Abstract
Severe pathophysiological changes in critical illness can lead to dramatically altered antimicrobial pharmacokinetics (PK). The additional effect of obesity on PK potentially increases the challenge for effective dosing. The aim of this prospective study was to describe the population PK of meropenem for a cohort of critically ill patients, including obese and morbidly obese patients. Critically ill patients prescribed meropenem were recruited into the following three body mass index (BMI) groups: nonobese (18.5 to 29.9 kg/m2), obese (30.0 to 39.9 kg/m2), and morbidly obese (≥40 kg/m2). Serial plasma samples were taken, and meropenem concentrations were determined using a validated chromatographic method. Population PK analysis and Monte Carlo dosing simulations were undertaken with Pmetrics. Nineteen critically ill patients with different BMI categories were enrolled. The patients' mean ± standard deviation (SD) age, weight, and BMI were 49 ± 15.9 years, 95 ± 22.0 kg, and 33 ± 7.0 kg/m2, respectively. A two-compartment model described the data adequately. The mean ± SD parameter estimates for the final covariate model were as follows: clearance (CL), 15.5 ± 6.0 liters/h; volume of distribution in the central compartment (V1), 11.7 ± 5.8 liters; intercompartmental clearance from the central compartment to the peripheral compartment, 25.6 ± 35.1 liters h−1; and intercompartmental clearance from the peripheral compartment to the central compartment, 8.32 ± 12.24 liters h−1. Higher creatinine clearance (CLCR) was associated with a lower probability of target attainment, with BMI having little effect. Although obesity was found to be associated with an increased V1, dose adjustment based on CLCR appears to be more important than patient BMI.
INTRODUCTION
The prevalence of obesity worldwide has continued to escalate during recent decades (1, 2). According to data from different organizations, more than two-thirds of adults in the United States are overweight or obese, and more than one-third are obese (3, 4). Obesity is thought to be a risk factor for mortality and morbidity from different types of infection in the intensive care unit (ICU), as shown for various types of surgical site infections (e.g., after hysterectomy [5] or spinal surgery [6]), community-acquired pneumonia (7), and peritonitis in peritoneal dialysis patients (8). Optimized drug dosing is likely to reduce the burden associated with infections in these patients, although there are only sparse data available for clinicians to guide antimicrobial dosing in obese patients.
Dosing in obese critically ill patients is considered highly challenging (9, 10). Indeed, the pathophysiological changes associated with both obesity and critical illness may have additive effects on altered pharmacokinetics (PK), although there are very limited published data on this topic (11, 12). The physiological differences in obese patients include changes in regional blood flow, increased cardiac output, and increased fat and lean mass (13). These changes may alter PK and PK/pharmacodynamics (PK/PD) of antimicrobials, necessitating dosing adjustment. As the prevalence of obesity increases, clinicians more frequently confront the dosing challenges in treating these patients.
Meropenem is a broad-spectrum antimicrobial of the carbapenem class which is frequently used as empirical or directed therapy in critically ill patients (14). Meropenem shows time-dependent antibacterial activity. To date, the PK data for meropenem have not been well described for critically ill obese patients. In vitro and animal infection model data suggest that maintaining unbound concentrations above the MIC for 40% of the dosing interval under steady-state PK conditions should be considered a minimum exposure target (40% TMIC). It remains unclear whether standard meropenem dosing regimens achieve this target in critically ill obese patients.
The aim of this prospective study was to describe the population PK of meropenem for a cohort of critically ill patients, including obese and morbidly obese patients.
MATERIALS AND METHODS
Setting.
This was an observational PK study using one-interval patient sampling at a tertiary referral ICU. Ethics approval was obtained from the local institutional Human Research Ethics Committee (approval no. HRC/14/QRBW/88). Written informed consent was obtained from all participants or from their substitute decision-makers.
Study population.
The inclusion criteria for this study were as follows: (i) age of ≥18 years, (ii) receiving meropenem (prophylaxis or treatment), and (iii) body mass index (BMI) of ≥18.5 kg/m2. The exclusion criteria were as follows: (i) patients on renal replacement therapy, (ii) pregnant women, (iii) actively bleeding patients, and (iv) patients with HIV or hepatitis.
Study protocol.
Meropenem was administered according to the intensivist's decision, with dosage regimens of 500 mg, 1 g, and 2 g. Participants were categorized into the following three groups according to BMI: nonobese (BMI = 18.5 to 29.9 kg/m2), obese (BMI = 30 to 39.9 kg/m2), and morbidly obese (BMI of ≥40 kg/m2). On a single occasion (one dosing interval), six blood samples were taken from each participant to determine plasma meropenem concentrations. Blood samples (about 3 ml) were drawn from the participants at the following times: predose and 30 min (end of infusion), 45 min, 1 h, 4 h, and 8 h after dose administration. Other clinical and demographic data were collected on the day of plasma sampling, including age, sex, weight, height, and BMI. Clinical data were also recorded, including SOFA and APACHE II scores, plasma albumin levels, and serum creatinine concentrations (Scr).
Sample handling, storage, and assay.
Collected blood samples were placed immediately in an ice bath and were centrifuged at 3,000 rpm for 10 min. Plasma samples were stored at −80°C until bioanalysis. Meropenem concentrations in plasma were determined by validated high-performance liquid chromatography with UV detection (HPLC-UV) on a Shimadzu Prominence instrument. Sample analysis was conducted in batches, with calibration standards and quality controls to which batch acceptance criteria were applied. Acetonitrile was added to a 100-μl aliquot of plasma combined with an internal standard (cefotaxime) to precipitate proteins. Following centrifugation, the supernatant was isolated and washed with dichloromethane to remove acetonitrile and lipophilic components. Following centrifugation, the upper layer was isolated for chromatographic analysis. The stationary phase was a Waters XBridge C18 2.1-mm by 50-mm column. The mobile phase was 4% acetonitrile-96% 50 mM phosphate buffer at pH 2.5 delivered isocratically. The eluent was monitored at 304 nm.
The calibration curve was linear, with a weighing of 1/x2 over the range of 0.2 to 100 μg/ml. The precision and accuracy at the lower limit of quantification (LLOQ) were ≤5.9%. The assay was validated against matrix effects (precision and accuracy within 4% at high and low concentrations). The assay's precision and accuracy were determined for both within-day and between-day comparisons and were within 6.5% at all three concentrations tested. Bioanalysis techniques were validated and conducted in accordance with the criteria of the U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (15).
Population pharmacokinetic modeling.
The plasma meropenem concentrations were fitted to one- and two-compartment models by using a nonparametric adaptive grid (NPAG) algorithm within the Pmetrics package for R (Laboratory of Applied Pharmacokinetics and Bioinformatics, Los Angeles, CA) (16, 17). Clearance (CL) from the central compartment and intercompartmental distribution were modeled as first-order processes.
Demographic and clinical characteristics that were considered biologically plausible for affecting meropenem PK were tested for inclusion as covariates. Age, total body weight (TBW), ideal body weight (IBW), lean body weight (LBW), sex, BMI, BMI category, Scr, creatinine clearance (CLCR) (estimated by the Cockcroft-Gault equation by separately using TBW, LBW, and IBW), albumin level, SOFA score, and APACHE II score were tested. Each of these covariates was plotted against the PK parameter estimates to assess the level of correlation. Covariates were retained in the model if they showed a significant improvement in the log likelihood (P < 0.05) and/or improved the goodness-of-fit plots.
Model diagnostics.
A visual prediction check (VPC) of the observed-predicted concentration scatterplot, the coefficient of determination of linear regression of observed-predicted values, and the log likelihood values for each run were used to evaluate the goodness of fit. Predictive performance evaluation was based on the means for both prediction error (bias) and bias-adjusted squared prediction error (imprecision) for the population and individual prediction models for the central compartment.
PTA.
Monte Carlo simulations (n = 1,000) were performed using Pmetrics software to determine the probability of target attainment (PTA) with a variety of MICs for CLCR values and BMI classes. Meropenem doses of 500 mg given intravenously (i.v.) every 8 h (q8h) as intermittent 30-min or 3-h prolonged infusions, 1,000 mg given i.v. q8h as intermittent or prolonged infusions, and 2,000 mg given i.v. q8h as intermittent or prolonged infusions were simulated at three different levels of renal function (CLCR = 30, 50, and 150 ml/min) and for three BMI categories (nonobese, obese, and morbidly obese). The PTA for achieving 40% TMIC (meropenem plasma concentration remains above the MIC for at least 40% of the dosing interval) was calculated for the first 24 h of therapy (3 doses given q8h). Unbound concentrations were calculated using previously published data on the free fraction of meropenem (98%) (18).
FTA calculation.
MIC data for pathogens that are commonly targeted for treatment with meropenem, i.e., Acinetobacter baumannii and Pseudomonas aeruginosa, were obtained from the European Committee for Antimicrobial Susceptibility and Testing (EUCAST) database (www.eucast.org) to determine the fractional target attainment (FTA). The FTA describes the pharmacodynamic exposure (PTA) of meropenem against a MIC distribution. The FTA threshold was achieved when the value exceeded 90%. Susceptible MIC distributions for both pathogens (MICs of ≤2 mg/liter) were used to determine the FTA for directed therapy. Additionally, we determined the FTA for the entire MIC distribution (including values for susceptible and resistant isolates) to describe dosing during empirical therapy.
Statistical analysis.
Continuous variables are presented as means (standard deviations [SD]) or medians (interquartile ranges), as appropriate. Categorical variables are expressed as absolute numbers and relative frequencies. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test for normality. One-way analysis of variance (ANOVA) was used to test for differences in demographic and clinical data between the BMI categories. Linear regression was used to describe correlations between patient weight metrics in the 3 BMI categories and the volume of distribution in the central compartment (V1) and CL values for meropenem. All statistical analyses were performed using the statistical software package IBM-SPSS Statistics 22.0 (IBM, New York, NY). P values of <0.05 were considered statistically significant.
RESULTS
Demographic and clinical data.
Nineteen critically ill patients (11 males) were enrolled in the study, including seven nonobese, six obese, and six morbidly obese patients. In total, 112 plasma samples were obtained from these patients. The demographic and clinical data for the respective BMI categorizations are shown in Table 1. Only patients' weights, BMIs, and APACHE II scores were significantly different between the three BMI categorizations (P < 0.05).
TABLE 1.
Demographic and clinical data for patients in this study
| Variablea | Mean value (SD)b |
P value | |||
|---|---|---|---|---|---|
| All patients (n = 19) | Nonobese patients (n = 6) | Obese patients (n = 7) | Morbidly obese patients (n = 6) | ||
| Age (yr) | 49 (15.9) | 41 (19.1) | 49 (16.2) | 58 (7.6) | 0.20 |
| Weight (kg) | 95 (22.0) | 71 (11.6) | 103 (6.8) | 109 (23.3) | <0.01 |
| Ideal body weight (kg) | 65 (12.9) | 65 (6.7) | 72 (6.3) | 55 (17.8) | 0.05 |
| Lean body weight (kg) | 60 (13.1) | 53 (7.3) | 70 (4.7) | 55 (17.7) | 0.03 |
| Height (cm) | 171 (12.2) | 173 (6.0) | 177 (7.0) | 162 (17.0) | 0.07 |
| Sex (male) | 11 (58) | 2 (33) | 7 (100) | 2 (33) | |
| BMI (kg/m2) | 33 (7.0) | 24 (3.4) | 33 (2.2) | 41 (1.4) | <0.01 |
| Scr (μmol/liter) | 72 (24.0) | 62 (26) | 89 (20.2) | 62 (16.2) | 0.05 |
| CG-TBW (ml/min) | 151 (62.6) | 139 (44.9) | 114 (39.3) | 206 (66.7) | 0.02 |
| CG-IBW (ml/min) | 100 (47.5) | 135 (59.9) | 80 (30.3) | 87 (34.2) | 0.07 |
| CG-LBW (ml/min) | 89 (33.5) | 107 (40.9) | 77 (27.9) | 86 (29.2) | 0.29 |
| Albumin level (g/liter) | 26 (6.1) | 24 (8.1) | 28 (5.1) | 24 (4.8) | 0.40 |
| SOFA score | 6 (3.9) | 5 (2.8) | 6 (4.9) | 6 (3.8) | 0.80 |
| APACHE II score | 20 (6.9) | 26 (3.7) | 15 (4.2) | 20 (6.9) | <0.01 |
APACHE II, acute physiology and chronic health evaluation; BMI, body mass index; CLCR, measured creatinine clearance; CG-TBW, estimated CLCR calculated using Cockcroft-Gault equation based on total body weight; CG-IBW, estimated CLCR calculated using Cockcroft-Gault equation based on ideal body weight; CG-LBW, estimated CLCR calculated using Cockcroft-Gault equation based on lean body weight; Scr, serum creatinine; SOFA, sequential organ failure assessment.
Data on male gender are presented as numbers (%) of patients.
Pharmacokinetic model building.
The meropenem PK was best described by a two-compartment linear model with zero-order input of drug into the central compartment (Fig. 1). Regarding covariates, the Cockcroft-Gault CLCR (CG-CLCR) was tested, with CG-CLCR calculated using TBW, LBW, and IBW separately. The CG-CLCR calculated using TBW (normalized to 100 ml/min) for meropenem CL improved the model fit best. For the meropenem volume of distribution in the central compartment (V1), we applied two categories of BMI (above and below 35 kg/m2), as this resulted in a more significant improvement in the model than using one or three categories. Furthermore, a scaling factor for the effect of obesity (O) on V1 in the group with BMIs of >35 kg/m2 was included. When these covariates were added, each resulted in a statistically significant improvement in the log likelihood from the previous model (P < 0.01). The final model was as follows:
| (1) |
| (2) |
| (3) |
where CG-CLCR is the estimated CLCR calculated using the Cockcroft-Gault equation, TVCL is the typical value for meropenem clearance, CL is the population parameter estimate for meropenem clearance, TVV1 is the typical value for the meropenem volume of distribution in the central compartment, V1 is the population parameter estimate for the volume of the central compartment, and O is a scaling factor for obesity.
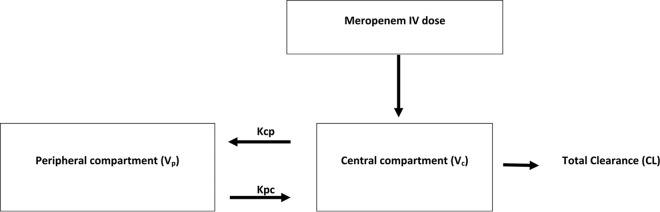
FIG 1.
Structural PK model for meropenem in critically ill obese and nonobese patients. The model contains the volumes of distribution for the central compartment (plasma; Vc) and the peripheral compartment (Vp), a rate constant for meropenem distribution from the central to the peripheral compartment (Kcp), and a rate constant for meropenem distribution from the peripheral to the central compartment (Kpc).
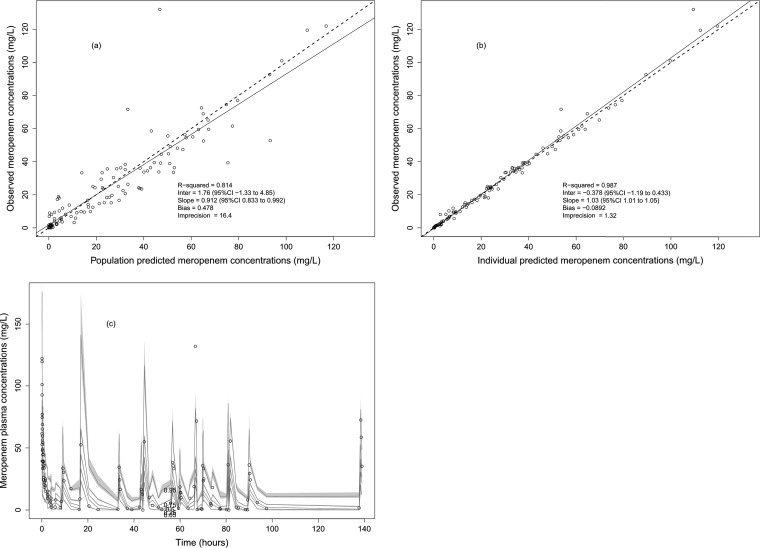
The mean ± SD population pharmacokinetic parameter estimates from the final covariate model are shown in Table 2. The diagnostic plots to confirm the goodness of fit of the model were considered acceptable and are shown in Fig. 2. The final covariate model was then used for Monte Carlo dosing simulations.
TABLE 2.
Parameter estimates for meropenem obtained from the final covariate two-compartment population pharmacokinetic model
| Parametera | Mean (SD) | Coefficient of variation (%) | Median |
|---|---|---|---|
| CL (liters/h) | 15.50 (5.99) | 38.8 | 14.3 |
| V1 (liters) | 11.66 (5.75) | 49.3 | 11.1 |
| kCP (h−1) | 25.60 (35.14) | 137.2 | 5.2 |
| kPC (h−1) | 8.32 (12.24) | 147.1 | 3.9 |
| O | 1.43 (0.46) | 32.0 | 1.5 |
CL, population clearance of meropenem; V1, population volume of distribution in the central compartment; kCP, rate constant for meropenem distribution from the central to the peripheral compartment; kPC, rate constant for meropenem distribution from the peripheral to the central compartment; O, scaling factor for obesity.
FIG 2.
Diagnostic plots for the final population pharmacokinetic covariate model. (a) Observed meropenem concentrations versus population predicted meropenem concentrations (R2 = 0.814). (b) Observed meropenem concentrations versus individual predicted meropenem concentrations (R2 = 0.987). (c) Visual predictive check.
Figure 3 shows the observed relationships between V1 and CL and the mean body weights for the three BMI categories. None of the measured correlations were statistically significant, although as described in our model building process, the inclusion of the effect of BMI on V1 improved the goodness-of-fit plots of the model.
FIG 3.
(Left) Relationship of meropenem clearance to the mean (SD) body weights for the BMI categorizations (for clearance versus BMI linear regression, r2 = 0.4915). (Right) Relationship of volume of distribution of the central compartment (V1) to the mean (SD) body weights for the prespecified BMI categorizations (nonobese, obese, and morbidly obese) (for V1 versus BMI linear regression, r2 = 0.4961).
Dosing simulations.
Monte Carlo simulations and PTA for achieving 40% TMIC for various meropenem doses are presented in Table 3. The results showed that increasing CLCR was associated with a lower PTA for different BMI categories. Furthermore, at the high CLCR of 150 ml/min, the intermittent dosing regimens of 500 mg and 1,000 mg consistently failed to achieve the PK/PD target for a MIC of 2 mg/liter in almost all BMI groups. In contrast, all prolonged-infusion doses as well as intermittent infusions of 2,000 mg achieved PK/PD targets up to a MIC of at least 2 mg/liter.
TABLE 3.
Meropenem probabilities of target attainment for different BMI groups, CLCR values, and dosage regimens and methods of administrationa
| Dose (mg) q8h (infusion interval) | BMI group | CLCR (ml/min) | Attainment of PTA for MIC (mg/liter) |
|||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 16 | |||
| 500 (II) | Nonobese | 30 | + | + | + | + | − | − |
| 50 | + | + | + | + | − | − | ||
| 150 | − | − | − | − | − | − | ||
| Obese | 30 | + | + | + | + | − | − | |
| 50 | + | + | + | + | − | − | ||
| 150 | − | − | − | − | − | − | ||
| Morbidly obese | 30 | + | + | + | + | − | − | |
| 50 | + | + | + | + | − | − | ||
| 150 | + | − | − | − | − | − | ||
| 500 (PI) | Nonobese | 30 | + | + | + | + | − | − |
| 50 | + | + | + | + | − | − | ||
| 150 | + | + | + | − | − | − | ||
| Obese | 30 | + | + | + | + | − | − | |
| 50 | + | + | + | + | − | − | ||
| 150 | + | + | + | − | − | − | ||
| Morbidly obese | 30 | + | + | + | + | − | − | |
| 50 | + | + | + | + | − | − | ||
| 150 | + | + | + | − | − | − | ||
| 1,000 (II) | Nonobese | 30 | + | + | + | + | + | − |
| 50 | + | + | + | + | + | − | ||
| 150 | − | − | − | − | − | − | ||
| Obese | 30 | + | + | + | + | + | − | |
| 50 | + | + | + | + | + | − | ||
| 150 | + | − | − | − | − | − | ||
| Morbidly obese | 30 | + | + | + | + | + | − | |
| 50 | + | + | + | + | + | − | ||
| 150 | + | + | − | − | − | − | ||
| 1,000 (PI) | Nonobese | 30 | + | + | + | + | + | − |
| 50 | + | + | + | + | + | − | ||
| 150 | + | + | + | + | − | − | ||
| Obese | 30 | + | + | + | + | + | − | |
| 50 | + | + | + | + | + | − | ||
| 150 | + | + | + | + | − | − | ||
| Morbidly obese | 30 | + | + | + | + | + | − | |
| 50 | + | + | + | + | + | − | ||
| 150 | + | + | + | + | − | − | ||
| 2,000 (II) | Nonobese | 30 | + | + | + | + | + | + |
| 50 | + | + | + | + | + | + | ||
| 150 | + | − | − | − | − | − | ||
| Obese | 30 | + | + | + | + | + | + | |
| 50 | + | + | + | + | + | + | ||
| 150 | + | + | − | − | − | − | ||
| Morbidly obese | 30 | + | + | + | + | + | + | |
| 50 | + | + | + | + | + | + | ||
| 150 | + | + | + | − | − | − | ||
| 2,000 (PI) | Nonobese | 30 | + | + | + | + | + | + |
| 50 | + | + | + | + | + | + | ||
| 150 | + | + | + | + | + | − | ||
| Obese | 30 | + | + | + | + | + | + | |
| 50 | + | + | + | + | + | + | ||
| 150 | + | + | + | + | + | − | ||
| Morbidly obese | 30 | + | + | + | + | + | + | |
| 50 | + | + | + | + | + | + | ||
| 150 | + | + | + | + | + | − | ||
The target was for the drug concentration to remain above the MIC for 40% of the dosage interval to achieve bactericidal activity. The meropenem target MIC was chosen according to the EUCAST breakpoint (2 mg/liter). BMI, body mass index; q8h, three-times-daily dosing; CLCR, creatinine clearance; II, intermittent infusion; IP, prolonged infusion.
Fractional target attainment.
The FTA values for different simulated dosing regimens and patient BMIs and CLCR for both directed and empirical coverage of A. baumannii and P. aeruginosa are shown in Table 4. For empirical therapy, meropenem at 500 mg q8h as intermittent or prolonged infusions failed to achieve 90% coverage of A. baumannii in all BMI groups at different CLCR levels. However, as CLCR increased (i.e., CLCR of ≥50 ml/min), meropenem given at 500 mg q8h as intermittent or prolonged infusions also failed to achieve 90% coverage of P. aeruginosa. Using the higher meropenem dose of 2,000 mg q8h as prolonged infusions enabled coverage of ≥90% of P. aeruginosa organisms in all BMI groups at different CLCR levels. However, this higher dose of meropenem failed to achieve 90% coverage of A. baumannii in obese and morbidly obese patients at CLCR levels of 150 ml/min or greater.
TABLE 4.
Fractional target attainment for various meropenem dosing regimens, CLCR values, and BMI groupsa
| Dose (mg) q8h (infusion interval) | BMI group | CLCR (ml/min) | FTA (%) |
|||
|---|---|---|---|---|---|---|
|
Acinetobacter baumannii |
Pseudomonas aeruginosa |
|||||
| MIC for directed therapy | MIC for empirical therapy | MIC for directed therapy | MIC for empirical therapy | |||
| 500 (II) | Nonobese | 30 | 98.93 | 86.22 | 99.02 | 90.92 |
| 50 | 97.86 | 81.64 | 98.06 | 86.44 | ||
| 150 | 74.31 | 57.03 | 77.91 | 62.90 | ||
| Obese | 30 | 99.15 | 85.62 | 99.25 | 90.52 | |
| 50 | 98.38 | 81.75 | 98.54 | 86.57 | ||
| 150 | 82.14 | 62.98 | 85.20 | 68.75 | ||
| Morbidly obese | 30 | 99.34 | 84.70 | 99.44 | 89.58 | |
| 50 | 98.69 | 81.67 | 98.88 | 86.46 | ||
| 150 | 87.65 | 67.14 | 90.06 | 72.61 | ||
| 500 (PI) | Nonobese | 30 | 99.93 | 87.66 | 99.95 | 92.29 |
| 50 | 99.89 | 84.40 | 99.92 | 89.28 | ||
| 150 | 99.61 | 77.69 | 99.73 | 81.98 | ||
| Obese | 30 | 99.83 | 86.51 | 99.87 | 91.34 | |
| 50 | 99.81 | 83.53 | 99.86 | 88.38 | ||
| 150 | 99.25 | 76.94 | 99.48 | 81.27 | ||
| Morbidly obese | 30 | 99.72 | 85.22 | 99.81 | 90.03 | |
| 50 | 99.67 | 82.79 | 99.77 | 87.58 | ||
| 150 | 98.60 | 76.14 | 99.06 | 80.57 | ||
| 1,000 (II) | Nonobese | 30 | 99.19 | 94.33 | 99.24 | 96.62 |
| 50 | 98.38 | 88.62 | 98.49 | 92.48 | ||
| 150 | 84.58 | 67.19 | 86.56 | 72.37 | ||
| Obese | 30 | 99.43 | 94.12 | 99.48 | 96.55 | |
| 50 | 99.67 | 91.84 | 99.72 | 95.07 | ||
| 150 | 90.77 | 72.44 | 91.99 | 77.36 | ||
| Morbidly obese | 30 | 99.63 | 92.65 | 99.67 | 95.61 | |
| 50 | 99.23 | 88.19 | 99.32 | 92.48 | ||
| 150 | 94.49 | 75.81 | 95.18 | 80.54 | ||
| 1,000 (PI) | Nonobese | 30 | 99.98 | 95.72 | 99.99 | 97.82 |
| 50 | 99.97 | 91.86 | 99.98 | 95.29 | ||
| 150 | 99.97 | 83.51 | 99.98 | 88.28 | ||
| Obese | 30 | 99.94 | 94.92 | 99.96 | 97.26 | |
| 50 | 99.94 | 90.56 | 99.96 | 94.39 | ||
| 150 | 99.93 | 82.97 | 99.95 | 87.71 | ||
| Morbidly obese | 30 | 99.95 | 92.99 | 99.97 | 95.94 | |
| 50 | 99.95 | 89.38 | 99.97 | 93.52 | ||
| 150 | 99.91 | 82.44 | 99.94 | 87.14 | ||
| 2,000 (II) | Nonobese | 30 | 99.33 | 96.62 | 99.38 | 98.18 |
| 50 | 98.68 | 94.80 | 98.75 | 96.75 | ||
| 150 | 90.15 | 75.28 | 91.19 | 80.01 | ||
| Obese | 30 | 99.58 | 96.75 | 99.61 | 98.33 | |
| 50 | 99.04 | 95.27 | 99.10 | 97.20 | ||
| 150 | 94.19 | 79.69 | 94.76 | 84.29 | ||
| Morbidly obese | 30 | 99.75 | 96.62 | 99.77 | 98.31 | |
| 50 | 99.47 | 95.46 | 99.52 | 97.45 | ||
| 150 | 96.38 | 82.37 | 96.72 | 87.02 | ||
| 2,000 (PI) | Nonobese | 30 | 100.00 | 97.52 | 100.00 | 98.99 |
| 50 | 100.00 | 97.09 | 100.00 | 98.73 | ||
| 150 | 100.00 | 90.09 | 100.00 | 94.14 | ||
| Obese | 30 | 100.00 | 97.81 | 100.00 | 99.45 | |
| 50 | 100.00 | 97.27 | 100.00 | 99.11 | ||
| 150 | 100.00 | 89.60 | 100.00 | 93.86 | ||
| Morbidly obese | 30 | 100.00 | 97.41 | 100.00 | 99.17 | |
| 50 | 100.00 | 96.79 | 100.00 | 98.77 | ||
| 150 | 100.00 | 88.84 | 100.00 | 93.23 | ||
BMI, body mass index; CLCR, creatinine clearance; II, intermittent infusion; PI, prolonged infusion.
For directed therapy, meropenem at 500 mg q8h as intermittent infusions failed to achieve 90% coverage of A. baumannii and P. aeruginosa when CLCR was 150 ml/min or higher. Using meropenem at 500 mg q8h as prolonged infusions or use of an increased dose (i.e., 1,000 mg and 2,000 mg) enabled coverage of ≥90% of A. baumannii and P. aeruginosa organisms in all BMI groups at different CLCR levels.
DISCUSSION
To the best our knowledge, this is the first prospective population PK study of meropenem in morbidly obese, obese, and nonobese critically ill patients. We found that BMI was a significant covariate describing meropenem V1, but when BMI was included in dosing simulations, the presence of different BMIs did not greatly affect PK/PD target attainment. Importantly, we observed that a higher CLCR (≥150 ml/min) was associated with a lower achievement of PK/PD targets for all patients, while a standard dosing regimen achieved PK/PD targets for patients with low and normal kidney function.
As with antibiotic studies, the susceptibility of the pathogen is of paramount importance to effective drug therapy. In this case, we evaluated the fractional target attainment against A. baumannii and P. aeruginosa with susceptible MIC distributions that are encountered as part of directed therapy. We found for almost all dosing scenarios that meropenem achieved PK/PD targets successfully, with the exception of lower doses in the presence of high CLCR (>150 ml/min). This suggests that when meropenem is used for directed therapy against pathogens with MICs of <2 mg/liter, dose adjustment is rarely necessary. However, when meropenem is used as part of empirical therapy before the susceptibilities of the pathogens are known, depending on local susceptibility patterns in the case of possible A. baumannii or P. aeruginosa infection, higher doses and/or use of prolonged infusion should be considered until the pathogen and susceptibility have been characterized.
At higher CLCR levels (≥150 ml/min) in critically ill patients in all BMI groups, intermittent meropenem dosing regimens consistently failed to achieve PK/PD targets. This failure could be remedied by adjusting either the dose or the duration of infusion. Specifically, meropenem at 500 mg or 1,000 mg q8h did not achieve the PK/PD target for the EUCAST breakpoint for A. baumannii and P. aeruginosa of a MIC of 2 mg/liter. When the doses were escalated to 2,000 mg q8h, the PK/PD target was achieved. Similarly, when meropenem was administered as a prolonged infusion (3 h), PK/PD target achievement increased significantly, even for lower doses of 500 mg q8h. This finding is not new for use of meropenem in critically ill patients, but it is novel in the context of the range of BMIs investigated in this study.
Of all the patient characteristics tested in this study, CLCR had by far the greatest influence on achievement of PK/PD targets for meropenem. Even increasing BMI, which has been proposed to likely be associated with reduced meropenem PK exposures, had a far smaller overall effect than CLCR. The BMI effect was described in our model as being associated with changes in the V1, which may indicate that altered concentrations are most likely in early dosing intervals but will be irrelevant thereafter, when dosing should be performed based only on CLCR. Interestingly, as shown in Fig. 3, BMI was not well correlated with meropenem CL or V1. Other weight descriptors were also not correlated, highlighting the variable effect that increasing body weight has on meropenem PK.
A recent retrospective study of 1,400 patients evaluated the effect of obesity on the unbound plasma concentrations of piperacillin and meropenem (19). For meropenem, the presence of obesity did not significantly affect unbound concentrations. The authors of that study used logistic regression to demonstrate that dosing based on CLCR was the strongest predictor of therapeutic concentrations in critically ill patients, including the obese (odds ratio [OR], 21.74; 95% confidence interval [CI], 6.02 to 76.92). Another study retrospectively evaluated the PK of broad-spectrum β-lactam antibiotics, including meropenem, in critically ill obese and nonobese patients. That study analyzed routine therapeutic drug monitoring (TDM) data from 17 obese (BMI of ≥30 kg/m2) and 17 nonobese (BMI of <25 kg/m2) patients and found that meropenem CL values were not significantly different between the critically ill obese and nonobese patients (20). However, the total volume of distribution was nonsignificantly higher in the obese group (40.0 liters versus 27.9 liters; P = 0.10). The authors concluded that TDM should be performed routinely for obese critically ill patients.
In a small prospective PK study of 9 morbidly obese critically ill patients (mean ± SD BMI, 54.7 ± 8.6 kg/m2), Cheatham et al. (21) also found that CL values for meropenem were similar between the enrolled morbidly obese patients and previously published data on nonobese patients. Like the present study and that of Hites et al. (20), the total volume of distribution (in that study described as the volume of distribution at steady state [Vss]) was numerically larger in the morbidly obese group (37.8 liters versus 21.7 liters). It was suggested that standard dosing regimens of meropenem can provided adequate PK/PD target attainment for susceptible pathogens (MICs of ≤2 mg/liter). The data from these previous studies generally support our results, with BMI, often described as an obesity category, affecting V but not CL and having little effect on achievement of PK/PD targets.
This analysis has some limitations. First, estimation of CLCR by using the Cockcroft-Gault method is known to be suboptimal for critically ill patients. However, it is still commonly used clinically, although 8- or 24-h urinary CLCR measurements should be used where possible for increased accuracy (22–24). Given that we did not have urinary CLCR values, we used Cockcroft-Gault CLCR values in our modeling process and found that they greatly improved the model, and thus they were retained. Notably, the weight descriptor for CG-CLCR was TBW, and clinicians should use this weight metric in calculating CG-CLCR for meropenem dosing. Second, although this is the largest prospective PK study of its type for meropenem, the sample size in this study is not sufficient for quantification of the effect of meropenem exposure on patient outcomes. Third, in the morbidly obese group (n = 6), the higher BMI was associated predominantly with a lower patient height rather than a higher weight. It is possible that a high BMI due to high weight may affect the PK of meropenem in a different way. Furthermore, increasing the sample size may also have helped to define additional patient factors associated with altered PK and to include a wider range of heights and weights, although these may not be clinically relevant.
Conclusions.
In summary, this analysis presents the first population pharmacokinetic study of meropenem in critically ill patients with three different BMI categories. BMI appears to have a minimal effect on PK/PD target attainment in critically ill patients, while increasing CG-CLCR (calculated using TBW) values were strongly associated with lower PK/PD target attainment rates. Higher doses or prolonged infusions should be applied for pathogens that have a higher MIC and/or for critically ill obese and nonobese patients with high CLCR values. TDM of meropenem should be used where possible to help to optimize meropenem dosing regimens accordingly.
ACKNOWLEDGMENTS
We recognize funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (grant APP1099452), from the Intensive Care Foundation, and from A.S.A. via The Saudi Arabian Culture Mission (SACM), affiliate to the Royal Embassy of Saudi Arabia. J.A.R. received a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1048652).
REFERENCES
- 1.Dixon JB. 2015. The global burden of obesity and diabetes, p 1–6. In Brethauer SA, Schauer PR, Schirmer BD (ed), Minimally invasive bariatric surgery. Springer, New York, NY. [Google Scholar]
- 2.Keating C, Backholer K, Gearon E, Stevenson C, Swinburn B, Moodie M, Carter R, Peeters A. 2015. Prevalence of class-I, class-II and class-III obesity in Australian adults between 1995 and 2011–12. Obes Res Clin Pract 9:553–562. doi: 10.1016/j.orcp.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Weight-control Information Network. 2012. Overweight and obesity statistics. U.S. Department of Health and Human Services, Weight-control Information Network, Bethesda, Maryland: http://www.niddk.nih.gov/health-information/health-statistics/Documents/stat904z.pdf. [Google Scholar]
- 4.Fryar CD, Carroll MD, Ogden CL. 2015. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. CDC, Atlanta, GA: http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm. [Google Scholar]
- 5.Löfgren M, Poromaa IS, Stjerndahl JH, Renström B. 2004. Postoperative infections and antibiotic prophylaxis for hysterectomy in Sweden: a study by the Swedish National Register for Gynecologic Surgery. Acta Obstet Gynecol Scand 83:1202–1207. [DOI] [PubMed] [Google Scholar]
- 6.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, Fraser VJ. 2003. Risk factors for surgical site infection in spinal surgery. J Neurosurg Spine 98:149–155. doi: 10.3171/spi.2003.98.2.0149. [DOI] [PubMed] [Google Scholar]
- 7.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. 2000. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 8.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. 2004. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 24:340–346. [PubMed] [Google Scholar]
- 9.Lisboa T, Rello J, Richart C, Anzueto A, El Solh AA. 2009. Obesity and critical care. Clin Pulm Med 16:202–211. doi: 10.1097/CPM.0b013e3181ad2171. [DOI] [Google Scholar]
- 10.Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. 2016. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents 47:259–268. doi: 10.1016/j.ijantimicag.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 12.Blot SI, Pea F, Lipman J. 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient— concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Chung S, Jain L, Khurana M, Lau S, Lee J, Vaidyanathan J, Zadezensky I, Choe S, Sahajwalla C. 2011. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther 90:77–89. doi: 10.1038/clpt.2011.104. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin CM, Lyseng-Williamson KA, Keam SJ. 2008. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs 68:803–838. doi: 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 15.Center for Drug Evaluation and Research. 2001. Guidance for industry: bioanalytical method validation. Center for Drug Evaluation and Research, Rockville, MD: www.fda.gov/downloads/Drugs/Guidances/ucm070107. [Google Scholar]
- 16.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neely M, van Guilder M, Yamada W, Schumitzky A, Jelliffe R. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a non-parametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig WA. 1997. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis 24:S266–S275. doi: 10.1093/clinids/24.Supplement_2.S266. [DOI] [PubMed] [Google Scholar]
- 19.Alobaid AS, Brinkmann A, Frey OR, Roehr AC, Luque S, Grau S, Wong G, Abdul-Aziz MH, Roberts MS, Lipman J, Roberts JA. 2016. What is the effect of obesity on piperacillin and meropenem trough concentrations in critically ill patients? J Antimicrob Chemother 71:696–702. doi: 10.1093/jac/dkv412. [DOI] [PubMed] [Google Scholar]
- 20.Hites M, Taccone FS, Wolff F, Cotton F, Beumier M, De Backer D, Roisin S, Lorent S, Surin R, Seyler L, Vincent JL, Jacobs F. 2013. Case-control study of drug monitoring of beta-lactams in obese critically ill patients. Antimicrob Agents Chemother 57:708–715. doi: 10.1128/AAC.01083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheatham SC, Fleming MR, Healy DP, Chung EK, Shea KM, Humphrey ML, Kays MB. 2014. Steady-state pharmacokinetics and pharmacodynamics of meropenem in morbidly obese patients hospitalized in an intensive care unit. J Clin Pharmacol 54:324–330. doi: 10.1002/jcph.196. [DOI] [PubMed] [Google Scholar]
- 22.Grootaert V, Willems L, Debaveye Y, Meyfroidt G, Spriet I. 2012. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother 46:952–959. doi: 10.1345/aph.1Q708. [DOI] [PubMed] [Google Scholar]
- 23.Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, Lipman J. 2011. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care 15:R139. doi: 10.1186/cc10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JH, Fay MF, Udy A, Roberts J, Kirkpatrick C, Ungerer J, Lipman J. 2011. Pitfalls of using estimations of glomerular filtration rate in an intensive care population. Intern Med J 41:537–543. doi: 10.1111/j.1445-5994.2009.02160.x. [DOI] [PubMed] [Google Scholar]