, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S, Ramires S, Piketty C, Dannaoui E. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J Antimicrob Chemother 59:1076–1083. doi: 10.1093/jac/dkm095. [DOI] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff A, Canton E, Pelaez T, Peman J. 2011. Comparison of micafungin MICs as determined by the Clinical and Laboratory Standards Institute broth microdilution method (M27-A3 document) and Etest for Candida spp. isolates. Diagn Microbiol Infect Dis 70:54–59. doi: 10.1016/j.diagmicrobio.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Marcos-Zambrano LJ, Escribano P, Rueda C, Zaragoza O, Bouza E, Guinea J. 2013. Comparison between the EUCAST procedure and the Etest for determination of the susceptibility of Candida species isolates to micafungin. Antimicrob Agents Chemother 57:5767–5770. doi: 10.1128/AAC.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Moet GJ, Jones RN. 2010. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Etest methods with the CLSI broth microdilution method for echinocandin susceptibility testing of Candida species. J Clin Microbiol 48:1592–1599. doi: 10.1128/JCM.02445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]