Abstract
Protective antigen (PA)-based vaccines are effective in preventing the development of fatal anthrax disease both in humans and in relevant animal models. The Bacillus anthracis toxins lethal toxin (lethal factor [LF] plus PA) and edema toxin (edema factor [EF] plus PA) are essential for the establishment of the infection, as inactivation of these toxins results in attenuation of the pathogen. Since the toxins reach high toxemia levels at the bacteremic stages of the disease, the CDC's recommendations include combining antibiotic treatment with antitoxin (anti-PA) immunotherapy. We demonstrate here that while treatment with a highly potent neutralizing monoclonal antibody was highly efficient as postexposure prophylaxis treatment, it failed to protect rabbits with any detectable bacteremia (≥10 CFU/ml). In addition, we show that while PA vaccination was effective against a subcutaneous spore challenge, it failed to protect rabbits against systemic challenges (intravenous injection of vegetative bacteria) with the wild-type Vollum strain or a toxin-deficient mutant. To test the possibility that additional proteins, which are secreted by the bacteria under pathogenicity-stimulating conditions in vitro, may contribute to the vaccine's potency, we immunized rabbits with a secreted protein fraction from a toxin-null mutant. The antiserum raised against the secreted fraction reacts with the bacteria in an immunofluorescence assay. Immunization with the secreted protein fraction did not protect the rabbits against a systemic challenge with the fully pathogenic bacteria. Full protection was obtained only by a combined vaccination with PA and the secreted protein fraction. Therefore, these results indicate that an effective antiserum treatment in advanced stages of anthrax must include toxin-neutralizing antibodies in combination with antibodies against bacterial cell targets.
INTRODUCTION
Two major systems are known to affect Bacillus anthracis virulence, the immunomodulating toxins (1, 2) and the phagocytosis-protecting capsule (3). The toxins consist of lethal factor (LF), a mitogen-activated protein (MAP) kinase-degrading metalloprotease, and edema factor (EF), a calmodulin-dependent adenylate cyclase, which combined with protective antigen (PA), a heptamer-forming transport protein, form lethal toxin (LT) and edema toxin (ET), respectively. The toxin components are encoded on the virulence plasmid pXO1 and are produced and secreted from the vegetative bacteria in the host. Toxin secretion starts locally in the infected tissue, early during the first stages of the infection, and then in the bloodstream in parallel with the appearance of bacteria in the bloodstream. The PA in the lymph or bloodstream binds to specific receptors, namely, ANTXR1 and ANTXR2, is cleaved by a membrane-bound protease (furin), and oligomerizes into heptamers. The PA heptamer binds a total of 3 units of LF and EF and is internalized into the cell via phagocytosis. Acidification of the phagosome following lysosomal fusion results in PA conformational change and injection of the toxin into the cytosol, leading to disruption of cell regulation and function. This toxic activity causes, among other things, immunosuppression, modification of vascular permeability, and cell death (for reviews, see references 1 and 2).
The antiphagocytic γ-poly-d-glutamic acid capsule is produced concomitantly with the toxins in response to host conditions (elevated CO2 and the presence of serum proteins) and has been reported to play a major role in protecting the bacteria from the innate immune response, mainly contributing to survival within the phagocytic immune cells (i.e., macrophages and neutrophils [3]). The capsule biosynthetic enzymes are encoded on the virulence plasmid pXO2, and deletion of the capsule results in major attenuation (the attenuated live vaccine strains, e.g., the Sterne strain, lack the pXO2 plasmid). The exact function of the capsule in pathogenicity (active or passive role) is not completely understood, and it has been proposed that short, capsule-derived, γ-poly-d-glutamic acid chains are secreted into the bloodstream and play a role in immunomodulation and immune evasion (4, 5).
The capsule is a very weak immunogen and probably cannot by itself serve as a vaccine against anthrax. Nevertheless, the first B. anthracis vaccine strain (Pasteur) did not contain pXO1, the toxin-encoding plasmid (6, 7). Repeated attempts to demonstrate the ability of such a vaccine to protect against a lethal virulent strain challenge in animal models failed (8). The efficacy of the Pasteur vaccine was assumed to result from impurities of the vaccine strain and contamination with pXO1-bearing strains (6, 7). Therefore, the current live attenuated vaccines, such as the Sterne and STI vaccines, are based on B. anthracis pXO1-positive, pXO2-negative strains and are widely used to vaccinate livestock and also, in large parts of the world, humans against deadly B. anthracis infections (for reviews, see references 6, 7, and 9). It was assumed that the main protective antibodies are toxin neutralizing, mainly against the protective antigen (PA) (9, 10). In fact, a cell-free PA-based anthrax vaccine was approved by the Food and Drug Administration (FDA) for at-risk adults before exposure to anthrax. There are several human PA-based vaccines (6, 7) that differ from each other by the producing strain, Sterne or ATCC 14185, and by the degree of PA purification. The currently licensed BioThrax vaccine (formerly known as anthrax vaccine adsorbed [AVA]) is produced from cell-free filtrates of microaerophilic cultures of the avirulent, nonencapsulated strain of B. anthracis ATCC 14185. In accordance with the pharmacopeia guidelines, the FDA started to examine SparVax, a defined human vaccine that relies on purified recombinant PA as a sole antigen (9, 11). During the last decade, publications from different laboratories reported attempts to improve the PA vaccine efficacy by incorporating spore antigens (12) or, recently, by production of conjugative vaccines that fuse PA epitopes with epitopes from strong immunogens such as the Neisseria meningitidis serotype B outer membrane protein complex (OMPC) or cellular antigens such as capsule-derived immunogens (8). Although these modifications were found to be beneficial, the traditional PA-based vaccine is still the only approved human preexposure vaccine.
The use of passive transfer of antiserum as a treatment for anthrax was reported as early as the beginning of the 20th century (13). Since the B. anthracis toxins were discovered only in the mid-20th century, the nature of these preparations is unclear. In the past decade, several incidences of combining PA-specific human antiserum with antibiotic treatment for individuals with anthrax in the United States have been reported (14). There are currently two antitoxins in the CDC Strategic National Stockpile: raxibacumab (GlaxoSmithKline, London, United Kingdom) and anthrax immune globulin intravenous (AIGIV) (Cangene Corporation, Winnipeg, Manitoba, Canada). Both antitoxins inhibit binding of PA to anthrax toxin receptors and translocation of the two primary toxins (LT and ET) into cells. Raxibacumab is a recombinant, fully humanized, immunoglobulin G1(λ) [IgG1(λ)] monoclonal antibody (15). AIGIV is a human polyclonal antiserum produced from the plasma of persons immunized with AVA, which might have some direct effect on LF and EF (16). In its recent recommendations for the treatment of systemic anthrax, the CDC recommends combining one of these antitoxin antibodies with antibiotic treatment.
Previously, we demonstrated that the toxins play different roles during the infection and systemic phases of anthrax. We showed that a mutant strain lacking the toxins can cause a deadly anthrax disease once injected as capsular vegetative bacteria directly into the bloodstream of rabbits (17, 18). One of the targets for this toxin-independent pathogenicity is the central nervous system (CNS), very similar to what we find in intranasal (i.n.) infection with the wild-type spores (19). In this study, we test the ability of antibodies to treat systemic anthrax (bacteremic stage) in rabbits. The results raise questions regarding the efficacy of the proposed monoantigenic antitoxin treatment, and we propose that the combination of PA with additional bacterial antigens might significantly improve this postexposure antitoxin treatment.
MATERIALS AND METHODS
B. anthracis strain.
The strains used in this study were ATCC 14578 (Vollum) (Tox+ Cap+), Vollum Δpag Δlef Δcya (Vollum ΔTox), and Vollum ΔpXO1 ΔpXO2 from the collection of the Israel Institute for Biological Research (IIBR) (18, 20). For the induction of toxins and capsule production, a modified Dulbecco's modified Eagle's medium (DMEM; supplemented with 10% normal rabbit serum, 4 mM l-glutamine, 1 mM sodium pyruvate, and 1% nonessential amino acids) was used.
Precipitation of toxin-free secreted protein fraction (TF-SPF).
Vollum Δpag Δlef Δcya cells were grown in NBY-bicarbonate broth (nutrient broth, 8 g/liter; yeast extract, 3 g/liter; glucose, 10 g/liter; NaOH, 0.5 g/liter; NaHCO3, 9 g/liter) overnight at 37°C in a 10% CO2 atmosphere. The culture was centrifuged, and the growth medium was filtered through a 0.45-μm filter. The soluble proteins were precipitated by 90% ammonium sulfate. Following centrifugation, the proteins were resuspended in double-distilled water and dialyzed against phosphate-buffered saline (PBS). The final protein concentration was determined by the Bradford method.
Animal experiments.
Female New Zealand White rabbits, weighing 2.2 to 2.5 kg, were obtained from Charles River Laboratories, USA. The animals received food and water ad libitum.
Treatment of exposed rabbits with cAb29.
A group of 12 rabbits was inoculated i.n. with 2 × 106 to 6 × 106 (100 LD50 [50% lethal doses]) Vollum spores (21, 22). At 24 h postinoculation, blood samples were drawn from the rabbits' ear veins to determine the level of bacteremia and the animals were immediately treated with 1 mg purified IgG chimeric monoclonal antibody 29 (cAb29) (23) intravenously (i.v.). The animals were observed daily for 14 days (22).
Challenge of rabbits immunized with PA-based vaccine or TF-SPF.
Rabbits were immunized by subcutaneous (s.c.) injection (prime and boost 4 weeks apart) of 0.5 ml of purified PA absorbed to Alhydrogel (24) (55 μg/ml). The TF-SPF was injected (prime and two boosts 4 weeks apart) as a 1-ml 1:1 emulsion in incomplete Freund's adjuvant (250 μg total protein). The immunization regimens are presented in Results. For systemic i.v. inoculation, spores were germinated by incubation in Terrific Broth for 1 h at 37°C and then incubated in modified DMEM in a 10% CO2 atmosphere for 2 h at 37°C to induce capsule formation. The capsule was visualized by negative staining with India ink. The capsulated vegetative cells (7 × 106 to 1.4 × 107 CFU) were injected i.v. via the ear vein, and a remaining sample was plated for total viable counts (in CFU/ml). The spore content of the capsulated vegetative cell inoculum was lower than 0.2%, as determined by parallel plating with and without heat shock treatment (70°C for 20 min). In any case, the spore content in the capsulated Vollum ΔTox inoculum will not affect the outcome of the infection, as i.v. inoculation of 105 CFU of the mutant spores will not kill the host (data not shown). As an evaluation of PA immunization, rabbits (n = 4) were challenged by an s.c. injection of 2 × 104 (100 LD50) Vollum spores (18). The animals were observed daily for 14 days or for the indicated period. Upon the animals' death, blood samples were plated and DNA was extracted, followed by PCR analysis in order to determine the identity of the strain responsible for the animals' death.
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council (25). The protocols were approved by the Committee on the Ethics of Animal Experiments of the IIBR. Animals were euthanized upon exhibiting severe respiratory distress or the loss of righting reflex. Rabbits were sacrificed by injection of sodium pentabarbitone.
ELISA for anti-PA and TF-SPF antibodies.
Serum levels of anti-PA or anti-TF-SPF antibodies were determined as previously reported (26). Briefly, specific antibody titers were determined by direct enzyme-linked immunosorbent assay (ELISA). Plates were coated with 10 μg/ml PA or TF-SPF in NaHCO3 buffer (50 mM, pH 9.6) and subsequently blocked with 5% skim milk (Becton Dickinson, Sparks, MD). The plates were washed with PBS containing 0.05% Tween 20 (PBST) and incubated with the tested sera (diluted 1:2 in 0.5% skim milk) for 1 h at 37°C. The plates were washed with PBST and developed with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma, St. Louis, MO) as the detecting reagent and p-nitrophenyl phosphate (Sigma, St. Louis, MO) as the substrate. Absorbance at 405 nm was determined using a Spectramax 190 micro plate reader (Molecular Devices, Sunnyvale, CA). The endpoint was defined as the highest dilution at which the absorbance was above twice the absorbance of the negative control (defined as a signal-to-noise ratio [S/N] of >2).
Immunofluorescence staining.
Bacteria were fixed with 4% paraformaldehyde in PBS on slides in a glass chamber for 20 min. The bacteria were stained with primary rabbit antisera (anti-PA, anti-TF-SPF, or anti-PA plus anti-TF-SPF) from the immunized rabbit for 1 h at 37°C and then incubated for 1 h at 37°C with a secondary antibody (DyLight 594-conjugated donkey anti-rabbit IgG; Thermo Fisher Scientific Inc., Waltham, MA). The bacterial cells were visualized using a confocal microscope.
Determination of bacteremia.
Bacterial levels in the blood were determined as previously reported (27). Briefly, each blood sample was plated undiluted and after serial dilutions in saline. The plates were incubated for 16 h at 37°C, and bacteremia was determined by colony counting. The lower limit of detection was 10 CFU/ml.
Statistical analysis.
The significance of the differences in survival rates between treated groups and untreated controls was determined by log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests, using Prism 6 software (GraphPad, USA).
RESULTS
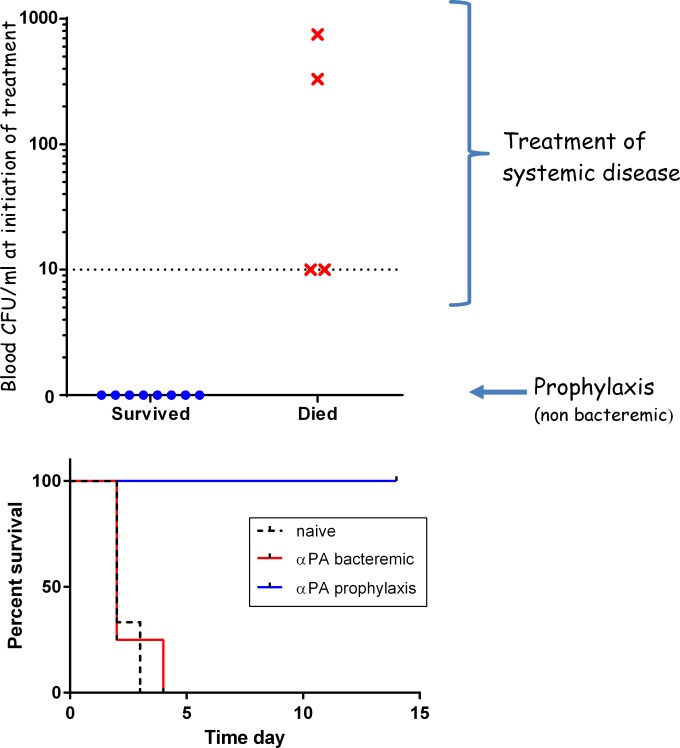
We tested the efficacy of a postexposure prophylactic therapy using a chimeric anti-PA neutralizing antibody as a sole treatment in the rabbit model following intranasal (i.n.) instillation of virulent Vollum spores. We previously demonstrated full protection (100%) by a postexposure prophylaxis treatment of nonbacteremic rabbits with two doses of 1 μg anti-PA IgG at the time of treatment initiation and at day 3 (22). Here we show that treatment of bacteremic animals (with systemic anthrax) did not prevent the death of any of the treated animals (Fig. 1). This lack of protection was observed even when the bacteremia at treatment initiation was as low as 10 CFU/ml, which is the limit of detection of the methodology used.
FIG 1.
Efficacy of postexposure treatment with chimeric anti-PA monoclonal antibody (cAb29). Rabbits (n = 12) were exposed i.n. to Vollum spores and at 24 h postinfection were treated i.v. with 1 mg/ml of the cAb29 antibodies. Upper panel, treatment efficacy relative to the bacteremia at treatment initiation; lower panel, survival curves of the nonbacteremic (blue), bacteremic (red), and untreated (dashed black) rabbits. α, anti-.
To evaluate the possibility that the limitation of neutralizing antibodies was the cause for the treatment failure, we tested the ability of active adaptive immunization to protect against systemic infection. We immunized rabbits with a PA-based vaccine (prime and boost 4 weeks apart), an immunization protocol that has previously been shown to protect rabbits against a lethal i.n. or s.c. challenge with B. anthracis spores (24). To determine the efficacy of the immunization protocol, we challenged the rabbits s.c. with 150 LD50. As shown in Fig. 2 and Table 1, this immunization protected 100% of the animals from a lethal spore challenge. To test the ability of this adaptive immune response to protect the animals from systemic disease (bacteremia), we bypassed the first steps of spore infection and injected capsular vegetative bacteria directly into the bloodstream by i.v. injection, as previously reported (17, 19). The results (Fig. 2; Table 1) demonstrate that active PA immunization did not protect against systemic anthrax, as 4 out of the 5 animals (80%) that were challenged succumbed to the challenge. However, the time to death (Fig. 2) was significantly longer than that of the control naive animals that were infected via the same route (P = 0.0047).
FIG 2.
Survival curves of the PA-vaccinated rabbits following systemic infection with the Vollum or Vollum ΔTox (Δpag Δlef Δcya) strain. PA-immunized rabbits were infected i.v. with vegetative encapsulated bacteria of the Vollum (n = 5; solid red) or Vollum ΔTox (n = 6; solid blue) strain. As a control, immunized rabbits were infected s.c. with the Vollum strain (green) and naive rabbits were infected i.v. with the Vollum (n = 4; dashed red) or Vollum ΔTox (n = 4; dashed blue) strain.
TABLE 1.
Efficacy of immunization with various soluble immunogens
| Immunogen | Challenge strain | Challenge routea | Challenge dose (CFU) | No. of protected rabbits/total no. challenged | Corresponding figure |
|---|---|---|---|---|---|
| PA | Vollum | s.c. | 2 × 103 | 4/4 | 2 |
| Vollum | i.v. | 7 × 106 | 1/5 | 2 | |
| Vollum Δpag Δlef Δcya | i.v. | 1 × 107 | 0/6 | 2 | |
| TF-SPF | Vollum Δpag Δlef Δcya | i.v. | 1.3 × 107 | 4/4 | 3 |
| Vollum | i.v. | 1.1 × 107 | 0/4 | 3 | |
| PA+TF-SPF | Vollum | i.v. | 1.4 × 107 | 4/4 | NAb |
The s.c. challenge was carried out with spores, while for the i.v. challenge, vegetative cells were used.
NA, not applicable.
To examine the role of the toxin in this process, we induced a systemic anthrax (artificial bacteremia) in PA-immunized rabbits by using a Vollum ΔTox (Δpag Δlef Δcya) strain. As expected, the PA-based immunization did not protect the animals against the systemic anthrax caused by a toxin-deficient strain, and 100% of the animals succumbed to the infection (Fig. 2; Table 1). Unexpectedly, the time to death of the animals infected with the toxin-deficient mutant was similar to that of the animals that succumbed to the wild-type Vollum infection and was significantly longer than that of the control group (P = 0.0143). These findings suggest that the immune response against other, nontoxin antigens in the vaccine (impurities) might have an effect and cause this prolonged time to death.
To test the possibility that additional bacterial immunogens might induce systemic protection, we looked at proteins that are secreted from the bacteria in the host in parallel to the toxins. Bacterial virulence factors are induced as a response to the unique environment in the mammalian host. In vitro, we can stimulate the production of virulence factors such as toxin secretion and capsule production by growing the bacteria in medium containing 0.9% sodium bicarbonate at 37°C in a 10% CO2 environment. We used these in vitro conditions to grow the Vollum ΔTox strain and precipitated the toxin-free secreted protein fraction (TF-SPF) from the growth medium with ammonium sulfate (90% final concentration). Rabbits were immunized with 250 μg of total protein, with the prime and two consequent boosts 30 days apart and the challenge 2 weeks after the last boost. We tested the ability of the immune response, specific to the TF-SPF, to protect the rabbits from systemic anthrax caused by the Vollum ΔTox mutant. The results (Fig. 3; Table 1) show that in contrast to the results with the PA vaccine, rabbits that were immunized with the TF-SPF were protected from a systemic infection with this mutant. We performed this experiment again, this time inducing the systemic infection with vegetative bacteria of the wild-type Vollum strain, and the outcome was different—all the animals succumbed to the infection (Fig. 3). The time to death was longer than that for naive rabbits (P = 0.0082), but the effect was smaller than that with the PA-vaccinated rabbits. The fact that the TF-SPF immunization protected against systemic infection with the toxin-deficient mutant but not the toxin-secreting wild type implied that for full protection, both the neutralization of the secreted proteins (and through them, possibly targeting the bacterial cells) and the PA are necessary. To test this hypothesis, we immunized rabbits with the secreted protein fraction and boosted them twice 30 days apart with combined doses of TF-SPF and PA. Two weeks after the last boost, the animals were systemically infected with capsular vegetative bacteria of the wild-type Vollum strain. This vaccination protocol protected 100% of the animals (Table 1).
FIG 3.
Survival curves of the TF-SPF-vaccinated rabbits following systemic infection with the Vollum or Vollum ΔTox (Δpag Δlef Δcya) strain. TF-SPF (toxin-free secreted protein fraction)-immunized rabbits (4 groups of 4 rabbits) were infected i.v. with vegetative encapsulated bacteria of the Vollum (solid red) or Vollum ΔTox (solid blue) strains. As controls, immunized rabbits were infected s.c. with the Vollum strain (green) and naive rabbits were infected i.v. with the Vollum strain (dashed red).
By the use of immunofluorescence assays, we demonstrated that the anti-TF-SPF antiserum reacts with the bacterial cells, both the capsular vegetative cells (Vollum ΔTox) and the noncapsular vegetative bacteria (Vollum ΔpXO1 ΔpXO2). Figure 4A demonstrates that while the anti-PA antiserum does not react with the bacterial cell, the anti-TF-SPF antiserum reacts strongly with the capsular and noncapsular bacterial cell. The ELISA titers of the different antisera are shown in Fig. 4C. PA, a strong immunogen, generates high anti-PA titers of specific antibodies in the range of 1 × 105 to 4 × 105 after a vaccination course of a prime and a single boost. On the other hand, the TF-SPF generates lower titers of only 6 × 103 to 12 × 103 after a vaccination course of a prime and two boosts. Reciprocal ELISA testing of PA-vaccinated sera against the TF-SPF and the anti-TF-SPF sera against PA supported this finding. It demonstrates that the PA-vaccinated sera completely failed to react with the TF-SPF. In comparison, significant titers of anti-TF-SPF sera against the PA vaccine were determined (∼800 ELISA units), indicating the presence of protein impurities in the PA-based vaccine. However, PA antigenicity is so dominant that animals given the PA-based vaccine generate little or no immunogenic reaction toward these impurities. In contrast, animals given the TF-SPF-based vaccine readily react with the same impurities present in the PA-based vaccine. Nevertheless, while the combined vaccination protocol that includes TF-SPF and the PA-based vaccine yielded sera with slightly lower ELISA titers, they induced protective immunity. These results indicate that the high immunogenicity of the PA does not interfere with the immune response against the secreted proteins but rather has a synergistic effect.
FIG 4.
Analysis of sera from rabbits that were immunized with PA, TF-SPF, or both. (A) Immunofluorescence assay of the different antisera (indicated on the left) with the different Vollum strains (indicated at the top). Superimposed images of DAPI (4′,6-diamidino-2-phenylindole) stain (blue indicates DNA) and the DyLight 594-labeled antisera (red indicates bacterial epitopes) are shown. (B) Western blot analysis of the TF-SPF (toxin-free secreted protein fraction) with different antisera (as marked at the top). Lane M, molecular size markers. (C) ELISA titers of the different antisera (as marked on the right) against PA, TF-SPF, or both (as indicated at the top). α, anti-.
Western blot (WB) analysis of the TF-SPF with the different antisera is presented in Fig. 4B. The anti-TF-SPF serum interacts with about six to seven major antigens in the TF-SPF fraction (Fig. 4B). In contrast to the ELISA and immunofluorescence results, WB analysis of the anti-PA serum demonstrates a low but significant reaction with specific antigens in the TF-SPF, some of them parallel to the antigenic profile of the anti-TF-SPF. The WB analysis of the anti-PA plus anti-TF-SPF is a combination of the two separate antigen profiles. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of this protein fraction identified about 100 B. anthracis proteins (see Dataset S1 in the supplemental material). At this point, we cannot identify any of the WB antigens due to low protein content and the absence of clearly distinct protein bands in SDS-PAGE.
Previously (19), we demonstrated that the PA-based vaccine does not protect guinea pigs against an i.n. challenge with the Vollum or Vollum ΔTox spores. In the guinea pig experiments, we demonstrated that animals that succumbed to Vollum infection had lower bacteremia and organ bacterial burden than the animals that succumbed to the Vollum ΔTox mutant. Nevertheless, the brain bacterial loads in all the animals were similar, implying that the lack of protection was due to failure of the PA vaccine to prevent brain invasion and lethal infection (as previously reported for animals that succumbed to infection during raxibacumab treatment [31]). In the present study, analysis of the bacteremia and brain bacterial burdens in rabbits (Fig. 5) revealed similar results. The bacteremias of the animals that succumbed to the Vollum strain are significantly different from those of animals that were challenged with the Vollum ΔTox strain (P = 0.03 in a t test, and P = 0.018 in one-way analysis of variance [ANOVA]). Brain bacterial burdens are similar and high, regardless of the challenge strain. The TF-SPF vaccine had little or no effect on the bacteremia following a Vollum challenge (Fig. 5).
FIG 5.
Blood and brain dissemination of the wild-type Vollum strain and the Vollum ΔTox mutant in PA- or TF-SPF-immunized rabbits. Bacteremia (in CFU/ml) and brain bacterial burden (in CFU/organ) were determined in rabbits that succumbed to the infection, as shown in Fig. 2 and 3. PA-vaccinated rabbits challenged with Vollum (red circles) and Vollum ΔTox (blue triangles) are indicated (the circled blue triangle represents an animal that was euthanized and excluded from the statistical analysis). TF-SPF (toxin-free secreted protein fraction)-vaccinated rabbits challenged with Vollum are indicated by green squares. Two additional rabbits that were immunized with TF-SPF and challenged with Vollum were highly bacteremic in a qualitative test. No brain bacterial burden was determined for these two rabbits.
DISCUSSION
The cell-free, PA-based vaccine is an efficient and safe vaccine proven to protect against inhalational or cutaneous anthrax and has been approved by the FDA for use in humans for the prevention of anthrax (6). Recently, the CDC updated the guidelines for prevention and treatment of anthrax, designating three treatment protocols for the disease: postexposure prophylaxis, antibiotic treatment for systemic anthrax without meningitis, and antibiotic treatment for systemic anthrax with suspected or confirmed meningitis (28). While postexposure prophylaxis is based on a monotherapy with a single antibiotic with the possibility of including active vaccination for exposures involving aerosolizing spores, the treatment of the systemic disease is more complex. For the systemic disease, the recommended treatment includes the i.v. administration of two or three antibiotics in combination with dexamethasone and antitoxin treatment-specific anti-PA monoclonal or polyclonal antibodies. Two antibody-based products are available: raxibacumab, which is a fully humanized anti-PA monoclonal antibody, and AIGIV (Arthrivig), affinity-purified human polyclonal anti-PA IgG. Raxibacumab binds to a specific domain on the PA protein, preventing the binding of the protein to the cellular receptor, and can therefore bind PA only in its soluble form, not when bound to the receptor or in the heptametrical form (15, 29). AIGIV is a polyclonal antiserum that probably reacts with different epitopes on the PA, and it might be able to interfere with protein activity even after the binding to the receptor. We previously determined (in rabbits) the contribution of combining neutralizing anti-PA polyclonal or monoclonal antibodies to ciprofloxacin treatment of inhalational anthrax (26). Our findings indicated that an additive effect could be obtained only with a specific monoclonal antibody, which was consequently shown to have the unique ability to neutralize PA at the final step of the toxin injection into the cytosol (22). The ability of a chimeric form of this antibody (cAb29) to treat anthrax in rabbits as a single treatment is shown in Fig. 1. Therefore, the ability of antibody-based antitoxin activity to improve the antibiotic therapy in the rabbit model depends on the target epitope of the specific monoclonal antibody.
Recent CDC recommendations and the goal of targeting PA for cotherapy rather than prevention might be of limited value. As we previously demonstrated in the guinea pig and rabbit models, B. anthracis has the capacity of causing a deadly systemic disease even in the absence of toxins (17–19). In this and a previous article, we demonstrated that a PA-based vaccine that is sufficient to protect rabbits from deadly s.c. infections (present study) or i.n. spore infections (24) fails to protect rabbits from a systemic infection with the wild type or the toxin-deficient mutant. These findings indicate that neutralization of the toxin at the systemic stage has little or no effect on the infection outcome. The finding that immunization with the bacterial secreted protein fraction (TF-SPF) protected the rabbits from deadly systemic infection with the toxin-deficient strain but not the wild-type strain suggests that the antibacterial immune responses play a major role in this protection. Therefore, these findings may indicate that the immunosuppression effect of the toxins revokes this protective effect. The time to death of the PA-vaccinated rabbits following systemic infections with vegetative bacteria of the Vollum or Vollum ΔTox strain was significantly prolonged compared to that of the unvaccinated control, and this may be attributed to impurities in the PA-based vaccine with bacterium-specific proteins (Fig. 4B and C). Notably, the PA vaccine did not protect any of the animals that were infected with the Vollum ΔTox mutant (n = 6) and protected only 1 out of 5 of the Vollum-infected rabbits (Table 1; Fig. 2). This finding may indicate that the PA protein is presented on the cell surface of the Vollum strain and therefore may contribute to the antibacterial immune response but is probably insufficient to ensure protection. However, it can explain the significantly lower bacteremia in the PA-vaccinated animals that succumbed to the Vollum strain challenge (Fig. 5).
The finding that all the immunized rabbits that succumbed to infection had high bacterial burdens in the brain and in some cases low or undetectable blood bacteremia (Fig. 5) implies that overcoming the blood-brain barrier (BBB) is the breach in protection. Effective immunity must prevent the bacterial BBB penetration, since antibodies usually do not cross the BBB and, as shown previously following brain penetration, Vollum strains will kill the host regardless of the toxins (17, 19).
Supporting the hypothesis that for effective protection both an effective antitoxin and antibacterial activity are necessary, the combined immunization with PA and the TF-SPF protected 100% of the rabbits against systemic infection with the Vollum strain. Three hypothetical pathways may explain this finding. First, a specific antibody can neutralize the essential activity of a soluble or surface-bound protein. Second, antibodies bind to the bacterial surface, mediating opsonization and phagocytosis (30). This second possibility is less likely, as encapsulated bacteria can survive phagocytosis by cells (5). On the other hand, immunization with the secreted protein alone was not effective in the presence of the immunosuppressive toxins, indicating that immune cells play a role in this process. Third, the antibodies that are specific to the bacterial surface (Fig. 4A) can induce the classical pathway of the complement reaction which is triggered by the antigen-antibody complex (30).
At the bacteremic stage of anthrax, when the host is overwhelmed with toxins, probably saturating the receptors on the host cells, the effect of antitoxins and antibodies might be limited and restricted to neutralizing the unbound toxins. The addition of neutralizing antibodies that will react with bacterial proteins to the antibiotic treatment may improve bacterial eradication and improve the efficacy of the antibacterial treatment. This additional activity becomes essential/required in cases of ineffective antimicrobial treatment due to bacterial resistance or late treatment initiation.
As expected, immunization with a mixture of about 100 proteins will result in generating antibodies that are directed against a variety of antigens (Fig. 4; see Dataset S1 in the supplemental material). At this stage, we cannot determine if this protection is due to the activity of a specific antibody or a general reaction to any surface antigen. Further characterization and purification of this soluble protein fraction may result in the identification of specific antigens that are involved in the protective activity and may improve the efficacies of both anti-anthrax cotreatment and the vaccine.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nili Rothschild for her excellent technical assistance. We give special thanks to Arie Ordentlich, Shmuel Yitzhaki, and Ohad Mazor for their support, fruitful discussions, and careful reading of the manuscript.
Funding Statement
This study was supported by Israel Institute for Biological Research self-funding.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00546-16.
REFERENCES
- 1.Liu S, Moayeri M, Leppla SH. 2014. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moayeri M, Leppla SH. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med 30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouet A. 2009. The surface of Bacillus anthracis. Mol Aspects Med 30:374–385. doi: 10.1016/j.mam.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Jang J, Cho M, Chun J-H, Cho M-H, Park J, Oh H-B, Yoo C-K, Rhie G-E. 2011. The poly-γ-d-glutamic acid capsule of Bacillus anthracis enhances lethal toxin activity. Infect Immun 79:3846–3855. doi: 10.1128/IAI.01145-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezzell JW, Welkos SL. 1999. The capsule of bacillus anthracis, a review. J Appl Microbiol 87:250. doi: 10.1046/j.1365-2672.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- 6.Tournier JN, Ulrich RG, Quesnel-Hellmann A, Mohamadzadeh M, Stiles BG. 2009. Anthrax, toxins and vaccines: a 125-year journey targeting Bacillus anthracis. Expert Rev Anti Infect Ther 7:219–236. doi: 10.1586/14787210.7.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Gordon Wright J, Quinn CP, Shadomy SV, Messonnier NE, Centers for Disease Control and Prevention. 2010. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 59(RR-6):1–30. [PubMed] [Google Scholar]
- 8.Chabot DJ, Joyce J, Caulfield M, Cook J, Hepler R, Wang S, Vietri NJ, Ruthel G, Shoop W, Pitt L, Leffel E, Ribot W, Friedlander AM. 2012. Efficacy of a capsule conjugate vaccine against inhalational anthrax in rabbits and monkeys. Vaccine 30:846–852. doi: 10.1016/j.vaccine.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Cybulski RJ Jr, Sanz P, O'Brien AD. 2009. Anthrax vaccination strategies. Mol Aspects Med 30:490–502. doi: 10.1016/j.mam.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ionin B, Hopkins RJ, Plune B, Silvko GS, Reid F, Clement KH, Rudge TL Jr, Stark GV, Innes A, Sari S, Guina T, Howard C, Smith J, Swoboda L, Vert-Wong E, Johnson V, Nabors GS, Skiadopoulos MH. 2013. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin Vaccine Immunol 20:1016–1027. doi: 10.1128/CVI.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffel KE, Bourdage SJ, Williamson ED, Duchers M, Fuerrst RT, Fusco CP. 2012. Recombinant protective antigen anthrax vaccine improves survival when administered as a postexposure prophylaxis countermeasure with antibiotic in the New Zealand White rabbit model of inhalation anthrax. Clin Vaccine Immunol 19:1158–1165. doi: 10.1128/CVI.00240-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brossier F, Levy M, Mock M. 2002. Anthrax spores make an essential contribution to vaccine efficacy. Infect Immun 70:661–664. doi: 10.1128/IAI.70.2.661-664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holty J-EC, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systemic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 14.Griffith J, Blaney D, Shadomy SV, Lehman M, Pesilk N, Tostenson S, Delaney L, Tiller R, DeVries A, Gomez T, Sullivan M, Blackmore C, Stanek D, Lynfield R. 2014. Investigation of inhalation anthrax case, United States. Emerg Infect Dis 20:280–283. doi: 10.3201/eid2002.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazumdar S. 2009. Raxibacumab. MAbs 1:531–538. doi: 10.4161/mabs.1.6.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mytle N, Hopkins RJ, Basu S, Malkevick NV, Meister GT, Sanford DC, Coner JE, Van Zandt KE, Al-Ibrahim M, Kramer WG, Howard C, Daczkowski N, Chakrabarti AC, Ionin B, Nabors GS, Skiadopoulos MH. 2013. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob Agents Chemother 57:5684–5692. doi: 10.1128/AAC.00458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy H, Glinert I, Weiss S, Sittner A, Schlomovitz J, Altboum Z, Kobiler D. 2014. Toxin-indendent virulence of Bacillus anthracis in rabbits. PLoS One 9:e84947. doi: 10.1371/journal.pone.0084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy H, Weiss S, Altboum Z, Schlomovitz J, Glinert I, Sittner A, Shafferman A, Kobiler D. 2012. Differential contribution of Bacillus anthracis toxins to pathogenicity in two animal models. Infect Immun 80:2623–2631. doi: 10.1128/IAI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy H, Glinert I, Weiss S, Bar-David E, Sittner A, Schlomovitz J, Altboum Z, Kobiler D. 2014. The central nervous system as target of Bacillus anthracis toxin independent virulence in rabbits and guinea pigs. PLoS One 9(11):e112319. doi: 10.1371/journal.pone.0112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy H, Fisher M, Ariel N, Altboum Z, Kobiler D. 2005. Identification of strain specific markers in Bacillus anthracis by random amplification of polymorphic DNA. FEMS Microbiol Lett 244:199–205. doi: 10.1016/j.femsle.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Levy H, Weiss S, Altboum Z, Schlomovitz J, Rothschild N, Glinert I, Sittner A, Kobiler D. 2012. The effect of deletion of the edema factor on Bacillus anthracis pathogenicity in guinea pigs and rabbits. Microb Pathog 52:55–60. doi: 10.1016/j.micpath.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Mechaly A, Levy H, Epstein E, Rosenfeld R, Marcus H, Ben-Arie E, Shafferman A, Ordentlich A, Mazor O. 2012. A novel mechanism for antibody-based anthrax toxin neutralization: inhibition of prepore-to-pore conversion. J Biol Chem 287:32665–32673. doi: 10.1074/jbc.M112.400473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfeld R, Marcus H, Ben-Arie E, Lachmi BE, Mechaly A, Reuveny S, Gat O, Mazor O, Ordentlich A. 2009. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus anthracis infection. PLoS One 4:e6351. doi: 10.1371/journal.pone.0006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S, Kobiler D, Levy H, Marcus H, Pass A, Rothschild N, Altboum Z. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect Immun 74:394–398. doi: 10.1128/IAI.74.1.394-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 26.Weiss S, Kobiler D, Levy H, Pass A, Ophir Y, Rothschild N, Tal A, Schlomovitz J, Altboum Z. 2011. Antibiotics cure anthrax in animal models. Antimicrob Agents Chemother 55:1533–1542. doi: 10.1128/AAC.01689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altboum Z, Gozes Y, Barnea A, Pass A, White M, Kobiler D. 2002. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect Immun 70:6231–6241. doi: 10.1128/IAI.70.11.6231-6241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, Rubinstein E, Holty J-EC, Messonnier NE, Smith TL, Pesilk N, Treadwell TA, Bower WA. 2014. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 20(2). doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kummerfeldt EC. 2014. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist 2014:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunkelberger RJ, Song W-C. 2010. Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration 2012. Raxibacumab prescribing information. U.S. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125349s000lbl.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.