Abstract
The apicomplexan parasites that cause malaria and babesiosis invade and proliferate within erythrocytes. To assess the potential for common antiparasitic treatments, we measured the sensitivities of multiple species of Plasmodium and Babesia parasites to the chemically diverse collection of antimalarial compounds in the Malaria Box library. We observed that these parasites share sensitivities to a large fraction of the same inhibitors and we identified compounds with strong babesiacidal activity.
TEXT
The apicomplexan phylum of eukaryotic microbial parasites is important in human and veterinary medicine. Apicomplexans cause malaria (Plasmodium spp.), babesiosis (Babesia spp.), toxoplasmosis (Toxoplasma gondii), and cryptosporidiosis (Cryptosporidium spp.), among other diseases. The Plasmodium and Babesia genera are relatively closely related among the apicomplexans (1) (last common ancestor, ∼55 million years ago [2]) (Fig. 1A) and share similar features in their biology, including mechanisms for host cell invasion and metabolism (3–6). Both Plasmodium and Babesia spp. are pathogenic during the stage of infection when parasites colonize host erythrocytes. Historically, drug development has focused more strongly on inhibitors for Plasmodium sp. parasites (7). Researchers have found that some antimalarial drugs also reduce proliferation of Babesia sp. parasites in erythrocytes as well (8, 9). The antimalarial atovaquone, a ubiquinone analog, is the preferred clinical treatment for human babesiosis in combination with azithromycin (10) and is used also in veterinary practice for babesiosis in dogs (11).
FIG 1.
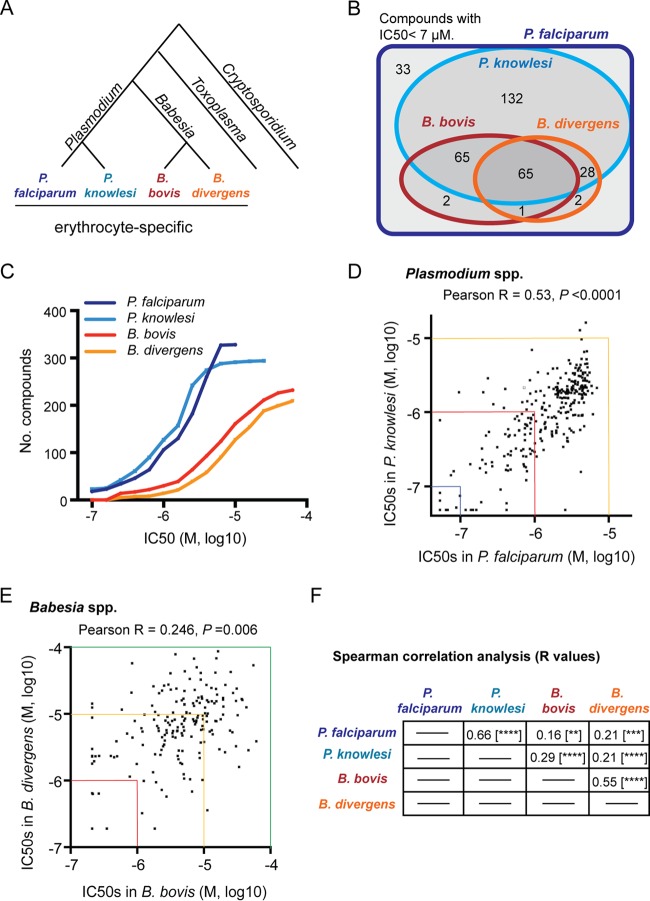
Comparative chemosensitivity analysis of Plasmodium and Babesia parasite species with the Malaria Box inhibitors. (A) Phylogeny of selected genera of apicomplexan parasites, including Plasmodium, Babesia, Toxoplasma, and Cryptosporidium (31). The erythrocyte-specific Plasmodium and Babesia species examined in this study are indicated. (B) Venn diagram summarizing the species specificity of Malaria Box compounds against the Plasmodium and Babesia parasite species tested. The number of compounds with an IC50 of <7 μM in each category is indicated. (C) For each of the Plasmodium and Babesia parasite species tested, the number of Malaria Box compounds with an IC50 value less than or equal to the indicated values on the x axis is shown. (D) Scatter plot comparing the IC50 values for Malaria Box compounds in P. falciparum (x axis) to P. knowlesi (y axis). Pearson's r and P values are shown (n = 294). (E) Scatter plot comparing the IC50 values for Malaria Box compounds in B. bovis (x axis) to B. divergens (y axis). Pearson's r and P values are shown (n = 190). In panels D and E, the axes are colored at specific IC50 values to permit comparison of scale between the two plots. (F) Summary of all Spearman correlation-based analyses between the parasite species tested. All 328 small molecules found to be inhibitory toward P. falciparum growth were included for each analysis. r values are indicated. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
A renewed focus on malaria eradication has led to the identification of an unprecedented number of bioactive compounds that block proliferation of Plasmodium falciparum in erythrocytes (12). In 2011, the nonprofit group Medicines for Malaria Venture (MMV) made available to the research community the Malaria Box, a collection of 400 chemically diverse, previously uncharacterized blood-stage antimalarials (13). Researchers have screened the antiparasitic activities of the Malaria Box compounds in nonerythrocytic host cells for the apicomplexans T. gondii, Cryptosporidium parvum, and Theileria annulata and identified a limited number of inhibitors (<3% of the library) active against each of these species (14–16). Here, we measured the susceptibilities of multiple blood-stage Plasmodium and Babesia parasite species to the Malaria Box compounds and found that erythrocyte-specific apicomplexans share considerable chemical sensitivities during the clinically relevant stages of parasitic infection.
To determine the species-specific action of the Malaria Box compounds, we measured the chemical susceptibility of Plasmodium knowlesi in parallel with the reference species P. falciparum (13) (see Dataset S1 in the supplemental material). Endemic to macaque monkeys in southeast Asia and an emerging zoonosis in humans, P. knowlesi is distinguished from P. falciparum by its shorter blood-stage cell cycle and reduced rate of parasite multiplication per cycle (17). Additionally, P. knowlesi is closely related to the second most important human malaria parasite, Plasmodium vivax, for which it is a useful experimental model parasite (18). We used a metabolic assay to measure biosynthetic incorporation of 3H-labeled hypoxanthine and parasite growth in the presence of Malaria Box compounds (19) and observed that 90% of inhibitors active against P. falciparum are also active against a human erythrocyte-adapted line of P. knowlesi (Fig. 1B). For 72 Malaria Box compounds, we observed limited or negligible activity against P. falciparum, and these molecules were excluded from all analyses. Compounds active against both P. falciparum and P. knowlesi exhibited similar well-correlated 50% inhibitory concentration (IC50) values up to ∼7 μM (Pearson's r = 0.53), with both species exhibiting sensitivity to ∼30% to 40% of the small molecules at submicromolar IC50 values (Fig. 1C and D). These results argue strongly that the majority of Malaria Box inhibitors are directed toward well-conserved targets in the blood stages of infection by divergent Plasmodium species.
To determine the efficacy of the Malaria Box inhibitors toward the Babesia parasite spp., we measured the chemical susceptibilities of the parasite species Babesia bovis and Babesia divergens growing in erythrocytes (see Data Set S1 in the supplemental material). Both species are cow parasites and cause major economic losses in the livestock industry in various parts of the world (20, 21). B. divergens occasionally causes severe zoonotic infections in splenectomized individuals (20). We used the [3H]hypoxanthine assay to measure growth of B. bovis in bovine erythrocytes and B. divergens in human erythrocytes (8, 22, 23). Of the 328 Malaria Box compounds that inhibit P. falciparum with an IC50 value of <7 μM, we observed that 65, or ∼20% of the total, inhibit growth of both B. bovis and B. divergens with an IC50 of <7 μM. An additional 65 molecules inhibit B. bovis selectively, and 28 molecules inhibit B. divergens selectively, perhaps reflecting species-specific differences between the Babesia species. Growth inhibition by the Malaria Box compounds administered at a single concentration (5 μM) is reproducible for either Babesia species (see Fig. S1A and B in the supplemental material).
Both Babesia parasite species tested are less sensitive than the Plasmodium species to the Malaria Box inhibitors, with blood-stage growth of either B. bovis or B. divergens sensitive to <10% of the small molecules at a submicromolar IC50 value compared to ∼30% to 40% sensitivity for the Plasmodium species (Fig. 1C). At an IC50 of <25 μM, each Babesia species is susceptible to ∼60% to 70% of the Plasmodium-active molecules. The lower sensitivities of the Babesia species to inhibitors compared to those of the Plasmodium species suggest variation in general features of these parasites (e.g., solute permeability of infected erythrocytes) (24). Significant correlation in the susceptibilities of B. bovis and B. divergens to Malaria Box compounds (Pearson's r = 0.246) (Fig. 1E) suggests frequent activity of these molecules against targets conserved within the Babesia genus. Additionally, the high number of Malaria Box compounds with inhibitory activity against all apicomplexan species tested (Fig. 1B and C) and significant correlation in the potencies of the compounds between the Plasmodium and Babesia parasite species (Fig. 1F) suggest targeting of features of blood-stage parasite biology common to the Plasmodium and Babesia genera.
To confirm the activities and determine the potencies of select babesiacidal Malaria Box compounds identified in our screen, we purchased nine compounds from commercial vendors and conducted dose-response susceptibility assays (Table 1). The compounds include imidocarb (Malaria Box compound MMV665810), which is used for treatment of babesiosis in livestock (20). We tested these small molecules in P. falciparum proliferating in human erythrocytes, B. bovis in cow erythrocytes, and B. divergens in both human and cow erythrocytes. Imidocarb exhibited IC50 values of 230 to 690 nM against the Babesia parasite species, and we observed IC50 values ranging from 30 nM to 2.4 μM for the other Malaria Box compounds in the Babesia species. In comparison, atovaquone demonstrated IC50 values of 12 to 32 nM in the Babesia species. Consistent with our primary screening data, the compounds are typically severalfold more potent against P. falciparum than against the Babesia species. Many of the compounds we tested exhibit IC90 values in all parasite species 4-fold or more lower than published IC50 values for inhibition of a human cell line (25) and do not violate the Lipinski rule of five parameters for the prediction of drug-like pharmacokinetics (13).
TABLE 1.
Confirmed inhibitory concentrations for selected Malaria Box compounds against P. falciparum and Babesia spp.
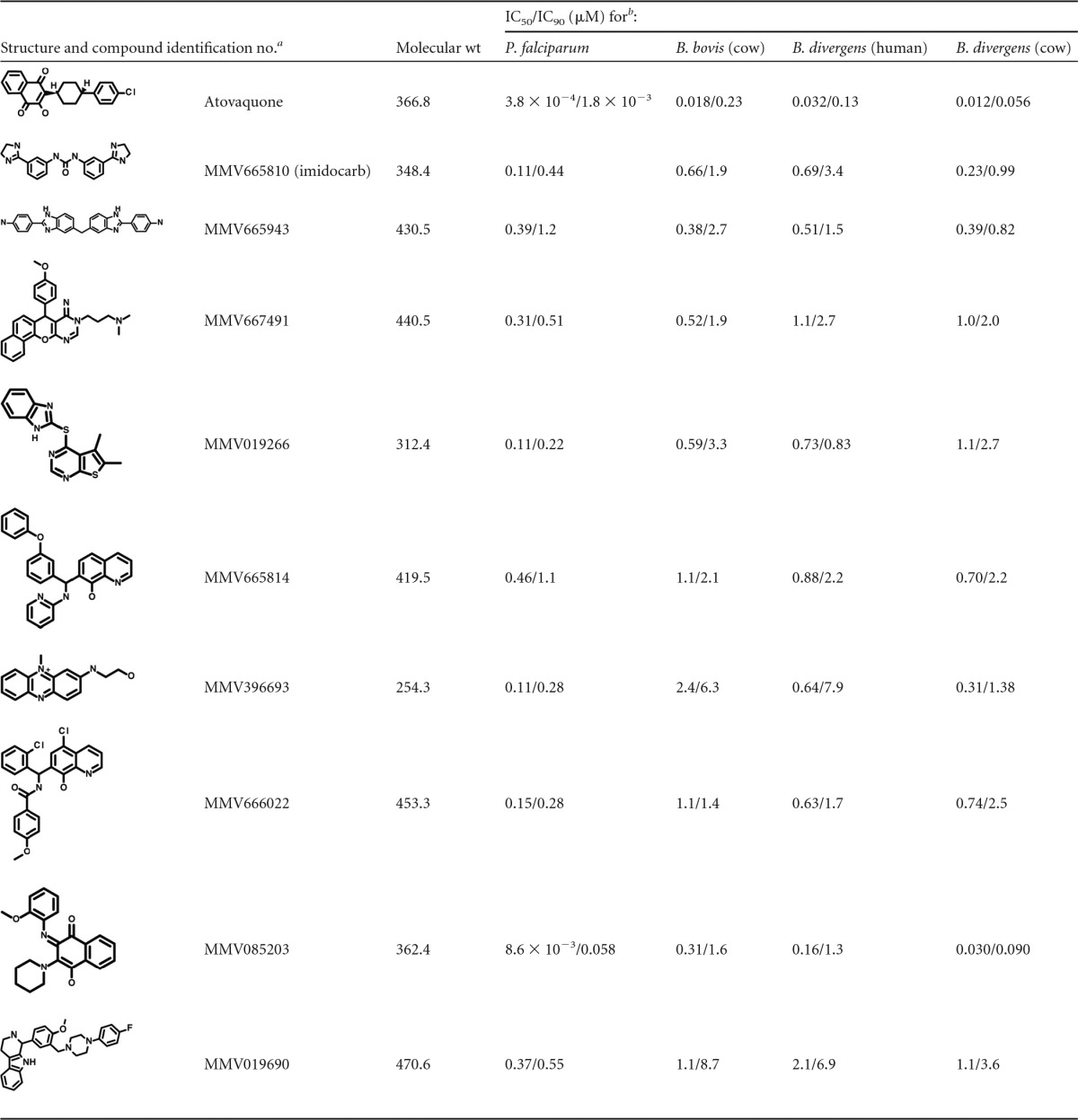
a From the supplemental material of the original report of the Malaria Box (13).
b Determined from 2 to 7 biological replicates.
The breadth of babesiacidal Malaria Box inhibitors is striking in relation to the comparatively few Malaria Box inhibitors reportedly active against nonerythrocyte apicomplexans, such as T. gondii, C. parvum, and T. annulata (14–16). We speculate that this difference may reflect the existence of conserved targets required for proliferation within a similar erythrocytic niche for diverse apicomplexan hemoprotozoan parasites and/or the close phylogenetic relatedness of Plasmodium and Babesia spp. Our results suggest that, with the discovery of novel antimalarial chemotypes at the blood stage (26–30), a substantial fraction is likely also to be babesiacidal and potentially lead to compounds to be repurposed for the treatment of babesiosis. The work discussed here has implications for chemotherapeutic strategies regarding malaria and babesiosis and should inspire more detailed investigation of the comparative biology of these parasites.
Supplementary Material
ACKNOWLEDGMENTS
We thank the MMV for providing us the Malaria Box library of small-molecule inhibitors; Stewart Rudnicki, Rachel Warden, and Jennifer Smith (ICCB-L, Harvard Medical School) for assistance with automated liquid handling; Kirk Deitsch and Laura Kirkman (Weill Cornell Medical College) for providing us with B. divergens; and members of the Duraisingh laboratory for valuable feedback.
A.S.P., C.K.M., and B.E. performed all the experiments. A.S.P. analyzed data and prepared figures. D.R.A. assisted with establishment of B. bovis cultures and with manuscript preparation. A.S.P. and M.T.D. designed experiments, interpreted data, and wrote the manuscript.
Funding Statement
M.T.D. was supported by NIH grant R01AI091787, and D.R.A. was supported by the University of Florida Foundation and departmental funds.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00928-16.
REFERENCES
- 1.DeBarry JD, Kissinger JC. 2011. Jumbled genomes: missing apicomplexan synteny. Mol Biol Evol 28:2855–2871. doi: 10.1093/molbev/msr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gou H, Guan G, Liu A, Ma M, Chen Z, Liu Z, Ren Q, Li Y, Yang J, Yin H, Luo J. 2013. Coevolutionary analyses of the relationships between piroplasmids and their hard tick hosts. Ecol Evol 3:2985–2993. doi: 10.1002/ece3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asada M, Goto Y, Yahata K, Yokoyama N, Kawai S, Inoue N, Kaneko O, Kawazu SI. 2012. Gliding motility of Babesia bovis merozoites visualized by time-lapse video microscopy. PLoS One 7:e35227. doi: 10.1371/journal.pone.0035227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo CA, Rodriguez M, Cursino-Santos JR. 2012. Babesia and red cell invasion. Curr Opin Hematol 19:170–175. doi: 10.1097/MOH.0b013e328352245a. [DOI] [PubMed] [Google Scholar]
- 5.Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, Bidwell SL, Brown WC, Crabtree J, Fadrosh D, Feldblum T, Forberger HA, Haas BJ, Howell JM, Khouri H, Koo H, Mann DJ, Norimine J, Paulsen IT, Radune D, Ren Q, Smith RK Jr, Suarez CE, White O, Wortman JR, Knowles DP, Mcelwain TF, Nene VM. 2007. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog 3:e148. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, Augagneur Y, Bres V, Duclos A, Randazzo S, Carcy B, Debierre-Grockiego F, Delbecq S, Moubri-Menage K, Shams-Eldin H, Usmani-Brown S, Bringaud F, Wincker P, Vivares CP, Schwarz RT, Schetters TP, Krause PJ, Gorenflot A, Berry V, Barbe V, Ben Mamoun C. 2012. Sequencing of the smallest apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res 40:9102–9114. doi: 10.1093/nar/gks700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells TN, Hooft van Huijsduijnen R, Van Voorhis WC. 2015. Malaria medicines: a glass half full? Nat Rev Drug Discov 14:424–442. doi: 10.1038/nrd4573. [DOI] [PubMed] [Google Scholar]
- 8.Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet JJ. 1998. In vitro evaluation of drug susceptibilities of Babesia divergens isolates. Antimicrob Agents Chemother 42:818–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marley SE, Eberhard ML, Steurer FJ, Ellis WL, McGreevy PB, Ruebush TK. 1997. Evaluation of selected antiprotozoal drugs in the Babesia microti hamster model. Antimicrob Agents Chemother 41:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannier E, Krause PJ. 2012. Human babesiosis. N Engl J Med 366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 11.Birkenheuer AJ, Levy MG, Breitschwerdt EB. 2004. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J Vet Intern Med 18:494–498. doi: 10.1111/j.1939-1676.2004.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 12.Guiguemde WA, Shelat AA, Garcia-Bustos JF, Diagana TT, Gamo FJ, Guy RK. 2012. Global phenotypic screening for antimalarials. Chem Biol 19:116–129. doi: 10.1016/j.chembiol.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TNC, Willis P. 2013. The open access Malaria Box: a drug discovery catalyst for neglected diseases. PLoS One 8:e62906. doi: 10.1371/journal.pone.0062906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access Malaria Box. Antimicrob Agents Chemother 58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyom FF, Fokou PV, Tchokouaha LR, Spangenberg T, Mfopa AN, Kouipou RM, Mbouna CJ, Donfack VFD, Zollo PH. 2014. Repurposing the open access Malaria Box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob Agents Chemother 58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostettler I, Müller J, Hemphill A. 2016. In vitro screening of the open source MMV Malaria Box reveals novel compounds with profound activities against Theileria annulata schizonts. Antimicrob Agents Chemother 60:3301–3308. doi: 10.1128/AAC.02801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar SB, Cox-Singh J. 2015. Human infections with Plasmodium knowlesi—zoonotic malaria. Clin Microbiol Infect 21:640–648. doi: 10.1016/j.cmi.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Grüring C, Moon RW, Lim C, Holder AA, Blackman MJ, Duraisingh MT. 2014. Human red blood cell-adapted Plasmodium knowlesi parasites: a new model system for malaria research. Cell Microbiol 16:612–620. doi: 10.1111/cmi.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidock DA, Nomura T, Wellems TE. 1998. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol 54:1140–1147. [DOI] [PubMed] [Google Scholar]
- 20.Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. 2003. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev 16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock R, Jackson L, de Vos A, Jorgensen W. 2004. Babesiosis of cattle. Parasitology 129(Suppl):S247–S269. [DOI] [PubMed] [Google Scholar]
- 22.Nott SE, O'Sullivan WJ, Gero AM, Bagnara AS. 1990. Routine screening for potential babesicides using cultures of Babesia bovis. Int J Parasitol 20:797–802. doi: 10.1016/0020-7519(90)90014-E. [DOI] [PubMed] [Google Scholar]
- 23.Nott SE, Bagnara AS. 1993. The toxicity of antifolates in Babesia bovis. Int J Parasitol 23:399–402. doi: 10.1016/0020-7519(93)90016-R. [DOI] [PubMed] [Google Scholar]
- 24.Alkhalil A, Hill DA, Desai SA. 2007. Babesia and plasmodia increase host erythrocyte permeability through distinct mechanisms. Cell Microbiol 9:851–860. doi: 10.1111/j.1462-5822.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser M, Maes L, Tadoori LP, Spangenberg T, Ioset JR. 2015. Repurposing of the open access Malaria Box for kinetoplastid diseases identifies novel active scaffolds against trypanosomatids. J Biomol Screen 20:634–645. doi: 10.1177/1087057115569155. [DOI] [PubMed] [Google Scholar]
- 26.Plouffe D, Brinker A, Mcnamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrián F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamo FJ, Sanz LM, Vidal J, De-Cózar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DVS, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 28.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jiménez-Díaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, Mazouni FE, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, DeRisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baragaña B, Hallyburton I, Lee MCS, Norcross NR, Grimaldi R, Otto TD, Proto WR, Blagborough AM, Meister S, Wirjanata G, Ruecker A, Upton LM, Abraham TS, Almeida MJ, Pradhan A, Porzelle A, Martínez MS, Bolscher JM, Woodland A, Norval S, Zuccotto F, Thomas J, Simeons F, Stojanovski L, Osuna-Cabello M, Brock PM, Churcher TS, Sala KA, Zakutansky SE, Jiménez-Díaz MB, Sanz LM, Riley J, Basak R, Campbell M, Avery VM, Sauerwein RW, Dechering KJ, Noviyanti R, Campo B, Frearson JA, Angulo-Barturen I, Ferrer-Bazaga S, Gamo FJ, Wyatt PG, Leroy D, Siegl P, Delves MJ, Kyle DE, Wittlin S, Marfurt J, et al. 2015. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522:315–320. doi: 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Moreno G, Cantizani J, Sánchez-Carrasco P, Ruiz-Pérez LM, Martín J, el Aouad N, Pérez-Victoria I, Tormo JR, González-Menendez V, González I, de Pedro N, Reyes F, Genilloud O, Vicente F, González-Pacanowska D. 2016. Discovery of new compounds active against Plasmodium falciparum by high throughput screening of microbial natural products. PLoS One 11:e0145812. doi: 10.1371/journal.pone.0145812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo CH, Wares JP, Kissinger JC. 2008. The apicomplexan whole-genome phylogeny: an analysis of incongruence among gene trees. Mol Biol Evol 25:2689–2698. doi: 10.1093/molbev/msn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.