Abstract
During infection, the sexually transmitted pathogen Neisseria gonorrhoeae (the gonococcus) encounters numerous host-derived antimicrobials, including cationic antimicrobial peptides (CAMPs) produced by epithelial and phagocytic cells. CAMPs have both direct and indirect killing mechanisms and help link the innate and adaptive immune responses during infection. Gonococcal CAMP resistance is likely important for avoidance of host nonoxidative killing systems expressed by polymorphonuclear granulocytes (e.g., neutrophils) and intracellular survival. Previously studied gonococcal CAMP resistance mechanisms include modification of lipid A with phosphoethanolamine by LptA and export of CAMPs by the MtrCDE efflux pump. In the related pathogen Neisseria meningitidis, a two-component regulatory system (2CRS) termed MisR-MisS has been shown to contribute to the capacity of the meningococcus to resist CAMP killing. We report that the gonococcal MisR response regulator but not the MisS sensor kinase is involved in constitutive and inducible CAMP resistance and is also required for intrinsic low-level resistance to aminoglycosides. The 4- to 8-fold increased susceptibility of misR-deficient gonococci to CAMPs and aminoglycosides was independent of phosphoethanolamine decoration of lipid A and the levels of the MtrCDE efflux pump and seemed to correlate with a general increase in membrane permeability. Transcriptional profiling and biochemical studies confirmed that expression of lptA and mtrCDE was not impacted by the loss of MisR. However, several genes encoding proteins involved in membrane integrity and redox control gave evidence of being MisR regulated. We propose that MisR modulates the levels of gonococcal susceptibility to antimicrobials by influencing the expression of genes involved in determining membrane integrity.
INTRODUCTION
Neisseria gonorrhoeae is a Gram-negative diplococcus and the causative agent of the sexually transmitted infection termed gonorrhea, which is currently the second most reported infection in the United States (1); an estimated 78 million new cases of gonorrhea occurred worldwide in 2012 (2). In addition to the high worldwide prevalence of gonorrhea, strains with resistance to currently or formerly used antibiotics have emerged, and concern has been voiced that without new effective antimicrobials, some cases of gonorrhea may be difficult to treat in future years (3). In addition to its ability to resist classical antibiotics used in treatment, gonococci have evolved mechanisms to evade the antimicrobial action of host compounds that participate in the innate host defense during infection. For instance, the ability of gonococci to resist the antibiotic-like action of host cationic antimicrobial peptides (CAMPs), such as defensins (4) or larger antimicrobial proteins (e.g., bactericidal permeability-increasing protein [5], cathepsin G [6], and CAP37 [7]), has been implicated in its survival within human polymorphonuclear granulocytes (PMNs) (8, 9).
Broadly, there are five known ways in which gonococci resist killing by CAMPs: (i) downregulation of host CAMP expression, (ii) delayed lysosomal fusion with gonococcal phagosomes, (iii) hindrance of CAMP access to the gonococcal surface, (iv) CAMP efflux, and (v) gonococcal surface modifications. These resistance mechanisms have been reviewed previously (10). Furthermore, recent evidence has shown that some gonococci can escape neutrophil extracellular traps (NETs) through the DNA-degrading action of a gonococcal thermonuclease, which is likely to diminish the bactericidal capacity of NET-associated antimicrobials, such as LL-37 and cathepsin G (11). Well-studied CAMP resistance mechanisms expressed by gonococci include efflux by the MtrCDE antimicrobial efflux pump (12) and surface modification at the lipid A moiety of lipooligosaccharide (LOS) with the small, positively charged molecule phosphoethanolamine (13). Both the efflux action of MtrCDE and phosphoethanolamine decoration of lipid A are important for gonococci to survive in the lower genital tract of experimentally infected female mice (14, 15), suggesting that these CAMP resistance systems are important for the in vivo survival of gonococci during genital tract infection in humans. In support of this hypothesis, Hobbs et al. showed that an lptA-null mutant was substantially less fit than the wild-type (WT) parental strain in the human male urethral infection model (16).
In addition to the presence and defined action of LptA and MtrCDE, gonococci have cis- and trans-acting control systems that modulate their expression at the transcriptional (mtrCDE) or translational (lptA) level; these regulatory systems have been reviewed elsewhere (10). In other bacteria, two-component regulatory systems (2CRS) consisting of a response regulator and a sensor kinase play prominent roles as regulators of CAMP resistance mechanisms. Well-studied examples include various outer membrane modifications of lipid A (e.g., decoration with small, positively charged molecules, such as 4-amino-4-deoxy-l-arabinose or phosphoethanolamine) through the action of the PhoP-PhoQ and PmrA-PmrB 2CRS in Gram-negative rods (17–19). Their sensing of environmental stimuli or stresses followed by transcriptional changes in target gene expression (e.g., arnT, eptA) has received considerable attention, especially with respect to the control of bacterial virulence. The MisR-MisS 2CRS in N. meningitidis (20–24) shares some, but not all, properties of PhoP-PhoQ and also bears some similarity to another 2CRS involved in antimicrobial resistance termed CpxR-CpxA (25, 26). Accordingly, we hypothesized that gonococci might use MisR-MisS to sense and adapt to stresses imposed by CAMPs as an additional mechanism for resisting nonoxidative killing systems of the host. To our surprise, we found that MisR, but not MisS, contributes to gonococcal resistance to CAMPs as well as aminoglycosides by a mechanism independent of mtrCDE regulation or phosphoethanolamine decoration of lipid A. Furthermore, the loss of MisR decreased the potency of MtrCDE overexpression as a mechanism of resistance to some antimicrobials. We propose that MisR-dependent gonococcal antimicrobial resistance involves the regulation of many genes whose products collectively influence membrane permeability.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
N. gonorrhoeae strain FA19 and isogenic mutant strains, along with the plasmids used and their Escherichia coli hosts, are listed in Table 1. The primers used in this study are listed in Table S1 in the supplemental material. E. coli strains were routinely cultured on Luria-Bertani (LB) agar or in LB broth (Difco) containing 50 μg/ml kanamycin or 100 μg/ml ampicillin as necessary (liquid cultures were shaken at 200 rpm). Gonococci were grown on gonococcal base (GCB) agar (Difco) containing Kellogg's supplements I and II (27) at 37°C under 5.0% (vol/vol) CO2. Liquid cultures of gonococci were begun by resuspending plate-grown cells in prewarmed 1× GCB broth; cells were then inoculated to a normalized optical density at 600 nm (OD600) of 0.08 in prewarmed 1× GCB broth containing Kellogg's supplements I and II and 0.043% (wt/vol) sodium bicarbonate and grown at 37°C with shaking at 200 rpm. Liquid cultures of gonococci contained a final concentration of 10 mM MgCl2 unless otherwise noted and were inoculated with plate-grown gonococci that were no more than 12 to 14 h old (we found that misR::kan gonococci grew poorly in broth unless these conditions were met; in contrast, misS::kan gonococci did not have a growth defect [unpublished observations]). In experiments using strains containing WT copies of genes expressed ectopically from integrated pGCC4-based vectors (28), all cultures were supplemented with isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM unless otherwise noted. When necessary, the presence of a single-base-pair deletion in the mtrR promoter (strain KH15 background) was confirmed by DNA sequencing of a PCR product representing the mtrR-mtrCDE intergenic region, generated using primers mtrC_promR and mtrJK1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| N. gonorrhoeae | ||
| FA19 | WT strain | 83 |
| JK100 | FA19 misR::kan | This study |
| JK101 | JK100 complementation (FA19 misR::kan/pGCC4-misR) | This study |
| JK102 | FA19 misS::kan | This study |
| JK103 | JK102 complementation (FA19 misS::kan/pGCC4-misS) | This study |
| FA19 lptA::spc | FA19 lptA::spc | 13 |
| KH14 | FA19 mtrD::kan | 84 |
| JF1 | FA19 ΔmtrR | 85 |
| JK200 | JF1 misR::kan | This study |
| KH15 | FA19 containing a single-base-pair deletion at the 13-bp inverted repeat between mtrR and mtrCDE (FA19 mtr−79) | 41 |
| JK300 | KH15 misR::kan | This study |
| FA19::PlptA-lacZ | FA19 containing a translational fusion of the promoter region of lptA to the lacZ gene integrated at the proAB locus of the chromosome | This study |
| JK100::PlptA-lacZ | FA19::PlptA-lacZ misR::kan | This study |
| Escherichia coli | ||
| One Shot TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 (ara leu)7697 galU galK rpsL (Strr) endA1 nupG (catalog no. C4040-03; Invitrogen) | Invitrogen |
| Plasmids | ||
| pUC19K | Plasmid containing the nonpolar aphA-3 kanamycin resistance cassette | 29 |
| pLES94 | pUC18 derivative containing a truncated lacZ gene for use in translational fusions; recombines at the proAB locus of the gonococcal chromosome | 35 |
| pGCC4 | Complementation vector for cloning of WT gene alleles downstream of an IPTG-inducible Plac promoter; recombines between the lctP and aspC genes in the chromosome | 28 |
| pLES94-lptA | pLES94 containing the FA19 lptA promoter | This study |
| pGCC4-misR | pGCC4 containing the WT FA19 misR gene under the control of Plac | This study |
| pGCC4-misS | pGCC4 containing the WT FA19 misS gene under the control of Plac | This study |
Generation of misR and misS mutants and complemented strains.
Construction of the FA19 misR::kan mutant (here termed JK100) and FA19 misR::kan/pGCC4-misR (here termed JK101) complemented strains was performed as described below. The misR gene was inactivated using the nonpolar aphA-3 kanamycin resistance cassette (29). JK100 was constructed by transforming FA19 with meningococcal genomic DNA from the N. meningitidis misR::kan mutant SZT1001 constructed previously (30). Plate transformations were performed as described previously (31). In general, misR-deficient transformants could be obtained with 3 to 4 days of incubation on GC agar containing kanamycin at 50 μg/ml. Loss of misR was confirmed by PCR using primers misR1, MisSR, and MisRrev. Mutants were further verified by Western blotting (anti-MisR antisera were kindly provided by Yih-Ling Tzeng, Emory University). JK100 was complemented with a WT copy of misR cloned into pGCC4 using primers misR3PacI and misR4PmeI and methods described previously (13) to generate strain JK101. MisR complementation in JK101 was confirmed by PCR and Western blotting.
Like misR, the misS gene was also inactivated using the nonpolar aphA-3 kanamycin resistance cassette. Strain JK102 (FA19 misS::kan) was constructed by transforming FA19 with genomic DNA from the N. meningitidis misS::kan mutant YT0310 constructed previously (24). The loss of misS in JK102 was confirmed by growth of the mutant on GCB agar containing kanamycin (50 μg/ml), followed by PCR and DNA sequencing across the misRS operon using primers misR1 and MisSR, as well as KanB and KanD aphA-3 cassette primers. JK102 was complemented with a WT copy of misS cloned into pGCC4 using primers misSPacI and misSPmeI and methods described previously (13) to generate strain JK103 (FA19 misS::kan/pGCC4-misS). Complementation in JK103 was confirmed by PCR.
MIC assays.
For MIC assays, overnight (O/N) plate cultures of gonococci were resuspended in unsupplemented 1× GCB broth, vortexed briefly, and normalized to an OD600 of 0.1 before 5 μl was spot plated onto GCB agar containing 2-fold serial dilutions of the test antimicrobial. Inoculated plates were grown for ∼48 h before growth was analyzed. The MIC for each strain was considered to be the lowest concentration of antimicrobial required to significantly impact growth compared to that on a medium-only control plate.
MBC assays and pretreatment of gonococci with PMB.
Polymyxin B (PMB) and LL-37 minimum bactericidal concentrations (MBCs) were determined as described previously (32) using gonococci grown in supplemented GCB broth containing 10 mM MgCl2 and 1 mM IPTG. For PMB sublethal pretreatments, broth cultures were inoculated, grown as described above, and then split at early log phase (OD600, 0.2 to 0.3) and treated with either carrier (sterile double-distilled H2O [ddH2O]) or a sublethal level (0.1× the strain's plate MIC) of PMB for 3 h prior to performing the PMB MBC assay (32) and plating on GCB agar supplemented with 1 mM IPTG. The MBC90 of each strain was considered to be the concentration of antimicrobial at which ≥90% of the gonococci were killed. Purified synthetic LL-37 peptide was a kind gift from Jan Pohl (Biotechnology Core Facility Branch, Centers for Disease Control and Prevention, Atlanta, GA).
RNA-Seq analysis and qRT-PCR validation.
The transcriptome sequencing (RNA-Seq) experiment comparing the enriched mRNA transcriptomes of WT strain FA19 and JK100 (33) and the analyzed data set containing fold changes in gene expression (10; J. L. Kandler, R. Vélez Acevedo, M. K. Dickinson, D. R. Cash, W. M. Shafer, and C. N. Cornelissen, submitted for publication) have been reported previously. Gene expression fold changes (between JK100 and WT strain FA19) were considered significant if they met a ≥2-fold cutoff and had a Bonferroni-corrected P value of <0.05. For quantitative reverse transcription-PCR (qRT-PCR) measurement of MisR target gene expression, 1 ml of broth-grown gonococci was harvested at mid- or late log phase by centrifugation at 10,000 rpm for 2 min, and the pellets were stored at −70°C. RNA was purified by RNeasy (Qiagen) and Turbo DNAfree (Ambion) treatment, and cDNA was generated using a QuantiTect reverse transcriptase kit (Qiagen). The levels of MisR target gene transcription were determined by quantitative PCR in a 25-μl SYBR green (Bio-Rad) reaction mixture using 2 μl of 1:1,000 cDNA as the template. The normalized expression of each target gene was calculated by the 2−ΔΔCT threshold cycle (CT) method (34) using 16S rRNA as a housekeeping reference gene. Mean fold change values were equivalent to the normalized expression ratio (MisR-repressed genes) or calculated as −1/normalized expression ratio (MisR-activated genes).
Construction of the lptA-lacZ fusion and qualitative assessment of CPRG uptake and cleavage.
An lptA-lacZ translational fusion was generated using the pLES94 system (35). Briefly, the proximal promoter (36) of lptA was amplified using primers 5′lptA-Z and 3′lptA-Z and used to generate a translational fusion of lptA to the truncated, promoterless lacZ gene in pLES94. The pLES94 construct was transformed into One Shot TOP10 chemically competent E. coli cells (Invitrogen) by heat shock, and transformants were selected on LB agar containing 100 μg/ml ampicillin and 40 μg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and screened by PCR and sequencing. A confirmed pLES94-lptA plasmid was purified by use of a miniprep kit (Qiagen) and transformed into strain FA19 to generate strain FA19::PlptA-lacZ. Gonococcal transformants were selected on GCB agar containing 1 μg/ml chloramphenicol and further verified by PCR. Strain JK100::PlptA-lacZ was generated by transforming FA19::PlptA-lacZ with a PCR product spanning the misR::kan-misS operon from strain JK100, which was amplified using primers misR1 and MisSR. Transformants were selected on GCB agar containing 50 μg/ml kanamycin and then replica plated onto separate GCB agar plates containing 1 μg/ml chloramphenicol, 50 μg/ml kanamycin, or 25 μg/ml PMB (JK100 cannot grow in the presence of this PMB concentration). Transformants with the correct antibiotic resistance phenotype were further confirmed for the absence of WT misR by PCR using primers misR1 and MisRrev. Strains FA19 and JK100 with and without the lptA-lacZ fusion were spot plated onto supplemented GCB agar containing 2-fold serial dilutions of chlorophenol red-β-d-galactopyranoside (CPRG; catalog number 884308; Boehringer Mannheim) as described above for the MIC experiments. After 24 h of incubation, areas of gonococcal growth on plates containing 100 μg/ml CPRG were photographed to assess CPRG cleavage by LacZ and the release of chlorophenol red.
Western blotting.
Mid-log-phase gonococci from broth cultures were pelleted by centrifugation at 10,000 rpm for 2 min, and whole-cell lysates were prepared in 1× Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.001 M MgSO4·6H2O) by freeze-thawing 3 times in a dry ice-ethanol bath, followed by thorough vortexing. A 12% SDS-polyacrylamide gel (with a 5% stacking gel) was run in duplicate, with the levels of protein being normalized by use of a NanoDrop spectrophotometer prior to dilution and boiling for 10 min in 2× SDS loading dye (100 mM Tris-HCl, pH 6.8, 4% [wt/vol] SDS, 0.2% [wt/vol] bromophenol blue, 20% glycerol, 200 mM dithiothreitol [DTT]). One gel was Coomassie stained to show that the wells were loaded with an equivalent amount of protein. The other gel was transferred to a nitrocellulose membrane in Bjerrum-Schafer-Nielsen buffer (48 mM Tris, 39 mM glycine, 20% methanol [vol/vol]) on a Trans-Blot SD semidry transfer cell (Bio-Rad) and was then blocked O/N at 4°C using 3% (wt/vol) bovine serum albumin (MtrE blot) or 4% (wt/vol) nonfat dried milk (MisR blot) in 1× TST buffer (0.01 M Trizma base, 0.150 M NaCl, 0.05% [vol/vol] Tween 20). Blocked membranes were washed 3 times (10 min each) in 1× TST buffer and probed with primary antibody O/N at 4°C against MtrE or MisR using 1:10,000 rabbit polyclonal antisera (antisera were generously provided by Ann E. Jerse and Yih-Ling Tzeng, respectively) diluted in 1× TST buffer. Anti-MtrE antiserum was generated using amino acids 110 to 120 of MtrE (RQGSLSGGNVS [37]). Anti-MisR antiserum was generated using purified MisR-His6× protein (24). The blots were washed as described above in 1× TST buffer and incubated with 1:2,500 horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (product number 32460; Thermo Scientific) diluted in 1× TST buffer for 1 h at room temperature. The blots were given final washes as described above in 1× TST buffer before 1 min of development with a 1:1 luminol-peroxide solution (product number 32209; Thermo Scientific). Bands were visualized by exposure of the membranes to autoradiography film.
MALDI-TOF MS analysis of gonococcal lipid A.
Overnight cultures of WT strain FA19, JK100, JK101, and FA19 lptA::spc (∼12 liters per strain) were grown with shaking O/N at 37°C in GCB broth containing 1 mM IPTG and 10 mM MgCl2, pelleted by centrifugation at 7,500 rpm for 15 min at 4°C, washed 3 times in 1× phosphate-buffered saline (PBS; pH 7.2), and fixed in 10% formalin diluted in 1× PBS. Processed pellets were frozen at −70°C, and LOS was extracted from the wet cell paste by the hot phenol-water method (38), followed by dialysis (3,500-Da-molecular-mass cutoff) against several exchanges of deionized H2O. Phospholipids were removed by three washes and precipitation of LOS with chilled ethanol-H2O (9:1, vol/vol). Nucleic acids and proteins were removed by treatment with DNase I, RNase A, and proteinase K (all reagents from Sigma-Aldrich) following the manufacturers' instructions, the LOS material was dialyzed (3,500-Da-molecular-mass cutoff), and the dialysate was ultracentrifuged at 100,000 × g for 6 h at 4°C. Lipid A was extracted from LOS by mild hydrolysis with 1% acetic acid for 1 h at 100°C and recovered from the reaction mixture by extraction with chloroform. Purified lipid A was analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) on an AB Sciex TOF/TOF 5800 system in the negative reflector mode ([M-H]−).
RESULTS
MisR, but not MisS, is required for constitutive and inducible levels of gonococcal resistance to CAMPs.
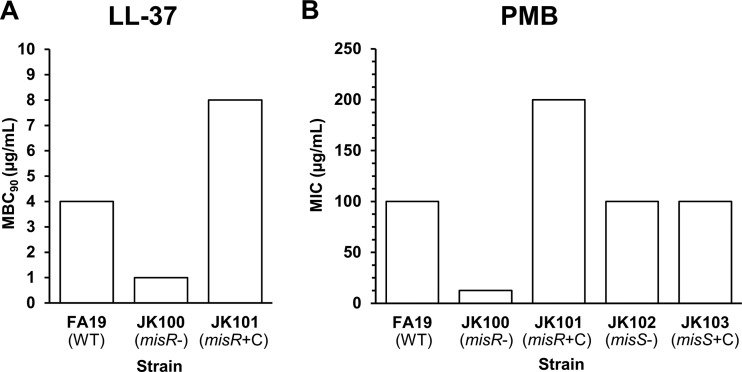
Earlier reports demonstrated that the loss of misR increases the susceptibility of N. meningitidis to a cationic lipopeptide antimicrobial, polymyxin B (PMB), which has served as a model CAMP (21, 22), and to the human defensin human neutrophil peptide-1 (HNP-1) (20). Since meningococcal MisR (GenBank accession number AAF41023.1; locus tag NMB0595) and gonococcal MisR (GenBank accession number EEZ45023.1; locus tag NGEG_00293) are 100% identical at the amino acid level (http://blast.ncbi.nlm.nih.gov/Blast.cgi), we hypothesized that gonococci would also display MisR-dependent CAMP resistance. Accordingly, we determined the MBC90 of the human cathelicidin LL-37 and the MIC of PMB against strain FA19 (WT) and the FA19 misR::kan mutant (JK100). We found that FA19 was 4- to 8-fold more resistant to LL-37 and PMB than JK100 and that the parental levels of LL-37 and PMB resistance could be restored if a WT copy of misR was expressed ectopically in complemented strain JK101 (FA19 misR::kan/pGCC4-misR) (Fig. 1A and B). Interestingly, the loss of misS in gonococcal strain JK102 (FA19 misS::kan) did not increase gonococcal susceptibility to PMB (Fig. 1B). Complementation of misS in strain JK103 (FA19 misS::kan/pGCC4-misS) also had no impact on PMB susceptibility.
FIG 1.
MisR is required for constitutive resistance to CAMPs. The MBC90s and MICs of LL-37 and PMB, respectively, were determined using gonococcal strains with and without a functional MisR-MisS 2CRS. (A) Loss of MisR increases susceptibility to the human cathelicidin LL-37. Shown are the modal results from two independent experiments performed in technical triplicate. (B) The loss of MisR, but not MisS, increases susceptibility to the model CAMP PMB. Shown are the modal results of three or more independent experiments. MisR and MisS genotypes are noted in parentheses. misR-, misR deficient; misS-, misS deficient; +C, complemented strain.
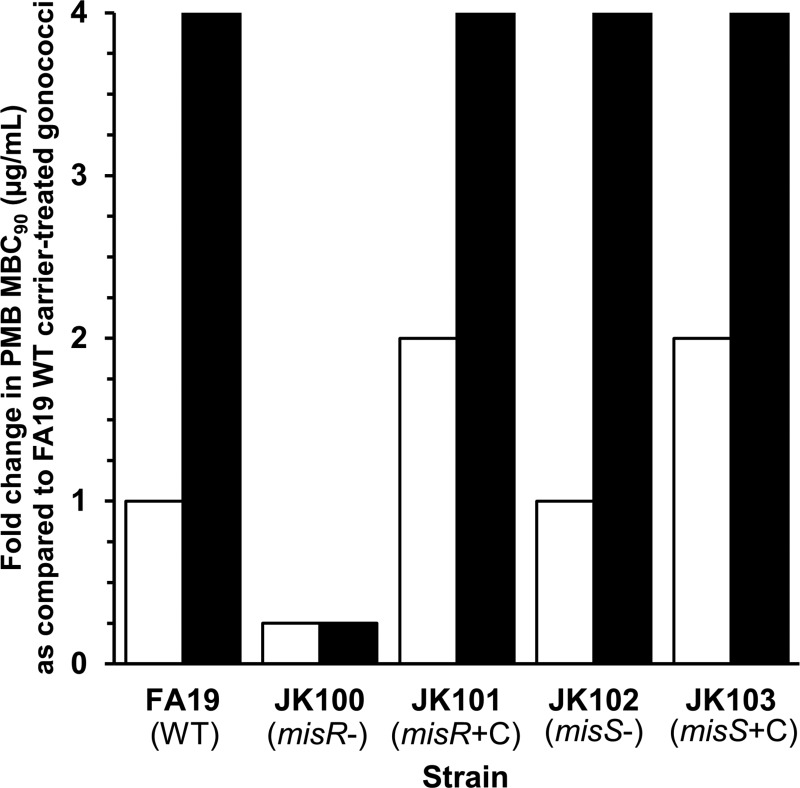
The result presented above suggested that the response regulator action of MisR did not require input from its cognate sensor kinase, MisS, to provide constitutive levels of gonococcal resistance to CAMPs under the conditions tested. In order to determine if inducible CAMP resistance in gonococci is possible and if such resistance requires both MisR and MisS, we grew the test strains in the absence or presence of a sublethal level (0.1× plate MIC against each strain) of PMB in broth culture and then quantified the resulting PMB susceptibility by determining the MBC90 for each strain. Briefly, we found that gonococci could indeed be induced to higher levels of PMB resistance (4-fold greater than that of carrier-treated WT strain FA19 gonococci) but that this induction required only MisR and not MisS (Fig. 2).
FIG 2.
MisR, but not MisS, is required for constitutive and inducible resistance to PMB. MBC90s for gonococci were determined after growth in broth in the presence or absence of sublethal levels of PMB. MBC90 values compared to those for carrier-treated FA19 (WT) gonococci are represented. White columns, strains treated with carrier (ddH2O); black columns, strains treated with a sublethal level of PMB (0.1× the plate MIC). Shown are modal results from three or more independent experiments (the JK103 [FA19 misS::kan/pGCC4-misS] complemented strain was tested in two independent experiments) performed in technical triplicate. MisR and MisS genotypes are noted in parentheses.
Gonococci exhibit MisR-dependent resistance to aminoglycosides and antimicrobials recognized by the MtrCDE efflux pump.
In order to determine if MisR-dependent antimicrobial resistance of gonococci was restricted to CAMPs, we assessed whether strain JK100 was more susceptible to other kinds of antimicrobials (i.e., antibiotic drugs, dyes, and detergents) than WT parent strain FA19. For this purpose, we also examined other genetic derivatives of strain FA19 that displayed increased CAMP susceptibility due to the loss of the MtrCDE efflux pump (strain KH14 [FA19 mtrD::kan]) or that lacked the ability to decorate lipid A with phosphoethanolamine (strain FA19 lptA::spc); the roles of the MtrCDE efflux system and phosphoethanolamine decoration of lipid A in gonococcal CAMP resistance have been described previously (12, 13, 16, 36, 39–41). With respect to differences in CAMP susceptibility, the misR::kan and mtrD::kan mutations (strains JK100 and KH14, respectively) significantly increased PMB susceptibility 8- and 4-fold, respectively, though the strains with these mutations were not as exquisitely PMB sensitive as the FA19 lptA::spc mutant (256-fold increased susceptibility; Table 2). However, the loss of the MtrCDE efflux pump in KH14 did result in gonococcal hypersusceptibility to a number of antimicrobials known from previous work to be substrates for this pump (41). In contrast, strain JK100 displayed only a modest (2-fold) increase in susceptibility to most tested MtrCDE efflux pump substrates and was not hypersusceptible to Triton X-100 (TX-100), which has been shown to be exported by this pump (42). A notable exception to this trend was ceftriaxone, the MIC of which was decreased 4-fold in strain JK100 compared with that in strain FA19.
TABLE 2.
Susceptibility of misR-deficient gonococci to substrates of the MtrCDE efflux pump and aminoglycosides
| Strain | MIC (μg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PMB | Ery | CRO | Pen G | CV | TX-100 | Cipb | Gent | Tob | Str | Spcc | |
| FA19 (WT) | 100 | 0.25 | 0.0006 | 0.016 | 0.625 | 125 | 0.0025 | 10 | 10 | 12.5 | 25 |
| JK100 (FA19 misR::kan) | 12.5 | 0.125 | 0.00015 | 0.008 | 0.313 | 125 | 0.0025 | 1.25 | 1.25 | 3.13 | 12.5 |
| JK101d (FA19 misR::kan/pGCC4-misR) | 200 | 4 | 0.0006 | 0.016 | 0.625 | 125 | 0.0025 | 10 | 10 | 25 | 25 |
| JF1 (FA19 ΔmtrR) | 200 | 1 | 0.0006 | 0.016 | 1.25 | 250 | 0.0025 | 10 | 10 | 12.5 | 25 |
| JK200 (JF1 misR::kan) | 25 | 0.5 | 0.0003 | 0.008 | 0.625 | 125 | 0.0025 | 1.25 | 1.25 | 3.13 | 12.5 |
| KH15 (FA19 mtr−79) | 200 | 2 | 0.0006 | 0.032 | 2.5 | 8000 | 0.0025 | 10 | 10 | 25 | 25 |
| JK300 (KH15 misR::kan) | 50 | 0.5 | 0.0003 | 0.008 | 0.625 | 250 | 0.0025 | 1.25 | 1.25 | 3.13 | 6.25 |
| KH14 (FA19 mtrD::kan) | 25 | 0.031 | 0.0012 | 0.032 | 0.078 | 15.6 | 0.0025 | 5 | 5 | 12.5 | 12.5 |
| FA19 lptA::spce | 0.39 | 0.25 | 0.0006 | 0.032 | 0.625 | 125 | 0.0025 | 5 | 5 | 50 | 100 |
Modal MIC values were determined from three or more independent experiments. Bold values indicate a 4-fold or greater increased susceptibility in a given misR-deficient mutant compared with the susceptibility of the parent strain. Antimicrobial abbreviations: PMB, polymyxin B; Ery, erythromycin; CRO, ceftriaxone; Pen G, penicillin G; CV, crystal violet; TX-100, Triton X-100; Cip, ciprofloxacin; Gent, gentamicin; Tob, tobramycin; Str, streptomycin; Spc, spectinomycin.
The fluoroquinolone ciprofloxacin was included as a control drug that is not subject to MtrCDE efflux.
The aminocyclitol spectinomycin was included as a control.
Strain JK101 is resistant to erythromycin due to the ermC gene expressed by the pGCC4 construct.
FA19 lptA::spc is resistant to both spectinomycin and streptomycin due to the aadA gene encoded in the Ω cassette that interrupts lptA (86).
Since the repression of mtrCDE transcription by MtrR in the WT strain FA19 background (43, 44) might mask MisR's impact on antimicrobial efflux, we also examined the effect of the misR::kan mutation in two genetic derivatives of strain FA19 (strain JF1 [FA19 ΔmtrR], which has a large deletion in the mtrR gene, and strain KH15 [FA19 mtr−79], which has a single-base-pair deletion in the mtrR-mtrCDE intergenic region [Table 2]). JF1 and KH15 have medium and high levels of expression of mtrCDE, respectively, and as a result display enhanced resistance to antimicrobials that are exported by MtrCDE. Strain JK200 (JF1 misR::kan) generally had 2-fold increased susceptibility to MtrCDE substrates compared to that of JF1. However, the MIC profile of strain JK300 (KH15 misR::kan) revealed the importance of MisR for the high-level resistance mediated by this efflux pump. Notable changes occurred in the MICs for crystal violet, erythromycin, penicillin G, and TX-100 (4-fold or more increased susceptibility in JK300 compared with that in KH15).
Having observed the impact of MisR on CAMP resistance and the efficient high-level efflux of antimicrobials, we wondered if gonococcal susceptibility to drugs that are not pump substrates of MtrCDE would also be affected by the loss of MisR. To test this, we determined the MICs of three aminoglycosides (gentamicin, tobramycin, and streptomycin) and an aminocyclitol (spectinomycin) against our test strains. As shown in Table 2, the loss of MisR in any mtrCDE background consistently increased gonococcal sensitivity to all aminoglycosides by 4- to 8-fold (in contrast to results for the aminocyclitol spectinomycin and the fluoroquinolone ciprofloxacin [Table 2]). We noted that this fold difference in aminoglycoside susceptibility was similar to that seen for CAMPs. Importantly, the loss of phosphoethanolamine decoration of lipid A in strain FA19 lptA::spc and the loss of MtrCDE efflux in strain KH14 resulted in only 2-fold increased susceptibility to aminoglycosides, which is not considered significant in 2-fold agar dilution MIC assays.
To ensure that the increased CAMP and aminoglycoside susceptibility of gonococci due to the loss of MisR was not restricted to the FA19 genetic background, we constructed a misR::kan transformant of strain MS11, which has a higher level of PMB resistance (MIC, 400 μg/ml) due to mutations at the mtrR-mtrCDE locus (mtr120 and mtrRA39T) that result in overexpression of the mtrCDE efflux pump (45, 46). In agreement with the results obtained with MisR-negative variants of strains FA19, JF1, and KH15, we found that the loss of MisR in strain MS11 misR::kan resulted in an 8-fold increase in gonococcal susceptibility to both PMB and gentamicin compared to the susceptibility of the MS11 parent strain.
MisR control of CAMP and aminoglycoside resistance is independent of LptA and MtrCDE.
The results from the MIC studies described above suggested that MisR modulation of gonococcal CAMP and aminoglycoside resistance is through a pathway independent of phosphoethanolamine decoration of lipid A or simple upregulation of MtrCDE efflux. To test this hypothesis, we asked if the loss of MisR might influence the lipid A structure (specifically, phosphoethanolamine decoration) or the levels of the MtrCDE efflux pump. With respect to lipid A, MALDI-TOF MS analysis of purified lipid A from gonococcal strains showed that phosphoethanolamine-decorated lipid A was readily detected in strains FA19, JK100, and JK101 (see Fig. S1 in the supplemental material). As expected, phosphoethanolamine-lipid A was undetectable in the FA19 lptA::spc control. No differences in lipid A attributable to altered MisR expression were found. To determine any impact of MisR on the levels of the MtrCDE efflux pump, we used anti-MtrE antiserum to probe for the levels of the outer membrane channel protein, MtrE (which is encoded by the last gene in the mtrCDE operon), in gonococcal whole-cell lysates. Results from Western immunoblotting experiments demonstrated that the levels of MtrE were identical in strain pairs FA19 and JK100, JF1 and JK200, and KH15 and JK300 (see Fig. S2 in the supplemental material). Finally, analysis of published RNA-Seq data comparing the mRNA-enriched transcriptomes of FA19 and JK100 (33; Kandler et al., submitted) confirmed that the loss of MisR did not alter the transcription of lptA or mtrCDE, nor did it change the expression of accessory efflux genes mtrR, mtrA, and mtrF. This observation is consistent with the biochemical data described above showing that MisR does not control the expression of these CAMP resistance systems. Taken together, we conclude that MisR does not influence the expression of two established CAMP resistance systems (phosphoethanolamine decoration of lipid A and MtrCDE efflux of antimicrobials) and likely modulates the levels of gonococcal antimicrobial susceptibility through a different mechanism.
MisR influences global transcription patterns and membrane permeability.
Additional analysis of a previously reported RNA-Seq data set (33; Kandler et al., submitted) revealed that 59% (55/94) of significantly MisR-regulated target genes (≥2-fold cutoff; Bonferroni-corrected P value, <0.05) encoded proteins that are predicted or known to localize to the cell envelope (e.g., laz, dca, nadC), act in the protein-folding/chaperone machinery (e.g., htpX, dsbD), participate in protein secretion (e.g., tatB, tatC), or contribute to redox reactions within the cell (e.g., bfrA, bfrB, grx3, trx1) (see Tables S2A and B in the supplemental material). As is summarized in Table 3, we validated the RNA-Seq data using qRT-PCR of strains FA19 and JK100 to confirm the MisR activation or MisR repression of several genes in this regulon. For most genes tested, the MisR-dependent activation (tatC, htpX, bfrA and bfrB, dsbD) or repression (nadC, clpB) observed in the RNA-Seq experiment was repeatable by qRT-PCR of total RNA prepared from both mid- and late-log-phase cultures. Additionally, complementation of MisR in strain JK101 returned the level of expression of these genes to nearly WT levels (see footnote d in Table 3). In contrast, grpE and dnaK expression was similar in FA19 and JK100 gonococci, as assessed by qRT-PCR. However, late-log-phase dnaK levels were >2-fold decreased in strain JK101 compared to those in FA19, indicating slight MisR repression of dnaK, which is consistent with the regulation seen in the RNA-Seq experiments. These observations highlight the importance of confirming transcriptomic data using alternative methods. Finally, a bioinformatics search for MisR consensus binding sites using the PRODORIC online tool (47) and a search for published DNA-binding experiments revealed the presence of a consensus or a nearly consensus MisR-binding site upstream of most of these genes, suggesting that they may be directly regulated by MisR. No such site was found upstream of grpE, which may explain the lack of MisR regulation as assessed by qRT-PCR.
TABLE 3.
Validation of the MisR RNA-Seq data set by qRT-PCR and bioinformatic prediction of MisR binding sites
| FA 1090 ORFa (gene) | Fold change in expression between misR-deficient and WT strains by: |
MisR binding site sequenceb | Position in FA 1090 |
||||
|---|---|---|---|---|---|---|---|
| RNA-Seqc | qRT-PCRd |
||||||
| Mid-log phase | Late log phase | Start | End | Relative to start codon | |||
| NGO0181(tatC) | −24.34 | −3.61 ± 1.34 | −4.03 ± 1.43 | GTATTGATAAGGGTT | 185530 | 185544 | −1017 |
| NGO0377 (nadC) | 10.42 | 7.01 ± 3.73 | 11.61 ± 2.23 | GATATGTAAGGGGAA | 372470 | 372484 | −164 |
| NGO0399 (htpX) | −3.84 | −20.37 ± 13.89 | −14.34 ± 2.79 | GAATCGTAAACCATC | 392453 | 392467 | −162 |
| NGO0794/NGO0795 (bfrA/bfrB) | −3.26/−3.79 | −6.36 ± 3.48/−6.40 ±4.28 | −4.57 ± 2.90/−7.27 ± 3.27 | GATTTGGAAGGCATC | 784521 | 784535 | −154 |
| NGO0978 (dsbD) | −9.21 | −51.30 ± 27.47 | −34.09 ± 10.67 | TTTATGTAAAACCCGe | 950416 | 950402 | −68 |
| NGO1046 (clpB) | 5.01 | 3.09 ± 1.50 | 2.70 ± 1.30 | ATTTTGAAAAGGAAA | 1006110 | 1006124 | −6 |
| NGO1422 (grpE) | 2.62 | 1.44 ± 0.54 | 1.64 ± 0.57 | No site found using the search parameters | NAf | NA | NA |
| NGO1429 (dnaK) | 2.20 | 1.53 ± 0.46 | 1.53 ± 0.42 | TATTCATAAAGTTAT | 1390419 | 1390433 | −119 |
ORF, open reading frame.
Potential MisR binding sites were determined bioinformatically using the PRODORIC online tool (http://prodoric.tu-bs.de/). Input parameters were as follows: strain ATCC 700825, N. gonorrhoeae FA 1090 (NCBI reference sequence, GenBank accession number NC_002946); single-pattern IUPAC code, KWWWTGTAARGNNWH; mismatch tolerance, 2; maximum distance to gene, 3,000 bp; ignored match orientation and removed palindromic matches. MisR binding site nucleotides that match the consensus sequence reported previously (23) are in bold, and mismatches are underlined. The consensus sequence is KWWWTGTAARGNNWH, where K is G or T, W is A or T, R is A or G, N is any nucleotide, and H is A, T, or C.
Shown are the mean fold changes in the number of reads per kilobase per million reads (RPKM) comparing gene expression in JK100 and WT strain FA19 gonococci from three independent experiments (see reference 33 for the methods). All RNA-Seq fold changes (between the misR-deficient strain and the WT) were statistically significant (Bonferroni-corrected P value, ≤0.05). Negative fold changes indicate MisR activation, and positive fold changes indicate MisR repression.
Shown are the mean ± standard deviation fold changes in expression between JK100 and WT strain FA19 gonococci at mid- or late log phase from three independent experiments. The mean normalized expression ratios comparing complemented strain JK101 and WT strain FA19 gonococci from three independent experiments are as follows: for mid-log-phase cells, 1.25 for tatC, 0.78 for nadC, 1.04 for htpX, 0.79 for bfrA, 1.85 for bfrB, 0.83 for dsbD, 0.74 for clpB, 1.13 for grpE, and 0.59 for dnaK; for late-log-phase cells, 1.08 for tatC, 0.58 for nadC, 0.78 for htpX, 0.75 for bfrA, 0.79 for bfrB, 1.04 for dsbD, 0.40 for clpB, 1.81 for grpE, and 0.46 for dnaK.
See the predicted MisR binding site upstream of dsbD in N. meningitidis (78). Note that the NCBI-annotated dsbD start codon in FA 1090 is upstream of the transcriptional start site experimentally determined previously (78) and is therefore likely to be incorrect. Thus, the numerical position of the MisR binding site shown here for dsbD is relative to that of the N. meningitidis dsbD start codon in the previous report (78).
NA, not available.
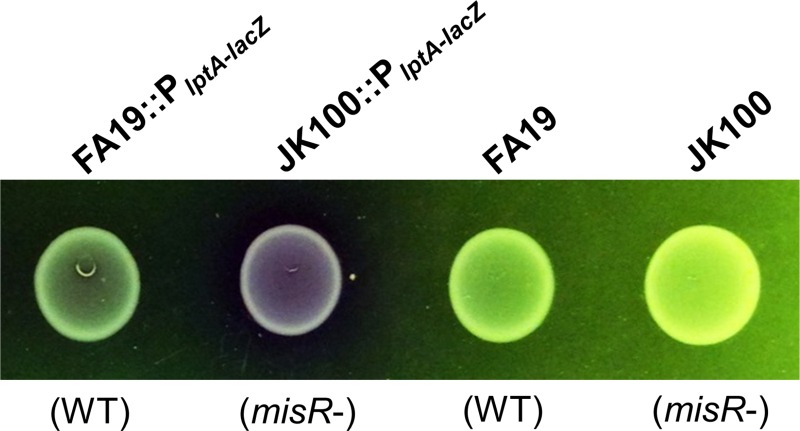
Although the RNA-Seq data did not pinpoint a specific molecular mechanism of MisR-dependent antimicrobial resistance, it did suggest that the overall physiology and integrity of the cell envelope in MisR-negative gonococci differed from those in MisR-positive gonococci. Based on this hypothesis, we asked if the loss of MisR would increase the general permeability of gonococci. For a qualitative assessment of this possibility, we used strains FA19 and JK100 expressing an lptA-lacZ translational fusion to test the cleavage of the beta-galactosidase substrate chlorophenol red-β-d-galactopyranoside (CPRG), which is analogous to the more commonly used beta-galactosidase substrate o-nitrophenyl-β-d-galactopyranoside (ONPG). When hydrolyzed by the cytosolic enzyme beta-galactosidase, the yellow-orange CPRG substrate releases the red chromophore chlorophenol red. CPRG is more sensitive to beta-galactosidase activity than ONPG (48). Like ONPG, CPRG is membrane impermeant and has previously been used to assess membrane integrity (49–51); importantly, previous work with these strains confirmed their expression of beta-galactosidase, and MIC experiments demonstrated that CPRG lacks antigonococcal action against the test strains employed (data not shown). As is shown in Fig. 3, after 24 h of growth on plates containing 100 μg/ml CPRG, the JK100::PlptA-lacZ strain had cleaved more CPRG than the FA19::PlptA-lacZ strain, as demonstrated by the purple-red coloring of the cells and surrounding medium. In contrast, parent strains lacking beta-galactosidase were unable to cleave CPRG and generated no red coloring. This result is consistent with the notion that the loss of MisR increases the permeability of the gonococci.
FIG 3.
Loss of MisR increases gonococcal envelope permeability. Gonococci expressing beta-galactosidase were grown in the presence of the membrane-impermeant beta-galactosidase substrate CPRG. Shown are buttons of gonococcal growth after 24 h of standard incubation on supplemented GCB agar containing 100 μg/ml CPRG. Note the increased cleavage of CPRG to release chlorophenol red for misR-deficient strain JK100::PlptA-lacZ. MisR genotypes are noted in parentheses.
DISCUSSION
We propose that the gonococcal MisR protein component of the MisR-MisS 2CRS functions as a transcriptional regulator of genes involved in determining cell envelope integrity. Herein, we provide phenotypic evidence that its loss increases gonococcal cell permeability and sensitizes gonococci to various antimicrobial agents, especially CAMPs and aminoglycosides. Additionally, we found that decoration of lipid A with phosphoethanolamine (a consequence of PhoP-PhoQ or PmrA-PmrB regulation in other bacteria [19, 52]) was not changed in the absence of MisR. It is also important to note that in preliminary experiments we did not observe an influence of Mg2+ levels on expression of the MisR autoregulatory target misRS in gonococci (data not presented), which is consistent with results from previous work performed with meningococci demonstrating that MisR-MisS is unresponsive to changes in Mg2+ (24). Taken together, these data suggest that despite some phenotypic overlap (e.g., importance for CAMP resistance, impaired growth in low-Mg2+ broth culture), MisR-MisS does not function as a gonococcal PhoP-PhoQ orthologue, nor does it appear to behave as such in meningococci (22–24).
With respect to CAMPs, our results indicate that MisR participates in constitutive and inducible resistance independently of MisS. We have not ruled out the possibility that MisS-independent phosphorylation of MisR can take place in gonococci and contribute to gene control. In other Gram-negative organisms, response regulators can be phosphorylated spontaneously by the phosphoryl donor molecule acetyl phosphate, leading to activation/repression of target genes (53, 54). This process is mediated by the activity of the Pta-AckA metabolic pathway and is robust when E. coli cells are grown in the presence of 0.4% glucose (55, 56), the very same concentration of glucose present in gonococcal GCB agar and broth (27). Since the Pta-AckA pathway is genetically intact in N. gonorrhoeae strains FA 1090 (http://www.genome.ou.edu/gono.html; http://www.kegg.jp/pathway/ngo01200) and FA19 (GenBank accession number CP012026), it is possible that gonococcal MisR could be constitutively phosphorylated by acetyl phosphate during growth in standard laboratory media. In this scenario, MisS-dependent MisR phosphorylation would be redundant, which might explain why we did not see a difference in PMB susceptibility due to the loss of the sensor kinase MisS. Additionally, even though MisR was required for inducible resistance to PMB, it is possible that other factors are directly responsible for sensing CAMP stress and that permeability differences in misR::kan mutants simply prevent those factors from conferring resistance.
We were intrigued that MisR-dependent resistance for most MtrCDE substrates was apparent only when MtrCDE was overexpressed (compare the MICs for strains KH15 and JK300 in Table 2). Since we demonstrated that the levels of this efflux pump were likely the same regardless of MisR expression (see the Results and Fig. S2 in the supplemental material), we concluded that MisR is required for efficient MtrCDE function but not expression. Whether MtrCDE impairment by the loss of MisR is due to improperly localized/misfolded MtrCDE components, decreased pumping power due to possible effects on the proton motive force, or an expedited passage of MtrCDE substrates through a flawed phospholipid bilayer is not yet clear. We also note the curious finding that ceftriaxone is most potent against misR::kan gonococci in an FA19 background (Table 2). Ceftriaxone is currently the front-line therapy for gonococcal infections in the United States, but several instances of ceftriaxone resistance around the world (see Fig. 13 in reference 57) hasten the approach of more treatment failures (3, 58). To our knowledge, this is the first report of a 2CRS being involved in modulating the levels of gonococcal susceptibility to a 3rd-generation cephalosporin (59, 60).
While our data suggest that gonococcal MisR is not regulating CAMP and aminoglycoside resistance through known mechanisms, our RNA-Seq data show that it does regulate the genes of numerous envelope-localized proteins, proteases/chaperones, and redox factors. That MisR targets these particular categories of genes is reminiscent of 2CRS regulators in other Gram-negative organisms, such as CpxR in Vibrio cholerae (61) and E. coli (reviewed in reference 26), AmgR in Pseudomonas aeruginosa (62), and, indeed, MisR in N. meningitidis (23). Gonococcal MisR (GenBank accession number EEZ45023.1; locus tag NGEG_00293) is 100% identical to meningococcal MisR (GenBank accession number AAF41023.1; locus tag NMB0595) at the amino acid level, and a simple BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) reveals that CpxR in E. coli strain K-12 and V. cholerae O1 biovar El Tor (50% and 48% amino acid sequence identity, respectively) and, likewise, AmgR in P. aeruginosa strain PAO1 (41% amino acid sequence identity) are the closest orthologues to MisR in these species. Similar to MisR in gonococci, CpxR and AmgR are required for intrinsic aminoglycoside (26, 62–64) and CAMP (65, 66) resistance. In contrast, expression of the well-characterized PhoP-PhoQ and PmrA-PmrB systems appears to be important primarily for CAMP but not aminoglycoside resistance in enteric pathogens (reviewed in reference 67).
A shared characteristic of CAMPs and aminoglycosides is that both classes of antimicrobials have been proposed to enter bacteria via self-promoted uptake in a membrane integrity-dependent manner (68–70). As the concentration of aminoglycoside increases, misreading of mRNA results in an accumulation of improperly folded proteins, which, if integrated into the membrane, are hypothesized to fit poorly between phospholipids; inappropriate membrane integration may generate nonspecific hydrophilic channels through which more aminoglycoside molecules (normally unable to efficiently permeate the phospholipid bilayer) may pass with relative ease (69, 71). Similarly, CAMPs, which initially bind parallel to the plane of bacterial membranes at the interface of the hydrophilic head groups and hydrophobic fatty acid tails, may associate at higher concentrations into membrane-disruptive aggregates (72). Thus, one possible explanation for the defective membrane integrity seen in the gonococcal misR::kan mutants is that aberrant protein folding or faulty translocation through the membranes, perhaps resulting from dysregulation of protein quality control genes, may predispose gonococci to CAMP and aminoglycoside entry by disrupting the normally stable packing of membrane phospholipids. Notably, the gene encoding the HtpX membrane protease, which is important for degrading misfolded cell envelope proteins (73), is a shared target for strong activation by MisR (Table 3), CpxR (74, 75), and AmgR (62–64, 76) across three different bacterial genera.
Additionally, the loss of MisR may result in an atypical redox environment due to MisR regulation of genes encoding redox factors, iron controllers, electron transport components, etc. (see Table S2 in the supplemental material), whose products are associated with reduction/oxidation reactions or oxidative stress responses. For example, we demonstrated (Table 3; see also Table S2A in the supplemental material) that the bacterioferritin genes bfrA and bfrB are strongly activated by MisR and recently reported with our collaborators that bidirectional MisR regulation of the transferrin binding protein genes tbpB and tbpA is important for iron uptake control (Kandler et al., submitted). Furthermore, a hallmark target for MisR activation is the gene dsbD (Table 3), which encodes the thiol:disulfide interchange protein DsbD important for proper disulfide bond formation in periplasmic proteins (23, 77). Published experiments performed with meningococci (78) have shown that misRS expression is increased in response to the presence of a reducing agent (DTT) in a MisS-dependent manner, which could indicate that MisS can directly sense changes to the periplasmic redox balance. Further work is needed to address this possibility in gonococci.
CAMPs are increasingly recognized as important components of the innate host defense during infection. Over the millennia, these antimicrobials have exerted selective pressure on bacteria to develop mechanisms to resist their action (79). As was recently reviewed (80), CAMP resistance mechanisms expressed by bacteria can have a significant influence on bacterial survival or fitness during infection. One of the early observations validating this concept in gonococci was that the loss of the MtrD efflux pump protein increased gonococcal susceptibility to LL-37 by approximately 8-fold in vitro (12) and led to a >100-fold reduction in the amount of viable gonococci at 3 days postinfection in vivo (14). While information regarding the importance of MisR during gonococcal infection is lacking, previous studies on meningococci showed that the loss of MisR significantly decreased the virulence of N. meningitidis in an experimental murine infection model (21), and we suspect that the CAMP-susceptible phenotype of misR-deficient gonococci would produce similar results. It is also important to emphasize that because some CAMP resistance systems (e.g., efflux by MtrCDE) can also influence bacterial susceptibility to antibiotics used in therapy, the capacity of bacteria to regulate the expression or function of mechanisms involved in such resistance could impact the clinical efficacy of antibiotics. The latter point is of importance for gonorrhea because the absence of a vaccine requires the continued availability of effective antibiotics, which is now threatened by the emergence of strains resistant to previously used or current front-line antibiotics (3, 81). The characterization herein of a novel CAMP/aminoglycoside/MtrCDE-substrate resistance mechanism in gonococci is of special interest in light of the dwindling number of curative antibiotics for gonorrhea, clinical isolates that overexpress mtrCDE (46), and the potential implementation of an aminoglycoside (gentamicin) as a first-line therapy in the United States (82).
Supplementary Material
ACKNOWLEDGMENTS
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States government.
We have no competing financial interest to declare.
We thank Yih-Ling Tzeng (Emory University School of Medicine, Atlanta, GA) for the kind gifts of DNA from misR::kan and misS::kan mutants of N. meningitidis and anti-MisR antiserum. We also thank Ann E. Jerse (Uniformed Services University of the Health Sciences, Bethesda, MD) for the kind gift of anti-MtrE antiserum.
Funding Statement
This work was supported by VA Merit Award 510 1BX000112-07 from the Biomedical Laboratory Research and Development Service of the U.S. Department of Veterans Affairs to W.M.S., by NIH grants R37 AI21150-31 (W.M.S.) and U19 AI113170-02 (to Ann E. Jerse), and by Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, U.S. Department of Energy, grant DE-FG02-93ER20097 (to R.W.C. and A.M.) to the DOE Center for Plant and Microbial Complex Carbohydrates at the Complex Carbohydrate Research Center. W.M.S. is the recipient of a Senior Research Career Scientist Award from the Biomedical Laboratory Research and Development Service of the U.S. Department of Veterans Affairs.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00823-16.
REFERENCES
- 1.CDC. 2014. Sexually transmitted disease surveillance 2014. CDC, Atlanta, GA: http://www.cdc.gov/std/stats14/surv-2014-print.pdf Accessed 4 April 2016. [Google Scholar]
- 2.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu XD, Harwig SS, Oren AM, Shafer WM, Lehrer RI. 1996. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect Immun 64:1240–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey SG, Shafer WM, Spitznagel JK. 1985. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun 47:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafer WM, Onunka VC, Martin LE. 1986. Antigonococcal activity of human neutrophil cathepsin G. Infect Immun 54:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafer WM, Martin LE, Spitznagel JK. 1986. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect Immun 53:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criss AK, Seifert HS. 2012. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Microbiol 10:178–190. doi: 10.1038/nrmicro2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MB, Criss AK. 2011. Resistance of Neisseria gonorrhoeae to neutrophils. Front Microbiol 2:77. doi: 10.3389/fmicb.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandler JL. 2014. Regulatory mechanisms that impact Neisseria gonorrhoeae survival of host innate immunity and antibiotics: the roles of LptA, TbpBA, and MisR. Ph.D. dissertation. Emory University, Atlanta, GA. https://etd.library.emory.edu/view/record/pid/emory:gshb0.
- 11.Juneau RA, Stevens JS, Apicella MA, Criss AK. 2015. A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J Infect Dis 212:316–324. doi: 10.1093/infdis/jiv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis LA, Choudhury B, Balthazar JT, Martin LE, Ram S, Rice PA, Stephens DS, Carlson R, Shafer WM. 2009. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect Immun 77:1112–1120. doi: 10.1128/IAI.01280-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packiam M, Yedery RD, Begum AA, Carlson RW, Ganguly J, Sempowski GD, Ventevogel MS, Shafer WM, Jerse AE. 2014. Phosphoethanolamine decoration of Neisseria gonorrhoeae lipid A plays a dual immunostimulatory and protective role during experimental genital tract infection. Infect Immun 82:2170–2179. doi: 10.1128/IAI.01504-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbs MM, Anderson JE, Balthazar JT, Kandler JL, Carlson RW, Ganguly J, Begum AA, Duncan JA, Lin JT, Sparling PF, Jerse AE, Shafer WM. 2013. Lipid A's structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 4:e00892–13. doi: 10.1128/mBio.00892-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prost LR, Miller SI. 2008. The salmonellae PhoQ sensor: mechanisms of detection of phagosome signals. Cell Microbiol 10:576–582. doi: 10.1111/j.1462-5822.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 18.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CR, Newcombe J, Thorne S, Borde HA, Eales-Reynolds LJ, Gorringe AR, Funnell SG, McFadden JJ. 2001. Generation and characterization of a PhoP homologue mutant of Neisseria meningitidis. Mol Microbiol 39:1345–1355. doi: 10.1111/j.1365-2958.2001.02324.x. [DOI] [PubMed] [Google Scholar]
- 21.Newcombe J, Eales-Reynolds LJ, Wootton L, Gorringe AR, Funnell SG, Taylor SC, McFadden JJ. 2004. Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity. Infect Immun 72:338–344. doi: 10.1128/IAI.72.1.338-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzeng YL, Datta A, Ambrose K, Lo M, Davies JK, Carlson RW, Stephens DS, Kahler CM. 2004. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J Biol Chem 279:35053–35062. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng YL, Kahler CM, Zhang X, Stephens DS. 2008. MisR/MisS two-component regulon in Neisseria meningitidis. Infect Immun 76:704–716. doi: 10.1128/IAI.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzeng YL, Zhou X, Bao S, Zhao S, Noble C, Stephens DS. 2006. Autoregulation of the MisR/MisS two-component signal transduction system in Neisseria meningitidis. J Bacteriol 188:5055–5065. doi: 10.1128/JB.00264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamet A, Rousseau C, Monfort JB, Frapy E, Nassif X, Martin P. 2009. A two-component system is required for colonization of host cells by meningococcus. Microbiology 155:2288–2295. doi: 10.1099/mic.0.027755-0. [DOI] [PubMed] [Google Scholar]
- 26.Raivio TL. 2014. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim Biophys Acta 1843:1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Dillard JP. 2011. Genetic manipulation of Neisseria gonorrhoeae. Curr Protoc Microbiol Chapter 4:Unit4A.2. doi: 10.1002/9780471729259.mc04a02s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehr IJ, Long CD, Serkin CD, Seifert HS. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ménard R, Sansonetti PJ, Parsot C. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol 175:5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao S, Montanez GE, Kumar P, Sannigrahi S, Tzeng YL. 2010. Regulatory role of the MisR/S two-component system in hemoglobin utilization in Neisseria meningitidis. Infect Immun 78:1109–1122. doi: 10.1128/IAI.00363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunn JS, Stein DC. 1996. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol Gen Genet 251:509–517. [DOI] [PubMed] [Google Scholar]
- 32.Zughaier SM, Kandler JL, Shafer WM. 2014. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PLoS One 9:e87688. doi: 10.1371/journal.pone.0087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vélez Acevedo RN, Ronpirin C, Kandler JL, Shafer WM, Cornelissen CN. 2014. Identification of regulatory elements that control expression of the tbpBA operon in Neisseria gonorrhoeae. J Bacteriol 196:2762–2774. doi: 10.1128/JB.01693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Silver LE, Clark VL. 1995. Construction of a translational lacZ fusion system to study gene regulation in Neisseria gonorrhoeae. Gene 166:101–104. doi: 10.1016/0378-1119(95)00605-6. [DOI] [PubMed] [Google Scholar]
- 36.Kandler JL, Joseph SJ, Balthazar JT, Dhulipala V, Read TD, Jerse AE, Shafer WM. 2014. Phase-variable expression of lptA modulates the resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides. Antimicrob Agents Chemother 58:4230–4233. doi: 10.1128/AAC.03108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner DM, Folster JP, Shafer WM, Jerse AE. 2007. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis 196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 38.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Methods Carbohydr Chem 5:83–91. [Google Scholar]
- 39.Rouquette-Loughlin C, Stojiljkovic I, Hrobowski T, Balthazar JT, Shafer WM. 2002. Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrC-MtrD-MtrE efflux pump requires TonB-ExbB-ExbD proteins. Antimicrob Agents Chemother 46:561–565. doi: 10.1128/AAC.46.2.561-565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohneck EA, Goytia M, Rouquette-Loughlin CE, Joseph SJ, Read TD, Jerse AE, Shafer WM. 2015. Overproduction of the MtrCDE efflux pump in Neisseria gonorrhoeae produces unexpected changes in cellular transcription patterns. Antimicrob Agents Chemother 59:724–726. doi: 10.1128/AAC.04148-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141(Pt 3):611–622. [DOI] [PubMed] [Google Scholar]
- 42.Lucas CE, Hagman KE, Levin JC, Stein DC, Shafer WM. 1995. Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Mol Microbiol 16:1001–1009. doi: 10.1111/j.1365-2958.1995.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 43.Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol 177:4162–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas CE, Balthazar JT, Hagman KE, Shafer WM. 1997. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J Bacteriol 179:4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohneck EA, Zalucki YM, Johnson PJ, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187-11. doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warner DM, Shafer WM, Jerse AE. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol 70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Münch R, Hiller K, Barg H, Heldt D, Linz S, Wingender E, Jahn D. 2003. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res 31:266–269. doi: 10.1093/nar/gkg037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eustice DC, Feldman PA, Colberg-Poley AM, Buckery RM, Neubauer RH. 1991. A sensitive method for the detection of beta-galactosidase in transfected mammalian cells. Biotechniques 11:739–740, 742–743. [PubMed] [Google Scholar]
- 49.Paradis-Bleau C, Kritikos G, Orlova K, Typas A, Bernhardt TG. 2014. A genome-wide screen for bacterial envelope biogenesis mutants identifies a novel factor involved in cell wall precursor metabolism. PLoS Genet 10:e1004056. doi: 10.1371/journal.pgen.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schieck E, Lartigue C, Frey J, Vozza N, Hegermann J, Miller RA, Valguarnera E, Muriuki C, Meens J, Nene V, Naessens J, Weber J, Lowary TL, Vashee S, Feldman MF, Jores J. 2016. Galactofuranose in Mycoplasma mycoides is important for membrane integrity and conceals adhesins but does not contribute to serum resistance. Mol Microbiol 99:55–70. doi: 10.1111/mmi.13213. [DOI] [PubMed] [Google Scholar]
- 51.Yun S, Lee EG, Kim SY, Shin JM, Jung WS, Oh DB, Lee SY, Kwon O. 2015. The CpxRA two-component system is involved in the maintenance of the integrity of the cell envelope in the rumen bacterium Mannheimia succiniciproducens. Curr Microbiol 70:103–109. doi: 10.1007/s00284-014-0686-5. [DOI] [PubMed] [Google Scholar]
- 52.Winfield MD, Groisman EA. 2004. Phenotypic differences between Salmonella and Escherichia coli resulting from the disparate regulation of homologous genes. Proc Natl Acad Sci U S A 101:17162–17167. doi: 10.1073/pnas.0406038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lima BP, Lennon CW, Ross W, Gourse RL, Wolfe AJ. 2016. In vitro evidence that RNA polymerase acetylation and acetyl phosphate-dependent CpxR phosphorylation affect cpxP transcription regulation. FEMS Microbiol Lett 363:fnw011. doi: 10.1093/femsle/fnw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfe AJ. 2010. Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr Opin Microbiol 13:204–209. doi: 10.1016/j.mib.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lima BP, Thanh Huyen TT, Basell K, Becher D, Antelmann H, Wolfe AJ. 2012. Inhibition of acetyl phosphate-dependent transcription by an acetylatable lysine on RNA polymerase. J Biol Chem 287:32147–32160. doi: 10.1074/jbc.M112.365502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Rensburg JJ, Fortney KR, Chen L, Krieger AJ, Lima BP, Wolfe AJ, Katz BP, Zhang ZY, Spinola SM. 2015. Development and validation of a high-throughput cell-based screen to identify activators of a bacterial two-component signal transduction system. Antimicrob Agents Chemother 59:3789–3799. doi: 10.1128/AAC.00236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.WHO. 2014. WHO antimicrobial resistance: global report on surveillance 2014. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 Accessed 4 April 2016. [Google Scholar]
- 58.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unemo M, Nicholas RA. 2012. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao S, Duncan M, Tomberg J, Davies C, Unemo M, Nicholas RA. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother 53:3744–3751. doi: 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acosta N, Pukatzki S, Raivio TL. 2015. The Vibrio cholerae Cpx envelope stress response senses and mediates adaptation to low iron. J Bacteriol 197:262–276. doi: 10.1128/JB.01957-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, Singh PK, Manoil C. 2009. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci U S A 106:14570–14575. doi: 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinz A, Lee S, Jacoby K, Manoil C. 2011. Membrane proteases and aminoglycoside antibiotic resistance. J Bacteriol 193:4790–4797. doi: 10.1128/JB.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau CH, Fraud S, Jones M, Peterson SN, Poole K. 2013. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2243–2251. doi: 10.1128/AAC.00170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Audrain B, Ferrieres L, Zairi A, Soubigou G, Dobson C, Coppee JY, Beloin C, Ghigo JM. 2013. Induction of the Cpx envelope stress pathway contributes to Escherichia coli tolerance to antimicrobial peptides. Appl Environ Microbiol 79:7770–7779. doi: 10.1128/AEM.02593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandez L, Alvarez-Ortega C, Wiegand I, Olivares J, Kocincova D, Lam JS, Martinez JL, Hancock RE. 2013. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:110–119. doi: 10.1128/AAC.01583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poole K. 2012. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother 67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 68.Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 69.Davis BD. 1987. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev 51:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hancock RE, Bell A. 1988. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis 7:713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 71.Davis BD, Chen LL, Tai PC. 1986. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A 83:6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hale JD, Hancock RE. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther 5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- 73.Akiyama Y. 2009. Quality control of cytoplasmic membrane proteins in Escherichia coli. J Biochem 146:449–454. doi: 10.1093/jb/mvp071. [DOI] [PubMed] [Google Scholar]
- 74.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol 191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimohata N, Chiba S, Saikawa N, Ito K, Akiyama Y. 2002. The Cpx stress response system of Escherichia coli senses plasma membrane proteins and controls HtpX, a membrane protease with a cytosolic active site. Genes Cells 7:653–662. doi: 10.1046/j.1365-2443.2002.00554.x. [DOI] [PubMed] [Google Scholar]
- 76.Lau CH, Krahn T, Gilmour C, Mullen E, Poole K. 2015. AmgRS-mediated envelope stress-inducible expression of the mexXY multidrug efflux operon of Pseudomonas aeruginosa. Microbiologyopen 4:121–135. doi: 10.1002/mbo3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tinsley CR, Voulhoux R, Beretti JL, Tommassen J, Nassif X. 2004. Three homologues, including two membrane-bound proteins, of the disulfide oxidoreductase DsbA in Neisseria meningitidis: effects on bacterial growth and biogenesis of functional type IV pili. J Biol Chem 279:27078–27087. doi: 10.1074/jbc.M313404200. [DOI] [PubMed] [Google Scholar]
- 78.Kumar P, Sannigrahi S, Scoullar J, Kahler CM, Tzeng YL. 2011. Characterization of DsbD in Neisseria meningitidis. Mol Microbiol 79:1557–1573. doi: 10.1111/j.1365-2958.2011.07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goytia M, Kandler JL, Shafer WM. 2013. Mechanisms and significance of bacterial resistance to human antimicrobial peptides. In Heimstra PS, Zaat SAJ (ed), Antimicrobial peptides and innate immunity. Springer, Basel, Switzerland. [Google Scholar]
- 80.Bauer ME, Shafer WM. 2015. On the in vivo significance of bacterial resistance to antimicrobial peptides. Biochim Biophys Acta 1848:3101–3111. doi: 10.1016/j.bbamem.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edwards JL, Jennings MP, Apicella MA, Seib KL. 23 January 2016. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit Rev Microbiol. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkcaldy RD, Weinstock HS, Moore PC, Philip SS, Wiesenfeld HC, Papp JR, Kerndt PR, Johnson S, Ghanem KG, Hook EW III. 2014. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 59:1083–1091. doi: 10.1093/cid/ciu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarubbi FA Jr, Blackman E, Sparling PF. 1974. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol 120:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC, Shafer WM. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143(Pt 7):2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 85.Folster JP, Shafer WM. 2005. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J Bacteriol 187:3713–3720. doi: 10.1128/JB.187.11.3713-3720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prentki P, Krisch HM. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.