Abstract
Our study aims to identify the clinical breakpoints (CBPs) of second-line drugs (SLDs) above which standard therapy fails in order to improve multidrug-resistant tuberculosis (MDR-TB) treatment. MICs of SLDs were determined for M. tuberculosis isolates cultured from 207 MDR-TB patients in a prospective cohort study in China between January 2010 and December 2012. Classification and regression tree (CART) analysis was used to identify the CBPs predictive of treatment outcome. Of the 207 MDR-TB isolates included in the present study, the proportion of isolates above the critical concentration recommended by WHO ranged from 5.3% in pyrazinamide to 62.8% in amikacin. By selecting pyrazinamide as the primary node (CBP, 18.75 mg/liter), 72.1% of sputum culture conversions at month four could be predicted. As for treatment outcome, pyrazinamide (CBP, 37.5 mg/liter) was selected as the primary node to predict 89% of the treatment success, followed by ofloxacin (CBP, 3 mg/liter), improving the predictive capacity of the primary node by 10.6%. Adjusted by identified confounders, the CART-derived pyrazinamide CBP remained the strongest predictor in the model of treatment outcome. Our findings indicate that the critical breakpoints of some second-line drugs and PZA need to be reconsidered in order to better indicate MDR-TB treatment outcome.
INTRODUCTION
Mycobacterium tuberculosis is a major public health problem worldwide (1). The emergence of multidrug-resistant (MDR) M. tuberculosis strains has complicated treatment and is associated with increased treatment failure (2). A reduction in the efficacy of second-line drugs (SLDs) against MDR tuberculosis (MDR-TB) strains with resistance to SLDs has been described in observational studies (2, 3). Subtle changes in drug susceptibility may be predictive of clinical failures, especially when the drug susceptibility testing (DST) result is at the borderline of the susceptibility range.
Susceptibility testing for M. tuberculosis is increasingly being utilized in diagnostic laboratories to guide TB treatment. However, there has been considerable debate regarding the critical concentrations used to define resistance of antituberculosis drugs (4, 5). Until now, the standard approach for identifying antibiotic susceptibility breakpoints has been the epidemiological cutoff method. This method is based on the MIC distribution of a drug, which identifies the upper 95% cutoff point on the Gaussian curve of wild-type susceptible M. tuberculosis isolates (6–8). However, Gumbo, using Monte Carlo simulations, concluded that current critical concentrations of first-line drugs were overoptimistic, and new susceptibility breakpoints should be defined considering microbiologic and clinical outcomes (4). Therefore, clinical outcome studies including MIC results are needed. The aim of this study was to identify the clinical breakpoints (CBPs) in a cohort of MDR-TB patients in China and to develop a decision tree to better predict treatment outcomes of MDR-TB patients.
MATERIALS AND METHODS
Study design.
We conducted a prospective cohort study including MDR-TB patients who visited two MDR-TB designated hospitals in China for treatment between January 2010 and December 2012. Patients were included if they had a positive acid-fast bacillus smear, were defined as MDR-TB by the DST results before receiving fluoroquinolone-containing regimens, and gave informed consent. Patients who were pregnant, were below 18 or above 65 years of age, had impaired liver or renal function, were receiving treatment with SLDs in the previous 6 months, or were infected with extensively drug-resistant M. tuberculosis strains were excluded. The patients were followed up monthly during the fluoroquinolone-containing treatment, which was given as a directly observed treatment short course (DOT).
Species identification and drug susceptibility testing.
Sputum samples were decontaminated and digested with 2% NaOH. The mixture was concentrated by centrifugation and inoculated on Lowenstein-Jensen (LJ) medium. Species identification of mycobacteria was performed by conventional biochemical tests (9).
DST for first-line anti-TB drugs was performed using the proportion method (10) on LJ medium with the following drug concentrations: isoniazid (INH), 0.2 mg/liter; rifampin (RIF), 40.0 mg/liter; streptomycin (STR), 4.0 mg/liter; and ethambutol (EMB), 2.0 mg/liter. Furthermore, MIC testing for SLDs and pyrazinamide (PZA) was performed on the mycobacterial growth indicator tube (MGIT) 960 platform according to standard manufacturer protocols. Briefly, bacterial suspensions were transferred to serially 1:2 diluted MGIT tubes with a range of 1 to 32 mg/liter for ofloxacin (OFX) and levofloxacin (LVX), a range of 0.5 to 256 mg/liter for capreomycin (CAP), amikacin (AMK), and kanamycin (KAN), and a range of 6.2 to 400 mg/liter for PZA. Control plates without any antibiotic were inoculated with 1:100 diluted bacterial suspensions. The MIC was defined as the lowest antibiotic concentration that showed less than 100 growth units when the 1:100 diluted control reached 400 growth units. Duplicates of the pansusceptible M. tuberculosis H37Rv reference strain were included in each run as an inter- and intrareplication quality control. The MIC determination was also repeated twice for 10% (n = 20) of in vitro randomly selected isolates (45% resistant to FQs and 40% resistant to second-line injective drugs) to ensure reproducibility. We used the critical concentrations for MGIT-based DST recommended by WHO guidelines, and the substances mentioned above were bought from Sigma-Aldrich (St. Louis, MO). As there were no published WHO-recommended critical concentrations for KAN DST by MGIT 960 at the time of the study, we used 2.5 mg/liter based on the existing literature (11).
Outcome definition.
Treatment outcome was evaluated by two endpoints: (i) sputum culture conversion within 4 months (early sputum culture conversion); (ii) cure or treatment completion by 2 years (treatment success) after commencement of second-line treatment. Sputum samples were cultured on LJ media by the TB laboratory in the TB designated hospital. Sputum culture conversion was defined as two consecutive negative cultures of samples taken at least 30 days apart with no subsequent recurrence of a positive culture (12). In accordance with WHO guidelines (13) and the Global Fund program protocol, cure was defined as completion of treatment with at least five consecutive negative cultures from sputum samples, collected at least 30 days apart in the last 12 months of treatment. Treatment completion was defined as treatment done with fewer than five consecutive negative cultures in the last 12 months of treatment. Second-line treatment referred to the use of a treatment regimen comprised of one or more drugs (except streptomycin) listed in groups 2 to 5 of the WHO classification (14).
CART analyses.
We utilized classification and regression tree (CART) analysis to identify the MIC threshold predictive of treatment outcome for each SLD and to develop the decision tree to predict sputum culture conversion after 4 months of treatment as well as treatment outcome. CART analysis is a nonparametric method that uses binary recursive partitioning to assign patients to homogenous groups and then presents results in the form of intuitive and easy-to-interpret decision trees (15–17). Furthermore, CART analysis has the advantage of handling missing data by identifying and using surrogate variables to minimize ascertainment bias (18). CART analysis searches through potential predictors and all possible cutoff values of the variables to identify the best predictor for classifying between patients with and without the designated outcome (i.e., sputum culture conversion after 4 months of treatment and treatment outcome). This results in an upside-down tree whose root node is the primary predictor. We utilized the Gini criterion function for splitting nodes and attaining the minimum cost tree. The resulting trees were pruned to avoid overmatching. The optimal trees were then chosen based on relative misclassification costs, complexity, and parsimony. We performed 10-fold validation of the results as previously described (5). We applied receiver operating characteristic (ROC) analysis and 10-fold cross-validation to evaluate the goodness-of-fit for each model as previously described (5). In the cross-validation, the data set was randomly split into learning and test databases, and CART analysis was performed using Salford Predictive Miner System software (San Diego, CA, USA).
Data collection and analysis.
Age, sex, history of prior TB, year of diagnosis, dispensing of TB drugs, and site of TB disease were obtained from the provincial reportable diseases registry. Information on deaths and causes was obtained from the death registration system. Furthermore, hospital medical records were reviewed to obtain additional clinical, epidemiological, treatment, and outcome data. Absent clinical outcomes were due to lack of patient examination or loss to follow-up during the treatment phase. Duration of treatment was defined as months from the first to the last antituberculosis drug dispensed. Multivariate models were reviewed for appropriateness using the Hosmer-Lemeshow goodness-of-fit test. IBM SPSS 20.0 (IBM Corp., Armonk, NY) was used to perform univariate analysis and multivariate binary logistic regression analysis.
RESULTS
Demographics and clinical characteristics.
All pulmonary TB cases were reviewed during the study period. In total, 226 patients with MDR-TB were identified during the period. Of these, six patients were immediately transferred to other hospitals and 13 had received SLDs previously. As a result, 207 diagnosed MDR-TB patients were included in the study (Table 1). The mean (± standard deviations [SD]) age was 50.1 years (±16.9), and 66.7% of the patients were male. Of the 207 studied MDR patients, 72 (34.8%) were previously treated with first-line anti-TB drugs. The most common comorbidity was cardiovascular disease (15.9%), followed by diabetes (11.1%).
TABLE 1.
Clinical and demographic factors of 207 patients treated for multidrug-resistant tuberculosis
| Characteristic | Valuea (n = 207) |
|---|---|
| Female | 69 (33.3) |
| Age, yrb | 50.1 ± 16.9 |
| Prior tuberculosis treatment | 72 (34.8) |
| Pulmonary cavity | 56 (27.1) |
| Severe pulmonary disease on CXR | 35 (16.9) |
| Extra pulmonary tuberculosis | 36 (17.4) |
| Comorbidities | 58 (28.0) |
| Cardiovascular disease | 33 (15.9) |
| Diabetes mellitus | 23 (11.1) |
Data are presented as number (percent) unless otherwise specified.
Continuous variable; means ± standard deviations are presented.
Treatment outcome.
With regard to sputum culture conversion, 121 patients (58.5%) still had a positive culture or smear after the first 4 months of treatment. As for treatment outcome of the 207 patients, 22 patients (10.6%) were lost to follow up and 68 patients (32.9%) had treatment failure (43 patients were persistently sputum smear positive, 2 patients died due to TB, and 23 patients relapsed). During the follow-up period, another four patients died, three from cardiovascular disease and one from lung cancer.
MIC distribution of the M. tuberculosis isolates for SLDs and PZA among the MDR-TB patients.
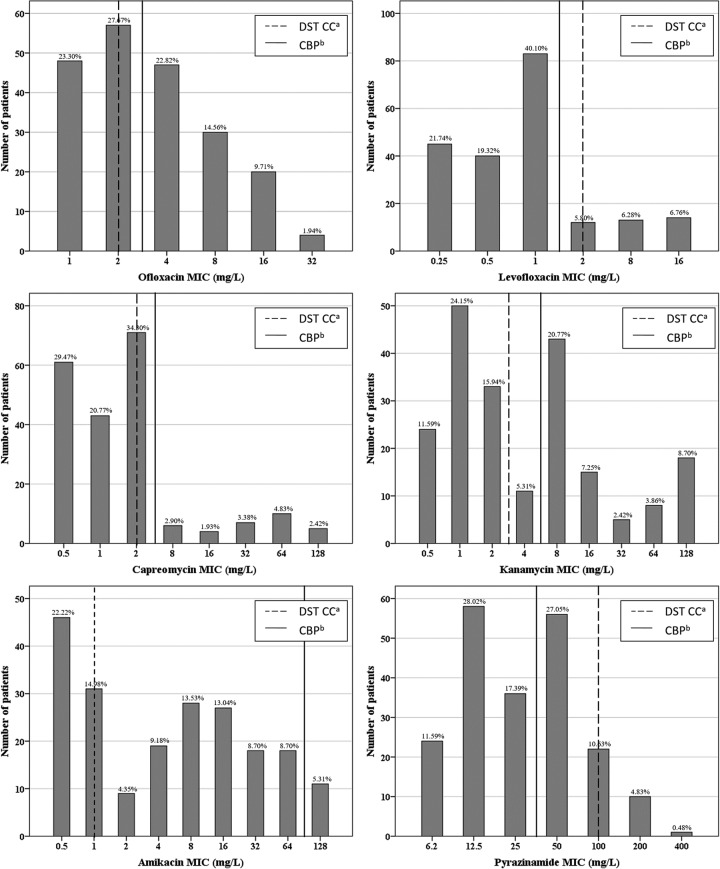
The MIC distributions for SLDs and PZA are presented in Fig. 1. The median OFX and LVX MICs were 2 mg/liter (range, 1 to 32 mg/liter) and 1 mg/liter (range, 0.25 to 16 mg/liter), respectively, with 49.0% and 13.0% of the M. tuberculosis isolates above the respective DST critical concentrations of 2 mg/liter for OFX and 2 mg/liter for LVX. As for the injectable drugs, the median MICs were 2 mg/liter (0.5 to 128 mg/liter) for KAN, 4 mg/liter (0.5 to 128 mg/liter) for AMK, and 1 mg/liter (0.5 to 128 mg/liter) for CAP, while 48.3%, 62.8%, and 15.5% of the M. tuberculosis isolates were above the DST critical concentrations for KAN (2.5 mg/liter), AMK (1 mg/liter), and CAP (2.5 mg/liter), respectively. For PZA, the median MIC was 25 mg/liter (6.2 to 400 mg/liter), and 5.3% of M. tuberculosis isolates were above the DST critical concentration of 100 mg/liter.
FIG 1.
MIC distribution of second-line drugs for M. tuberculosis isolates from 207 MDR-TB patients. The Gaussian distribution is skewed toward the right and is different from the MIC distribution summarized by EUCAST. Footnotes: a, the critical concentration of DST recommended by WHO except kanamycin, which was derived from existing literature (11); b, the suggested clinical breakpoints derived by CART analysis.
Our tentative CBPs with respect to sputum culture conversion by 4 months and treatment outcome are summarized in Table 2. The CBPs for OFX (3 mg/liter), LVX (1.5 mg/liter), and CAP (5 mg/liter) were close to WHO-recommended DST critical concentrations, while the CBP for PZA (37.5 mg/liter) in our study was lower than the WHO-recommended DST critical concentration.
TABLE 2.
CART analysis-derived MIC clinical breakpoints for M. tuberculosis isolates from 207 MDR-TB patientsa
| 2nd drug | Sputum culture conversion after 4 mo |
Treatment success |
||||
|---|---|---|---|---|---|---|
| MIC CBP (mg/liter) | Se | Sp | MIC CBP (mg/liter) | Se | Sp | |
| Pyrazinamide | 18.75 | 0.721 | 0.835 | 37.5 | 0.889 | 0.926 |
| Ofloxacin | 3 | 0.616 | 0.567 | 3 | 0.650 | 0.627 |
| Levofloxacin | 5 | 0.930 | 0.174 | 1.5 | 0.906 | 0.265 |
| Capreomycin | 48 | 0.977 | 0.107 | 5 | 0.880 | 0.250 |
| Amikacin | 3 | 0.523 | 0.661 | 96 | 0.983 | 0.118 |
| Kanamycin | 96 | 0.977 | 0.132 | 6 | 0.658 | 0.618 |
Se, sensitivity; Sp, specificity.
Identification of MIC decision tree to predict treatment outcome.
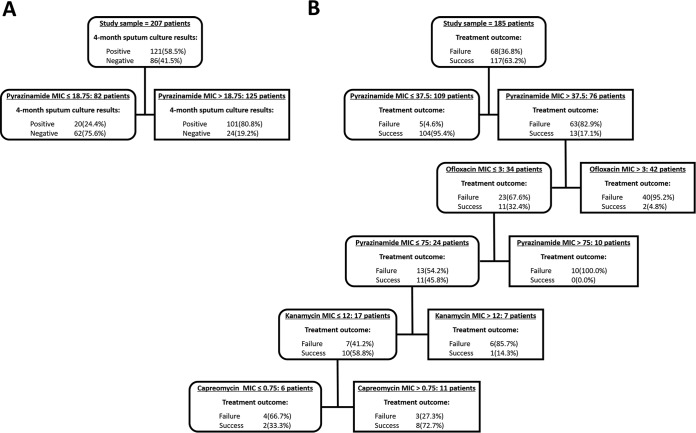
Since combination therapy is used for MDR-TB treatment, we included all of the MIC values of SLDs and PZA to illustrate a decision tree to predict treatment outcome (Fig. 2). By selecting PZA as the primary node (CBP, 18.75 mg/liter), 72.1% of the sputum culture conversion at month four could be correctly predicted. As for the treatment outcome, pyrazinamide was selected as the primary node (CBP, 37.5 mg/liter) to predict 89% of patients with treatment success, followed by OFX (CBP, 3 mg/liter) improving the predictive capacity of the primary node by 10.6%. The strongest predictor in treatment outcome of MDR-TB was a MIC of 37.5 mg/liter for PZA with a variable importance of 100.0%, while the variable importance of the second node of OFX was just 9.1%. Overall, the decision tree was capable of predicting 83.5% of the failure to sputum culture conversion by month four and predicting 85.3% of the treatment failure, with a specificity of 72.1% and 94.9%, respectively. Based on the test samples, the ROC scores of short-term and long-term treatment efficacy in the CART model were 0.777 and 0.946, respectively.
FIG 2.
Variables predictive of sputum culture conversion after 4 months of treatment (A) and long-term treatment outcome (B) in 207 and 185 MDR-TB patients, respectively. MICs of second-line anti-TB drugs and confounders were examined in the classification and regression trees. In tree A, the MIC of pyrazinamide (18.75 mg/liter) was the best predictor of sputum culture conversion after 4 months of treatment. Furthermore, 75.6% of patients with a pyrazinamide MIC below the threshold were sputum negative after 4 months of treatment. In tree B, the decision nodes demonstrate that the primary node was the MIC of pyrazinamide (37.5 mg/liter), followed by the ofloxacin MIC. The MIC cutoff values that identified as important predictive factors are shown. In those who had a pyrazinamide MIC below the threshold (37.5 mg/liter), only 4.6% failed the treatment.
Evaluation of decision tree in a multivariate model of MDR-TB treatment outcome.
Since several factors affect treatment outcome, we compared the distribution of these factors between patients with M. tuberculosis isolates with an MIC above our CART analysis-derived breakpoints and those below (see Table S1 in the supplemental material). The two groups of patients had very similar risk factors, except for pulmonary cavities and severe pulmonary disease on chest X-ray (CXR), which were more frequent in the group of patients with M. tuberculosis isolates with higher MICs. After adjusting for these variables, any differences in the sputum culture conversion by 4 months and treatment outcome can be attributed to the CART analysis-derived susceptibility breakpoints.
Adjusted for cavity and severe pulmonary disease on CXR in the binary logistical regression model and comparing the groups of patients with M. tuberculosis isolates with MICs higher and lower than the CART-derived breakpoints (Table 3), the CBPs of PZA (odds ratio [OR], 0.07; 95% confidence interval [CI], 0.04 to 0.15), OFX (OR, 0.48; 95% CI, 0.27 to 0.84), and AMK (OR, 0.46; 95% CI, 0.26 to 0.81) were the most statistically significant indicators for sputum culture conversion after 4 months of treatment. The association between the CBPs of PZA, OFX, LVX, KAN, and MDR-TB treatment success was strong, and the odds ratios (95% CI) for these drugs were 0.01 (0.003 to 0.03), 0.30 (0.15 to 0.57), 0.30 (0.13 to 0.69), and 0.29 (0.15 to 0.55), respectively. Furthermore, the CART decision tree remained the strongest indicator for 4-month-treatment sputum conversion (OR, 0.07; 95% CI, 0.04 to 0.15) as well as treatment success (OR, 0.01; 95% CI, 0.002 to 0.02). Additionally, M. tuberculosis isolates with MICs below the DST critical concentrations for PZA, OFX, and AMK were associated with a higher rate of sputum culture conversion after 4 months of treatment and treatment success, although the association was weaker than our suggested CBPs. Furthermore, the DST critical concentrations of CAP and KAN were more likely to predict sputum culture conversion after 4 months of treatment than the treatment outcome.
TABLE 3.
Univariate and multivariate analysis of factors associated with multidrug-resistant tuberculosis treatment outcome
| Drug DST CC or CBP | Univariate logistic regression analysis |
Multivariate logistic regression analysisa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sputum culture conversion after 4 mo |
Treatment success |
Sputum culture conversion after 4 mo |
Treatment success |
|||||||||
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | |
| CART Model | 0.000 | 0.08 | 0.04–0.15 | 0.000 | 0.01 | 0.002–0.02 | 0.000 | 0.07 | 0.04–0.15 | 0.000 | 0.01 | 0.002–0.02 |
| PZA CBP | 0.000 | 0.08 | 0.04–0.15 | 0.000 | 0.01 | 0.003–0.03 | 0.000 | 0.07 | 0.04–0.15 | 0.000 | 0.01 | 0.003–0.03 |
| PZA DST CC | 0.000 | 0.32 | 0.18–0.58 | 0.000 | 0.24 | 0.13–0.45 | 0.000 | 0.32 | 0.18–0.59 | 0.000 | 0.23 | 0.12–0.45 |
| OFX CBP | 0.010 | 0.48 | 0.27–0.84 | 0.000 | 0.32 | 0.17–0.60 | 0.010 | 0.48 | 0.27–0.84 | 0.000 | 0.30 | 0.15–0.57 |
| OFX DST CC | 0.003 | 0.27 | 0.11–0.65 | 0.001 | 0.24 | 0.10–0.56 | 0.003 | 0.27 | 0.11–0.65 | 0.001 | 0.23 | 0.10–0.55 |
| LVX CBP | 0.034 | 0.36 | 0.14–0.93 | 0.003 | 0.29 | 0.13–0.66 | 0.032 | 0.35 | 0.13–0.91 | 0.005 | 0.30 | 0.13–0.69 |
| LVX DST CC | 0.275 | 0.57 | 0.21–1.56 | 0.388 | 1.81 | 0.47–6.91 | 0.262 | 0.56 | 0.21–1.54 | 0.554 | 1.51 | 0.38–5.98 |
| CAP CBP | 0.036 | 0.20 | 0.04–0.90 | 0.025 | 0.41 | 0.19–0.89 | 0.037 | 0.20 | 0.04–0.90 | 0.015 | 0.36 | 0.16–0.82 |
| CAP DST CC | 0.004 | 0.11 | 0.03–0.50 | 0.949 | 0.97 | 0.34–2.79 | 0.003 | 0.11 | 0.03–0.48 | 0.657 | 0.78 | 0.26–2.32 |
| KAN CBP | 0.015 | 0.16 | 0.04–0.70 | 0.000 | 0.32 | 0.17–0.60 | 0.015 | 0.16 | 0.04–0.69 | 0.000 | 0.29 | 0.15–0.55 |
| KAN DST CC | 0.002 | 0.26 | 0.12–0.60 | 0.173 | 0.61 | 0.30–1.24 | 0.001 | 0.25 | 0.11–0.59 | 0.071 | 0.50 | 0.24–1.06 |
| AMK CBP | 0.008 | 0.47 | 0.27–0.82 | 0.012 | 0.13 | 0.03–0.63 | 0.008 | 0.46 | 0.26–0.81 | 0.019 | 0.15 | 0.03–0.73 |
| AMK DST CC | 0.006 | 0.31 | 0.14–0.72 | 0.048 | 0.47 | 0.22–0.99 | 0.006 | 0.31 | 0.13–0.71 | 0.021 | 0.40 | 0.18–0.87 |
Adjusted for the presence of pulmonary cavity and severe pulmonary disease on CXR.
Other factors associated with treatment outcome.
The frequency of elderly patients (≥65 years) in the treatment failure group and the treatment success group was not significantly different (22.1% versus 21.4%; P = 0.912). Patients with previous treatment history were more frequent in the treatment failure group than in the treatment success group, and this was statistically significant (42 [61.8%] versus 29 [24.8%]; P = 0.000). Furthermore, the severity of the disease was also significantly associated with treatment failure.
DISCUSSION
Susceptibility testing is an important guide for clinicians to evaluate the patient's likely response to a particular drug (22). The critical concentration of SLDs recommended by the WHO are based on wild-type, susceptible M. tuberculosis isolates. However, our research indicates that the MIC distribution of MDR-TB isolates shows disparity with the MIC distribution summarized by EUCAST (Fig. 1). These differences may be caused by phenotypic characteristics or genetic mutations in MDR-TB isolates. Considering the low cure rates of MDR-TB patients, guiding treatment merely based on efficacy of bacterial killing may not be sufficient. Therefore, we performed CART analyses to identify tentative CBPs of SLDs and develop a MIC decision tree to better predict sputum culture conversion after 4 months of treatment as well as the treatment outcome.
We propose that the CBPs of some of the SLDs, as well as PZA, should be considered to be lower than the current standards. In China, MDR-TB treatment is guided by DST results. However, the current DST critical concentration is selected based on epidemiological cutoff value (ECOFF) (5), which is used to define microbiological resistance. As indicated in our study, the MIC above which therapeutic failure occurs is not necessarily linked to the ECOFF derived from the MIC distribution. As previous authors suggested (19), the DST critical concentration of PZA (100 mg/liter) needs to be lowered, and we also propose the inclusion of an intermediate category showing MICs at 64 to 128 mg/liter. In our study, the CBPs for LVX and PZA were close to the mean MIC; thus, half of the patients in our current study would be considered to have isolates with LVX or PZA clinical resistance. Moreover, our proposed breakpoints are based on the failure of patients to respond to therapy and therefore are not defined by chromosomal mutations in the classic resistance genes, as is the case with Gene-Xpert or line probe assays, such as GenoType MTBDRplus and MTBDRsl (Hain Lifescience, Nehren, Germany). Not all drug resistance is due to mutations; the MICs for some M. tuberculosis isolates are naturally high, while other mechanisms of drug resistance, such as efflux pump induction, could also lead to drug resistance (20, 21). Our results suggest that adjustment of critical concentrations of some SLDs should be considered to better guide MDR-TB treatment.
We assessed whether CBPs derived by CART analysis can improve predictive accuracy to better guide MDR-TB treatment. In Fig. 1, the CBP of AMK derived by CART analysis was 96 mg/liter while the ECOFF was much lower, 1.0 mg/liter. The predictive sensitivity and specificity of the CBP for AMK was 98.3% and 11.8%, respectively. In other words, 98.3% of the treatment success, and only 11.8% of the treatment failure, can be predicted correctly. Therefore, the clinical significance of the CBP of AMK deserves further demonstration, especially in local settings. The CBP of PZA showed excellent accuracy (sensitivity, 88.9%; specificity, 92.6%) with more potential clinical significance than other SLDs, including LVX, CAP, AMK, and KAN. However, MDR-TB treatment consists of four to five effective drugs, so the decision tree based on all SLDs may be more useful than those based on a single drug.
In our study, the decision tree based on PZA and/or MICs of SLDs had excellent predictive accuracy of clinical outcomes, both after 4 months treatment and at the end of MDR-TB treatment (Fig. 2). This relationship has been shown in previous studies (2, 22). In addition, there were no differences in important variables related to treatment outcome, except for the higher frequency of pulmonary cavities and severe pulmonary disease on CXR in the group of patients with MICs above the CART-derived breakpoints. After adjustment for these variables, the treatment outcome of MDR-TB is influenced mainly by the drug susceptibilities of the M. tuberculosis isolate. In vitro data have suggested that PZA or FQs are less active against M. tuberculosis strains with higher MICs (23, 24). Strains with high MICs of a drug might have a thicker cell wall, which could cause a suboptimal response to the drug (25). Although high drug MICs in M. tuberculosis isolates have been related to previous exposure to these drugs (26), none of the patients with high MICs in our study had been exposed to SLDs in the previous 6 months. This emphasizes the necessity of adequate management of these patients. As expected, clinical manifestations, like the presence of pulmonary cavity and severe pulmonary disease on CXR, were associated with treatment outcome. Despite the importance of these variables, the relationship between high MIC for PZA and negative treatment outcome remained in the multivariate model. Therefore, as with many other pathogens, such as standard Gram-negative and Gram-positive bacteria, the MICs of antituberculosis drugs and their interactions could affect clinical outcome of MDR-TB.
Causal inferences between the MICs of the SLDs and worse treatment outcomes should be made with caution. Although drug resistance requires modification of the drug regimen and is known to be associated with worse treatment outcomes, other factors, such as nonadherence, medical comorbidities, and pharmacokinetic variability, also contribute to poor outcome of tuberculosis treatment (2, 27–29). Several factors showed at least a weak association with early sputum culture conversion or treatment outcome. In a univariate analysis, we found that prior TB treatment was significantly associated with both the failure to convert on sputum culture by 4 months of treatment and poor treatment outcome, highlighting the importance of appropriate treatment for MDR-TB. The relationship between the previous TB treatment and poor treatment outcome had been confirmed already in a meta-analysis (2). Additionally, indicators of disease severity, such as cavity and severe pulmonary disease on CXR, increase the risk of poor treatment outcome by inadequate penetration of the drug into the most diseased tissue due to the damaged lung parenchyma (30–33). These factors might also need to be taken into account when predicting treatment outcomes of MDR-TB.
Our study has some limitations. First, the relatively small sample size could limit the generalizability of the findings. However, CART has been able to correctly identify thresholds with similarly small populations in the past (19). Second, several other clinical factors also determine clinical outcomes, such as the presence of pulmonary cavities. However, these factors do not exclude a role for MICs in outcome prediction. Indeed, our CART analysis also examined some other possible predictors, but they were outranked by MICs. Third, one potential limitation of CART analysis is fitting and biasing toward covariates with many possible splits. Thus, our findings should be taken with these factors in mind. Nevertheless, cross-validation identified the same MIC thresholds, which were virtually identical to Monte Carlo simulation results published in recent years (4). Another limitation is the absence of pharmacokinetics-pharmacodynamics (PK/PD) data in our study. Measuring drug concentrations is not the standard of care in most medical facilities in China. However, population-based study design reduces the possible bias from the PK/PD variables to some degree. We have an ongoing prospective clinical study where PK/PD data as well as MIC distributions will be available and the clinical significance of these variables in MDR-TB treatment can be determined.
Conclusions.
This study revealed that MDR-TB patients infected by an M. tuberculosis isolate with higher MIC (especially for PZA and FQs) compared to patients whose isolates had lower MICs had increased risk of negative treatment outcome. These results suggest that the critical concentration of PZA could be reconsidered. In addition, the use of a MIC decision tree might have significance in guiding MDR-TB treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank the health staff in collaborative district CDCs for collecting data and microbiological laboratory work. We thank Brian Davies for language correction.
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (principal investigator [PI], Y.H., no. 81373063), TDR small grant (PI, Y.H., no. PO201377850), and the Swedish-Chinese VR-NSFC joint program (PI, B.X. and S.H., no. 81361138019).
We have no conflict of interest to declare.
Funding Statement
This work, including the efforts of Sven Hoffner and Biao Xu, was funded together by the Swedish-Chinese VR-NSFC joint program from Svenska Forskiningsradet Formas (Swedish Research Council Formas) and National Natural Science Foundation of China (NSFC) (81361138019).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03008-15.
REFERENCES
- 1.Zumla A, George A, Sharma V, Herbert RH, Oxley A, Oliver M. 2015. The WHO 2014 global tuberculosis report–further to go. Lancet Glob Health 3:e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Bastos ML, Hussain H, Weyer K, Garcia-Garcia L, Leimane V, Leung CC, Narita M, Pena JM, Ponce-de-Leon A, Seung KJ, Shean K, Sifuentes-Osornio J, Van der Walt M, Van der Werf TS, Yew WW, Menzies D, Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. 2014. Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: an individual patient data meta-analysis. Clin Infect Dis 59:1364–1374. doi: 10.1093/cid/ciu619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong-Min W, Xiao-Hong Z. 2015. Association of drug susceptibility testing results for first- and second-line drugs with treatment outcomes in patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis 60:1285–1286. doi: 10.1093/cid/ciu1169. [DOI] [PubMed] [Google Scholar]
- 4.Gumbo T. 2010. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 54:1484–1491. doi: 10.1128/AAC.01474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. 2014. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 58:6111–6115. doi: 10.1128/AAC.03549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werngren J, Sturegard E, Jureen P, Angeby K, Hoffner S, Schon T. 2012. Reevaluation of the critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against pyrazinamide using wild-type MIC distributions and pncA gene sequencing. Antimicrob Agents Chemother 56:1253–1257. doi: 10.1128/AAC.05894-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schon T, Jureen P, Chryssanthou E, Giske CG, Sturegard E, Kahlmeter G, Hoffner S, Angeby KA. 2011. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int J Tuberc Lung Dis 15:502–509. doi: 10.5588/ijtld.10.0238. [DOI] [PubMed] [Google Scholar]
- 8.Angeby KA, Jureen P, Giske CG, Chryssanthou E, Sturegard E, Nordvall M, Johansson AG, Werngren J, Kahlmeter G, Hoffner SE, Schon T. 2010. Wild-type MIC distributions of four fluoroquinolones active against Mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamic data. J Antimicrob Chemother 65:946–952. doi: 10.1093/jac/dkq091. [DOI] [PubMed] [Google Scholar]
- 9.Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, Mahler HT, Meissner G, Mitchison DA, Sula L. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ 29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 10.Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory. U.S. Department of Health and Human Services/Public Health Service/Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 11.Rodrigues C, Jani J, Shenai S, Thakkar P, Siddiqi S, Mehta A. 2008. Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the Bactec MGIT 960 system. Int J Tuberc Lung Dis 12:1449–1455. [PubMed] [Google Scholar]
- 12.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HS, Alarcon E, Cegielski JP, Grzemska M, Gupta R, Espinal M. 2005. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 9:640–645. [PubMed] [Google Scholar]
- 13.Anonymous. 2013. Definitions and reporting framework for tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.Anonymous. 2010. Treatment of tuberculosis, Guidelines for National Programmes, 4th ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 15.Grajski KA, Breiman L, Viana Di Prisco G, Freeman WJ. 1986. Classification of EEG spatial patterns with a tree-structured methodology: CART. IEEE Trans Biomed Eng 33:1076–1086. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D, Colla P. 1995. CART: tree-structured non-parametric data analysis. Salford Systems, San Diego, CA. [Google Scholar]
- 17.Breiman L, Friedman JH, Olshen RA, Stone CJ. 1984. Classification and regression trees. Chapman & Hall/CRC, Washington, DC. [Google Scholar]
- 18.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumbo T, Chigutsa E, Pasipanodya J, Visser M, van Helden PD, Sirgel FA, McIlleron H. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 69:2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasipanodya JG, Gumbo T. 2011. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr Opin Pharmacol 11:457–463. doi: 10.1016/j.coph.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmalstieg AM, Srivastava S, Belkaya S, Deshpande D, Meek C, Leff R, van Oers NS, Gumbo T. 2012. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 56:4806–4815. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen CM, Kurbatova EV, Tupasi T, Caoili JC, Van Der Walt M, Kvasnovsky C, Yagui M, Bayona J, Contreras C, Leimane V, Ershova J, Via LE, Kim H, Akksilp S, Kazennyy BY, Volchenkov GV, Jou R, Kliiman K, Demikhova OV, Vasilyeva IA, Dalton T, Cegielski JP. 2015. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med 12:e1001932. doi: 10.1371/journal.pmed.1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosset J, Almeida D, Converse PJ, Tyagi S, Li SY, Ammerman NC, Pym AS, Wallengren K, Hafner R, Lalloo U, Swindells S, Bishai WR. 2012. Modeling early bactericidal activity in murine tuberculosis provides insights into the activity of isoniazid and pyrazinamide. Proc Natl Acad Sci U S A 109:15001–15005. doi: 10.1073/pnas.1203636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarlier V, Nikaido H. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett 123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 26.Ho J, Jelfs P, Sintchenko V. 2014. Fluoroquinolone resistance in non-multidrug-resistant tuberculosis-a surveillance study in New South Wales, Australia, and a review of global resistance rates. Int J Infect Dis 26:149–153. doi: 10.1016/j.ijid.2014.03.1388. [DOI] [PubMed] [Google Scholar]
- 27.Jain K, Desai M, Solanki R, Dikshit RK. 2014. Treatment outcome of standardized regimen in patients with multidrug resistant tuberculosis. J Pharmacol Pharmacother 5:145–149. doi: 10.4103/0976-500X.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marais E, Mlambo CK, Lewis JJ, Rastogi N, Zozio T, Grobusch MP, Duse A, Victor T, Warren RW. 2014. Treatment outcomes of multidrug-resistant tuberculosis patients in Gauteng, South Africa. Infection 42:405–413. doi: 10.1007/s15010-013-0572-2. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unsal E, Guler M, Ofluoglu R, Capan N, Cimen F. 2013. Factors associated with treatment outcome in 64 HIV negative patients with multidrug resistant tuberculosis. J Thorac Dis 5:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie B, Yang Y, He W, Xie D, Jiang G. 2013. Pulmonary resection in the treatment of 43 patients with well-localized, cavitary pulmonary multidrug-resistant tuberculosis in Shanghai. Interact Cardiovasc Thorac Surg 17:455–459. doi: 10.1093/icvts/ivt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhingra VK, Rajpal S, Mittal A, Hanif M. 2008. Outcome of multi-drug resistant tuberculosis cases treated by individualized regimens at a tertiary level clinic. Indian J Tuberc 55:15–21. [PubMed] [Google Scholar]
- 33.Choi H, Lee M, Chen RY, Kim Y, Yoon S, Joh JS, Park SK, Dodd LE, Lee J, Song T, Cai Y, Goldfeder LC, Via LE, Carroll MW, Barry CE III, Cho SN. 2014. Predictors of pulmonary tuberculosis treatment outcomes in South Korea: a prospective cohort study, 2005-2012. BMC Infect Dis 14:360. doi: 10.1186/1471-2334-14-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.