Abstract
Human cytomegalovirus (HCMV) infection is usually benign in healthy individuals but can cause life-threatening disease in those with compromised immune systems. Approved drugs available to treat HCMV disease, including ganciclovir, cidofovir, and foscarnet, have significant toxicities that limit their use in certain patient populations. LJP538 and LJP539 are human monoclonal antibodies that are being evaluated as immunoglobulin therapeutics. The antibodies target glycoproteins gB and the gH/gL/UL128/UL130/UL131a pentameric complex, respectively. Here we present an in vitro characterization of these antibodies. We show that LJP538 and LJP539 are more potent than a marketed immunoglobulin at inhibiting HCMV infection of various cell lines relevant to pathogenesis. We find that LJP538 and LJP539 are active against a panel of clinical isolates in vitro and demonstrate minor-to-moderate synergy in combination. Passage of HCMV in the presence of LJP538 or LJP539 alone resulted in resistance-associated mutations that mapped to the target genes. However, no loss of susceptibility to the combination of antibodies was observed for >400 days in culture. Finally, the binding regions of LJP538 and LJP539 are conserved among clinical isolates. Taken together, these data support the use of LJP538 and LJP539 in combination for clinical trials in HCMV patients.
INTRODUCTION
Human cytomegalovirus (HCMV) can cause significant disease in immunocompromised individuals, including transplant recipients, those infected with HIV, and neonates infected in utero. Available HCMV therapies, such as ganciclovir (and its prodrug valganciclovir), cidofovir, and foscarnet, are effective but associated with serious toxicities. No therapy is available to prevent or treat congenital HCMV.
HCMV hyperimmune globulin (a polyclonal IgG preparation purified from human plasma pools) can be used to prevent infection and disease in some transplant recipients. It is safe and well tolerated, but it is less effective than other antiviral therapies. In solid-organ transplant recipients, the lower efficacy of hyperimmune globulin than ganciclovir or valganciclovir limits its use to selected high-risk situations (1). Treatment of hematopoietic stem cell transplant recipients with HCMV hyperimmune globulin is not recommended because of its limited efficacy and association with veno-occlusive disease (2). However, immunoglobulin or HCMV hyperimmune globulin is often added to ganciclovir or foscarnet when treating HCMV pneumonia. HCMV hyperimmune globulin has also been tested as a treatment for congenital HCMV. A prospective cohort study of pregnant women with primary HCMV infections showed that hyperimmune globulin was safe and that it appeared to reduce the incidence of congenital disease (3, 4). A follow-up randomized, placebo-controlled study of 123 pregnancies also showed a trend toward efficacy, although the benefit of this therapy was not statistically significant (5). More potent immunoglobulin preparations may better prevent and treat HCMV disease.
HCMV can infect a wide variety of cell types, including monocytes, endothelial or epithelial cells, smooth muscle cells, fibroblasts, stromal cells, neuronal cells, neutrophils, and hepatocytes (6). Entry into these various cell types is mediated by different HCMV glycoproteins. Glycoprotein B (gB) is required for entry into all physiologically relevant cell types, while gH and gL form two different complexes that mediate the infection of distinct cell populations. A pentameric complex consisting of gH, gL, UL128, UL130, and UL131a allows infection of myeloid, epithelial, and endothelial cells, whereas a three-member complex consisting of gH, gL, and gO is essential for entry into fibroblasts and likely mediates a preceding fusion process necessary for the infection of all cell types (7–9). This broad cellular tropism means that HCMV disease can occur in a variety of organs. Furthermore, infection of endothelial and hematopoietic cells appears to facilitate the systemic spread of virus, while infection of epithelial cells and fibroblasts seems to contribute to high-level replication (6).
LJP538 and LJP539 are human monoclonal antibodies that target gB and the gH/gL/UL128/UL130/UL131a pentameric complex, respectively. LJP538 and LJP539 are currently being developed as a combination (termed CSJ148) for the treatment of HCMV, and clinical evaluation for safety and efficacy in stem cell transplant patients is under way. Using these antibodies in combination has several advantages. First, the gB-targeting antibody LJP538 inhibits HCMV infection of all of the cell types tested, whereas LJP539 may reduce systemic spread by targeting the pentameric complex. Second, although antibodies directed against gB correlate with neutralizing activity (10), the pentameric complex may be the major neutralizing determinant in natural infections and for hyperimmune globulin (11, 12).Targeting of both gB and the pentameric complex should therefore maximize control of HCMV in vivo. Finally, using the combination of antibodies may decrease the development of resistance.
Here we present an in vitro characterization of LJP538 and LJP539, confirming that they block viral infection and syncytium formation in culture. We demonstrate that LJP538 and LJP539 are more potent than a marketed immunoglobulin, Cytotect, at inhibiting HCMV infection of various cell lines and against a variety of clinical isolates in vitro. In addition, we show that passage of HCMV in the presence of LJP538 or LJP539 individually leads to mutations in the glycoprotein targets but that reduced susceptibility was not observed when the antibodies were used in combination.
MATERIALS AND METHODS
Antibodies and compounds.
LJP538 and LJP539 (previously designated 7H3 and 4I22, respectively) were isolated from HCMV-infected human donors (13). These antibodies were synthesized at Novartis. Cytotect was acquired from Paviour Pharmaceuticals. A human anti-chicken lysozyme IgG1 control antibody was obtained from Abcam. Cetuximab is a chimeric mouse-human antibody with mouse Fab and human Fc portions that recognizes the human epidermal growth factor receptor (EGFR) (14). Cetuximab was purchased as Erbitux (lot 10C00002B; Eli Lilly) from McKesson. MSL-109 (synthesized at Novartis) is a human monoclonal IgG that recognizes a conformational epitope on gH (15). Ganciclovir was synthesized at Novartis.
Preparation of HCMV stocks.
HCMV reference strain VR1814 was obtained from the American Type Culture Collection (ATCC). The AD169rUL131 reference strain was created at Novartis from parent strain AD169 (ATCC). As parent strain AD169 contains a mutation in UL131 that renders the virus incapable of forming a pentameric complex, a version of AD169 was created in which the UL131 mutation was reversed by using a pAD/Cre bacterial artificial chromosome (BAC) system that was a generous gift from the laboratory of Thomas Shenk, Princeton University (see the supplemental material). The resulting virus, referred to here as AD169rUL131, was used for experiments.
Reference strain viral stocks were produced by infection of adult retinal pigment epithelial (ARPE-19) cells or normal human dermal fibroblasts (NHDF) as previously described (16). Briefly, culture medium was removed and the cell monolayer (80 to 90% confluence) was rinsed with 10 ml of serum-free Dulbecco's modified Eagle's medium (DMEM)/F-12. Virus (0.3 to 1 ml) was mixed with 7 ml of serum-free medium and added to the cell monolayer at a multiplicity of infection (MOI) of 0.01. Cells were incubated at 37°C in 5% CO2 for 3 h, and then 7 ml of growth medium was added. Cells were incubated at 37°C in 5% CO2 for 20 to 50 days, and the medium was exchanged once per week until the appearance of plaques. Virus was harvested once the cytopathic effect (CPE) was complete. Cells were removed by scraping, and the mixture was sonicated (169 W) for 5 min in an ice-water bath and then centrifuged at 1,000 × g at 4°C for 15 min. Supernatants were stored at −80°C. HCMV clinical isolates from anonymous donors were generous gifts from Mark Prichard, University of Alabama (MP series); Thomas Mertens, Ulm University Medical Center, Ulm, Germany (TM series); and Peter Barry, University of California at Davis (8816 series). For HCMV clinical isolates, human samples (e.g., lung fluid) were initially used to infect either NHDF or ARPE-19 cells as described above to generate high-titer virus. Typically, high-titer virus was not obtained on the first passage. Culture supernatants (5 to 10 ml) were therefore serially passaged three to eight times, after which viral titers were markedly increased and isolates could be tested in a neutralization assay.
Neutralization assay.
Neutralization of HCMV infection was assayed as described previously (16, 17), with modifications for the 96-well format and high-content imaging. Neutralization was measured by high-content immunofluorescence imaging of the HCMV immediate-early 1/2 (IE1/2) proteins as a marker of infected cells (see the supplemental material). Infection with HCMV reference strains (VR1814 and AD169rUL131) and most clinical isolates was carried out at an MOI of 0.1 to 1.0 so that 10 to 64% of the cells in the control wells were infected. Certain low-titer clinical isolates were infected with a target MOI of 0.01 to 0.1. The day before infection, ARPE-19 cells were seeded into 96-well plates at 1 × 104 per well in 100 μl of growth medium. On the day of infection, test antibodies and Cytotect were prepared as a six- or eight-point 10-fold dilution series in serum-free DMEM/F-12 or as a five-point 20-fold dilution series for low-titer viruses. Antibodies were mixed with virus and incubated at 37°C for 1 h. The antibody-virus mixture (50 μl per well, in triplicate) was then added to a cell monolayer that had been washed once with 100 μl of serum-free medium. Following 3 h of incubation at 37°C in 5% CO2, the inoculum was removed, the monolayer was washed once with 100 μl of serum-free medium, and 100 μl of growth medium was added per well. The plates were incubated at 37°C in 5% CO2 for 18 to 48 h.
Generation of HCMV with reduced susceptibility to antibodies.
HCMV strain VR1814 with reduced susceptibility to monoclonal antibodies was produced by serial passage in ARPE-19 cells or NHDF. The 90% effective concentration (EC90) of each antibody for VR1814 was determined by neutralization assay, and the virus (MOI of 0.5) was incubated with this concentration of antibody in 7 ml of serum-free medium for 1 h at room temperature. Cells (80 to 90% confluent) were then inoculated with the virus-antibody complex and incubated for 3 h at 37°C in 5% CO2. After inoculation, 7 ml of growth medium containing each antibody at a concentration equal to the EC90 was added and cells were monitored for the appearance of a CPE. Virus was harvested from tissue culture medium by sorbitol pelleting once cell death was complete. In cases where virus replication was greatly reduced, as assessed by the inability of the virus to cause a complete CPE, the virus was harvested from the cells and supernatants after a maximum of 50 to 60 days in culture. Cells were lysed by sonication in an ice bath, and virus-containing supernatants were obtained after sedimentation of cellular debris. For passage in the presence of a combination of LJP538 and LJP539 in ARPE-19 cells, antibody concentrations corresponded to ∼1× EC50 (0.648 μg/ml) of LJP538 and ∼65× EC50 (0.0648 μg/ml) of LJP539. This dose of the antibodies was chosen to simulate the dose predicted to be efficacious in clinical trials. The cultures were maintained over 11 passages for 439 days.
The titer of the virus was determined and its antibody susceptibility was tested on the cell type that was used for resistance selection. If the virus was found to retain sensitivity to the selecting antibody, the infection protocol was repeated with gradually increasing amounts of antibody. Emergence of resistant virus was assessed by observation of a complete CPE within 2 to 3 weeks in the presence of antibody, at which point the virus was harvested.
RESULTS
LJP538 and LJP539 neutralize HCMV infection of various cell types.
We tested the abilities of LJP538 and LJP539 to neutralize infection in cell types relevant to viral pathogenesis. Wild-type HCMV reference strain VR1814 was incubated with 10-fold dilutions of LJP538, LJP539, or Cytotect for 1 h and then used for infection. After 16 to 20 h of infection, cells were fixed and stained to detect IE1/2 proteins as a marker of HCMV infection. Both LJP538 and LJP539 neutralized HCMV strain VR1814 on the full range of physiologically relevant cell types (Table 1). In the two fibroblast types tested, the LJP538 EC90s were 331- and 450-fold more potent than that of Cytotect. On epithelial and endothelial cell types, LJP538 neutralized infection with a potency equivalent to its EC90 activity on fibroblasts and an average of 9.4-fold more potently than Cytotect. As expected, LJP539 did not neutralize the infection of fibroblasts, as the pentameric complex is not required for entry into these cells. Across epithelial and endothelial cell types, LJP539 neutralized VR1814 at an average EC90 1,226-fold lower than that of Cytotect.
TABLE 1.
Neutralization of HCMV reference strain VR1814 infection in various cell types
| Cell type | LJP539 |
LJP538 |
Cytotect |
|||
|---|---|---|---|---|---|---|
| EC50 | EC90 | EC50 | EC90 | EC50 | EC90 | |
| Adult retinal pigment epithelial | 0.001a | 0.0052 | 0.84 | 2.37 | 4.56 | 13.81 |
| Renal medullary epithelial | 0.022 | 0.149 | 2.29 | 8.68 | 30.22 | 75.19 |
| Renal cortical epithelial | 0.004 | 0.012 | 0.68 | 2.33 | 12.54 | 31.84 |
| Renal proximal tubule epithelial | 0.006 | 0.022 | 1.62 | 4.9 | 11.05 | 57.69 |
| Placental epithelial | 0.008 | 0.043 | 2.79 | 6.64 | 19.64 | 70.76 |
| Uterine microvascular endothelial | 0.003 | 0.019 | 1.76 | 2.96 | 16.64 | 34.69 |
| Human umbilical vein endothelial | 0.001 | 0.0083 | 0.92 | 3.77 | 10.18 | 25.09 |
| Human coronary artery endothelial | 0.005 | 0.009 | 0.86 | 3.01 | 2.54 | 18.92 |
| Placental fibroblast | >100 | >100 | 0.28 | 10.35 | 22.13 | 3,432.99 |
| Neonatal normal human dermal fibroblast | >100 | >100 | 1.4 | 2.81 | 280.3 | 1,264.35 |
Values are in micrograms per milliliter.
LJP538 and LJP539 neutralize geographically and temporally distinct HCMV clinical isolates.
We next investigated whether the neutralization ability of LJP538 and LJP539 was conserved among more than 20 geographically and temporally distinct clinical isolates of HCMV. Diverse subsets of HCMV glycoproteins gB and gH have previously been described for clinical isolates on the basis of phylogenetic and restriction fragment length polymorphism analyses (18, 19). We confirmed that the strain panel represented the four major gB N-terminal and furin cleavage site genotypes, the two gB C-terminal genotypes, and the two gH groups (data not shown). HCMV isolates were incubated with dilutions of LJP538, LJP539, or Cytotect for 1 h and then used to inoculate various cell types. LJP538 neutralized all of the isolates tested on all of the cell types tested, with EC90s 1 to 2 orders of magnitude more potent than those of Cytotect on epithelial and endothelial cell types and 3 orders of magnitude more potent than Cytotect on fibroblasts (see Tables S1, S2, and S3 in the supplemental material). Similarly, LJP539 neutralized clinical isolates an average of 3 to 4 orders of magnitude more potently than Cytotect on all of the epithelial and endothelial cell types tested; it was not tested on fibroblasts (see Tables S1 and S2 in the supplemental material). These data indicate that LJP538 and LJP539 are active against a diverse panel of HCMV clinical isolates.
LJP538 and LJP539 are not cytotoxic on stationary or proliferating cells.
Potential cytotoxic effects of LJP538 and LJP539 were tested in uninfected ARPE-19, NHDF, RPTE, HepG2, Huh7, and MT-4 cells (see the supplemental material). LJP538 and LJP539 were not cytotoxic either alone or in combination at the highest concentrations tested (500 and 50 μg/ml, respectively) in any of the cell types tested under either stationary or proliferating conditions. Puromycin showed 50% cytotoxic concentrations of 0.105 to 2.373 μg/ml (see Table S4 in the supplemental material).
Activity of LJP538 and LJP539 in combination.
To test the antiviral effects of LJP538 and LJP539 in combination, a synergy analysis was performed. Wild-type HCMV reference strain VR1814 was incubated with 10-fold dilutions of antibodies alone or in combination for 1 h. Human umbilical vein endothelial cells (HUVEC) or ARPE-19 cells were infected with the virus-antibody mixture, and neutralization capacity was analyzed by measuring the immunofluorescence of IE1/2 proteins to quantify the IE protein-positive cells (see the supplemental material). At the 95% confidence level, minor-to-moderate synergistic neutralization activity of LJP538 and LJP539 in combination was observed on ARPE-19 cells and HUVEC (Table 2). Notably, no antagonism was observed.
TABLE 2.
Neutralization activity of LJP538 and LJP539 in combination against HCMV reference strain VR1814
| Cell type | Synergy vola | Antagonism vol | Synergy level |
|---|---|---|---|
| ARPE-19 | 46 | 0 | Minor |
| HUVEC | 74 | 0 | Moderate |
Synergy volumes at the 95% confidence level were calculated from eight replicates by using the MacSynergy II program and interpreted in accordance with the MacSynergy II manual (33).
LJP538 and LJP539 treatment inhibits HCMV infection in long-term culture.
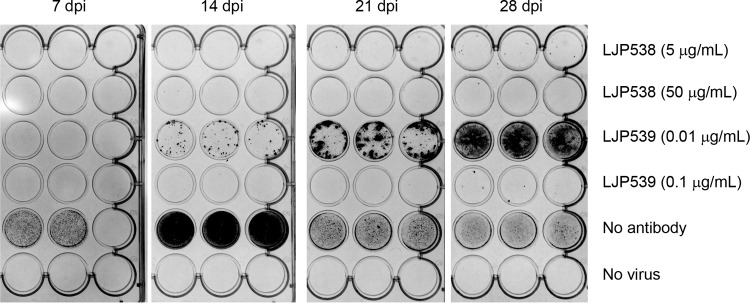
We investigated the abilities of LJP538 and LJP539 to suppress HCMV infection over an extended time period. ARPE-19 cells were infected at an MOI of 0.005 with HCMV strain VR1814 preincubated with LJP538 or LJP539 at approximately 1× and 10× EC90 (5 and 50 μg/ml for LJP538 and 0.01 and 0.1 μg/ml for LJP539). Antibody-containing medium was removed and replaced every 7 days, at which time viral replication was monitored by immunostaining for gB. After 28 days, infected cells grown in the presence of LJP538 (∼1× or ∼10× EC90) or LJP539 (∼10× EC90) showed minimal staining for gB (Fig. 1). LJP539 at ∼1× EC90 inhibited VR1814 replication to a lesser extent. gB expression was detectable in cells infected in the absence of antibody but not in uninfected controls.
FIG 1.
LJP538 and LJP539 inhibit HCMV infection in long-term cell culture. ARPE-19 cells were continuously cultured in the presence of HCMV reference strain VR1814 (MOI of 0.005) for up to 28 days postinfection (dpi) in the presence of LJP538 (5 or 50 μg/ml), LJP539 (0.01 or 0.1 μg/ml), or no antibody (as a control). Antibody-containing medium was removed and replaced every 7 days, at which time immunostaining for gB was performed to monitor viral replication.
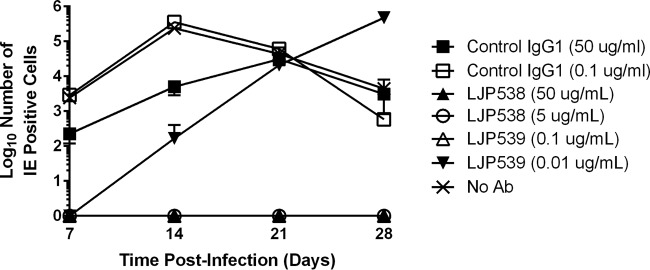
LJP538 and LJP539 treatment leads to decreased infectious HCMV production.
The ability of LJP538 or LJP539 to block HCMV entry should be reflected in a decrease in virion production. To test this, ARPE-19 cells were infected at an MOI of 0.005 with reference strain VR1814 preincubated with antibody and infectious HCMV titers were determined on ARPE-19 cells by staining for IE1/2 protein expression. No infectious HCMV was detectable in the supernatants of VR1814-inoculated cells cultured in the presence of LJP538 (∼1× and ∼10× EC90) or LJP539 (∼10× EC90) (Fig. 2). Delayed production of infectious HCMV was observed in the presence of 0.01 μg/ml LJP539 (∼1× EC90); in this case, the number of IE1/2 protein-positive cells observed at 28 days of culture with LJP539 was similar to that observed at 14 days of culture without the antibody. In the absence of the antibody or in the presence of a control IgG1 antibody, high titers of HCMV were seen at 7 to 14 days postinfection; titers decreased as the CPE overtook the culture (Fig. 2).
FIG 2.
LJP538 and LJP539 inhibit infectious HCMV production. ARPE-19 cells inoculated with HCMV reference strain VR1814 (cells 80 to 90% confluent in a six-well plate; MOI of 0.005) were grown in the presence of LJP538 (5 or 50 μg/ml), LJP539 (0.01 or 0.1 μg/ml), or human anti-chicken lysozyme IgG1 antibody (Ab) or no antibody (as negative controls) for up to 28 days. Progeny virus production was measured by harvesting virus every 7 days, followed by infection of naive cells and immunofluorescence staining for IE1/2 proteins at 18 h postinfection to measure infectious virus production.
LJP538 and LJP539 inhibit syncytium formation in HCMV glycoprotein-expressing cells.
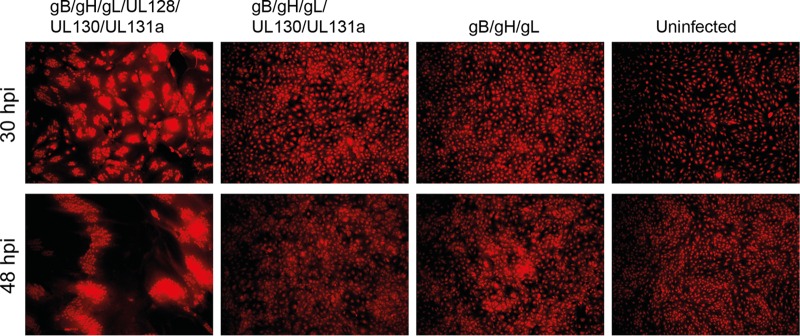
In addition to viral entry from the supernatant, HCMV has the ability to spread directly from cell to cell through syncytium formation. Syncytia are conglomerates caused by fusion of cells expressing viral glycoproteins on their surface. To determine whether LJP538 and LJP539 could inhibit syncytium formation, we expressed HCMV glycoproteins in ARPE-19 cells and monitored fusion by high-content imaging. Six recombinant adenoviruses, each expressing HCMV glycoprotein gB, gH, gL, UL128, UL130, or UL131a, were used to transduce cells alone or in combinations (see the supplemental material). Western blotting showed that each adenovirus expressed the encoded HCMV envelope protein in an MOI-dependent manner (data not shown). The glycoproteins adopted physiologic conformations on the ARPE-19 cell surface, as shown by flow cytometry analysis with antibodies known to bind to conformational epitopes on gB and gH/gL/UL128/UL130/UL131a and through the ability of glycoprotein-expressing cells to block superinfection by VR1814 (data not shown). Syncytium formation was readily observed at 30 and 48 h postransduction in cells expressing gB in combination with gH/gL/UL128/UL130/UL131a; other combinations of HCMV glycoproteins did not lead to cell-cell fusion (Fig. 3). Using the high-content assay, we showed that LJP538 and LJP539 inhibited cell-cell fusion at 30 h postransduction with EC50s of 4.77 and 0.076 μg/ml, respectively, while Cytotect was potent at 311.34 μg/ml (Table 3).
FIG 3.
The pentameric complex and gB are required for cell-cell fusion. The HCMV glycoproteins indicated were expressed by recombinant adenovirus infection of ARPE-19 cells; the far right panel (uninfected) does not express HCMV proteins. Cell-cell fusion was monitored by cytoplasmic staining with Cellmask stain, followed by high-content imaging at 30 and 48 h postinfection (hpi) to count individual cell bodies. Whole-cell staining is shown in red. Syncytia are visible as large fused cell conglomerates.
TABLE 3.
Activities of neutralizing antibodies in an assay of syncytium formation in ARPE-19 cells
| Antibody | EC50 | EC90 |
|---|---|---|
| LJP538 | 4.77a | 182.85 |
| LJP539 | 0.076 | 3.93 |
| Cytotect | 311.34 | 31,541.93 |
Values are in micrograms per milliliter.
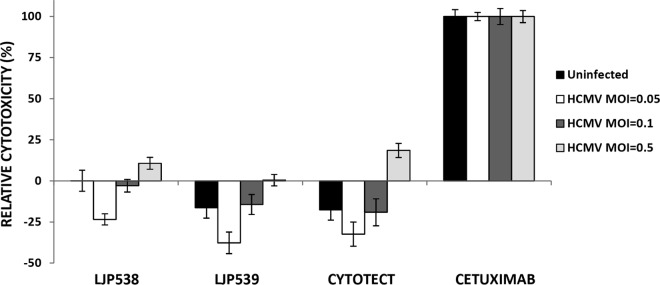
LJP538, but not LJP539, shows a low yet detectable level of ADCC in vitro.
Antibody-dependent cell-mediated cytotoxicity (ADCC) is an immune defense mechanism whereby certain peripheral blood mononuclear cells (PBMC), in particular, natural killer (NK) cells, mediate cytotoxicity of antibody-coated microorganisms or host cells. Targeting of cells that express HCMV antigens for antibody-dependent destruction may be a benefit of immunoglobulin therapy by helping to deplete cells that are actively generating infectious virus. The possibility of LJP538- or LJP539-mediated ADCC in vitro was investigated by incubating ARPE-19 cells with antibodies and then coculturing them with PBMC from HCMV-seronegative donors (see the supplemental material). A low level of ADCC was observed in HCMV-infected cells in the presence of LJP538 (Fig. 4). A similar profile was observed with Cytotect. No ADCC was observed in the presence of LJP539 or in uninfected cells. A chimeric mouse-human antibody that recognizes the human EGFR, cetuximab, was used as a control and induced a high level of ADCC, as previously reported (14).
FIG 4.
ADCC mediated by LJP538, LJP539, and Cytotect. ADCC effects of antibodies were tested in ARPE-19 cells with a lactate dehydrogenase release assay. ARPE-19 cells infected with HCMV reference strain VR1814 at differing MOIs for 72 h were subsequently treated with LJP538, LJP539, or Cytotect at ∼10× EC90 for 3 h. Cetuximab and an IgG1 isotype control (human anti-chicken lysozyme) were used at 100 μg/ml. Cells were washed and then cocultured with PBMC freshly isolated from HCMV-seronegative donors. Lactate dehydrogenase release measured by a commercial optical density (OD) assay was used to calculate relative cytotoxicity, where cytotoxicity was calculated as (ODtest sample – ODmedium only)/(ODTriton-lysed cells – ODmedium only) and relative cytotoxicity was determined as (Cytotoxicity of Sample – Cytotoxicity of Isotype Control)/Cytotoxicity of Cetuximab). Each data point is the average value from four independent experiments with PBMC derived from unique HCMV-seronegative human donors. Error bars show the standard error of the mean.
Passage of HCMV in the presence of monoclonal antibodies.
HCMV strain VR1814 was passaged in ARPE-19 cells or NHDF in the presence of LJP538 or LJP539 to investigate the emergence of resistance. Reduced susceptibility to the LJP538 antibody over four to six passages was seen in both NHDF and epithelial ARPE-19 cells and to LJP539 in ARPE-19 cells (Table 4); LJP539 was not tested in fibroblasts, as the pentameric complex is not required for entry into these cells. The reductions in susceptibility correlated with the detection of mutations in the coding regions of gB for LJP538 and UL130 for LJP539 (Table 4). By population sequencing, gB E361K and D362N mutations were detected in LJP538-selected virus from fibroblasts, while a deletion of gB amino acid E381 was observed in virus selected in epithelial cells. Sequencing of the coding region of UL130 in LJP539-selected virus from epithelial cells detected a Q191K mutation.
TABLE 4.
HCMV selection in the presence of antibody
| Antibody or combination | Cell type | No. of days in culture | No. of passages | Fold shift in EC50 vs wild typea | Protein; resulting mutation(s) |
|---|---|---|---|---|---|
| LJP538-LJP539 | ARPE-19 | 439 | 11 | —b | NDc |
| LJP538 | NHDF | 115 | 6 | >41 | gB; E361K, D362N |
| LJP538 | ARPE-19 | 158 | 5 | 22 | gB; E381 deletion |
| LJP539 | ARPE-19 | 76 | 4 | >50,000 | UL130; Q191K |
Fold shifts were calculated by dividing antibody EC50s for selected virus by those for wild-type VR1814. A greater-than sign before a fold shift value indicates that the antibody EC50 for the selected virus was greater than the highest concentration tested.
—, viral titer insufficient for assessment.
ND, none detected.
Viruses with reduced susceptibility to LJP538 and LJP539 in combination could not be isolated.
Compared to the individual antibodies, passaging in ARPE-19 cells in the presence of LJP538 and LJP539 in combination inhibited viral infection to a greater extent. This was indicated by a significant delay in the appearance of a CPE and much lower viral titers at each round of propagation (1 × 102 to 1 × 103 infectious units [IU]/ml) than typical for wild-type VR1814 (1 × 106 to 1 × 107 IU/ml). After 439 days in culture, titers were too low for the virus to be analyzed in the neutralization assay. However, we were able to PCR amplify gB, gH, gL, UL128, UL130, and UL131a; no mutations were detected (Table 4).
Engineering of resistance-associated mutations.
We investigated the roles of the resistance-associated mutations by engineering each variant (UL130 Q191K, gB E361K, gB D362N, gB E361K D362N, and gB E381 deletion) into HCMV reference strain AD169rUL131 by using BAC mutagenesis. The wild-type and mutant viruses reached comparable titers in culture (data not shown). The abilities of LJP538 and LJP539 to neutralize the wild-type and mutant viruses were then compared (Table 5). When introduced individually into the HCMV genome, two of the gB mutations observed on selection with LJP538 were found to result in decreased susceptibility to the antibody (E361K and E381 deletion). In contrast, the single D362N mutation conferred no significant resistance. Both mutations together, however, reduced susceptibility more than the E361K mutation alone did. The UL130 Q191K mutation also resulted in a decrease in susceptibility to LJP539.
TABLE 5.
Phenotypic characterization of mutations identified during resistance selection
| Antibody used during selection and protein (mutation[s]) | Fold shift in EC50 relative to wild-type BAC-derived HCMVa |
||
|---|---|---|---|
| ARPE-19 | NHDF | HUVEC | |
| LJP538 | |||
| gB (E361K) | 19.1, 26.5 | 1.0, 9.5 | 140.9, 25.1 |
| gB (E381 deletion) | >195.5, >109.2 | >128.2, >149.7 | >284.7, >114.9 |
| gB (D362N) | 0.3, 0.2 | 0.8, 3.2 | 1.8, 2.0 |
| gB (E361K D362N) | 34.3, >109.2 | >128.2, >149.7 | >284.7, >114.9 |
| LJP539, UL130 (Q191K) | >493.7, >405.7 | NAb | >4,714.6, >1,475.1 |
EC50 shift was calculated on the basis of the potency of the antibody indicated. Duplicate fold shift values are shown for each mutation. They were obtained from two independent neutralization assays done in triplicate.
NA, not applicable.
LJP538- and LJP539-resistant variant sites are conserved in clinical isolates.
To investigate the natural occurrence of the mutations identified in vitro, 22 clinical isolates susceptible to neutralization by LJP538 or LJP539 (see Tables S1 to S3 in the supplemental material) were analyzed for the presence of the gB and UL130 amino acid mutations detected in passaged virus by Sanger sequencing and the dominant sequences were compared. The gB E361K, D362N, and E381 deletion mutations and the UL130 Q191K mutation were not detected by population sequencing in any of the HCMV clinical isolates tested. One conservative change (D to E at gB residue 362) was found in isolates 8816 and 8824. The UL130 region of one clinical isolate, MP-MD-805, could not be amplified.
The natural prevalence of these amino acid changes was also analyzed in sequences deposited in the NCBI database. Fifty-eight unique sequences of gB, 74 of UL130, and 12 complete HCMV genomes were aligned with corresponding gB and UL130 sequences. Two of these sequences had the conservative mutation D362E, while two had E361K. The UL130 Q191K mutation found in the LJP539-selected virus was not present in any of the sequences analyzed (data not shown).
HCMV passaged in the presence of LJP538 or LJP539 remains susceptible to the nonselecting antibody.
Pooled HCMV resistant to LJP538 after passage in the presence of antibody on NHDF was tested for neutralization by LJP538 on NHDF and LJP539 on ARPE-19 cells. As expected, the virus had reduced susceptibility to LJP538; however, it remained susceptible to LJP539 (Table 6). Similarly, pooled virus resistant to LJP538 after passage in epithelial cells was not as readily neutralized by LJP538 on NHDF and remained sensitive to LJP539 on ARPE-19 cells. Virus resistant to LJP539 after passage in epithelial cells showed decreased susceptibility to LJP539 on ARPE-19 cells but remained susceptible to LJP538 on ARPE-19 cells. These results indicate an absence of cross-resistance between LJP538 and LJP539, consistent with the antibodies targeting distinct glycoproteins.
TABLE 6.
Absence of cross-resistance between LJP538 and LJP539a
| Antibody used for selection | Cell type used for: |
Fold shift in potency relative to wild-type VR1814 for: |
||||
|---|---|---|---|---|---|---|
| Viral selection | EC50 generation | LJP538 |
LJP539 |
|||
| EC50 | EC90 | EC50 | EC90 | |||
| LJP538 | NHDF | ARPE-19 | NDb | ND | 0.9 | 1.6 |
| LJP538 | NHDF | NHDF | >35.7 | >17.7 | NAc | NA |
| LJP538 | ARPE-19 | ARPE-19 | 11.3 | 5.8 | 1.1 | 0.7 |
| LJP539 | ARPE-19 | ARPE-19 | 1.2 | 1.2 | >666.6 | >192.3 |
The neutralization potencies of LJP538 and LJP539 against wild-type VR1814 in ARPE-19 cells and NHDF are shown in Table 1.
ND, not determined.
NA, not applicable.
Absence of cross-resistance between LJP538 or LJP539 and ganciclovir.
We assessed the potency of ganciclovir against virus pools with decreased susceptibility to LJP538 or LJP539 generated in both NHDF and ARPE-19 cells. In all cases, both mutant and wild-type viruses remained susceptible to ganciclovir (see Table S5 in the supplemental material). HCMV with reduced susceptibility to ganciclovir was generated by passaging strain AD169 in the presence of the drug. Reduced susceptibility to ganciclovir in NHDF was confirmed, with an EC50 shift of 23.74-fold (data not shown). Virus with decreased sensitivity to ganciclovir was then tested for the ability to be neutralized by antibodies (see Table S5 in the supplemental material). Both LJP538 and HCMV hyperimmunoglobulin (Cytotect) neutralized HCMV with decreased susceptibility to ganciclovir as potently as the wild-type virus. Parental AD169 virus does not express the pentameric complex and thus cannot infect ARPE-19 cells or be tested for susceptibility to LJP539.
The resistance mechanism of LJP538 is different from that of MSL-109.
MSL-109 is an IgG1 monoclonal antibody that targets HCMV glycoprotein gH and neutralizes infection in vitro (20). MSL-109 failed to prevent HCMV infection after allogeneic hematopoietic stem cell transplantation. Subsequent in vitro studies demonstrated that MSL-109 is subject to an unusual nongenetic escape mechanism in which the antibody is taken up by HCMV-infected cells and incorporated into the virion. The virus subsequently uses the Fc domain of the incorporated MSL-109 antibody to infect naive cells (16). This escape mechanism manifests itself in the acquisition of resistance within a single passage in cell culture and equally rapid reversion to the wild type after antibody withdrawal.
As candidates with a resistance mechanism similar to that of MSL-109 would not be clinically viable, LJP538 was experimentally compared to this antibody. We first investigated the ability of HCMV to rapidly escape neutralization on fibroblasts. Wild-type VR1814 virus was grown in the presence of MSL-109 (10 μg/ml [∼10× EC50]) or LJP538 (10 μg/ml [∼10× EC50]) in NHDF for a single passage. As previously observed (16), growth in the presence of MSL-109 for one passage resulted in viral titers similar to those reached in the absence of the antibody. In addition, the recovered virus had lost sensitivity to MSL-109 (see Table S6 in the supplemental material). In contrast, very little virus was detected after a single passage in the presence of LJP538 and the titer was too low to test for a shift in antibody susceptibility (see Table S6 in the supplemental material). These results indicate that, unlike MSL-109, reduced susceptibility to LJP538 did not occur in a single passage.
To further compare the resistance mechanisms of MSL-109 and LJP538, we investigated the tendencies of virus with reduced susceptibility to the antibodies to revert to the wild type after antibody withdrawal. NHDF-derived VR1814 virus resistant to MSL-109 was grown in the absence of the antibody in fibroblasts for a single passage and then tested for neutralization by MSL-109. Whereas virus resistant to MSL-109 was not neutralized by MSL-109, resistant virus grown in the absence of the antibody for one passage had regained susceptibility to the antibody (see Table S7 in the supplemental material). To investigate whether virus with reduced susceptibility to LJP538 behaved in a similar manner, NHDF-derived VR1814 virus resistant to LJP538 was propagated in the absence of the antibody in fibroblasts. The resulting virus was not neutralized by LJP538, even after three passages in the absence of the antibody (data not shown); LJP538-resistant viruses isolated from three independent plaques and grown in the absence of the antibody behaved similarly. These results emphasize that the in vitro mechanism of resistance to LJP538 is different from the nongenetic escape of MSL-109.
Modeling of LJP538 binding to gB.
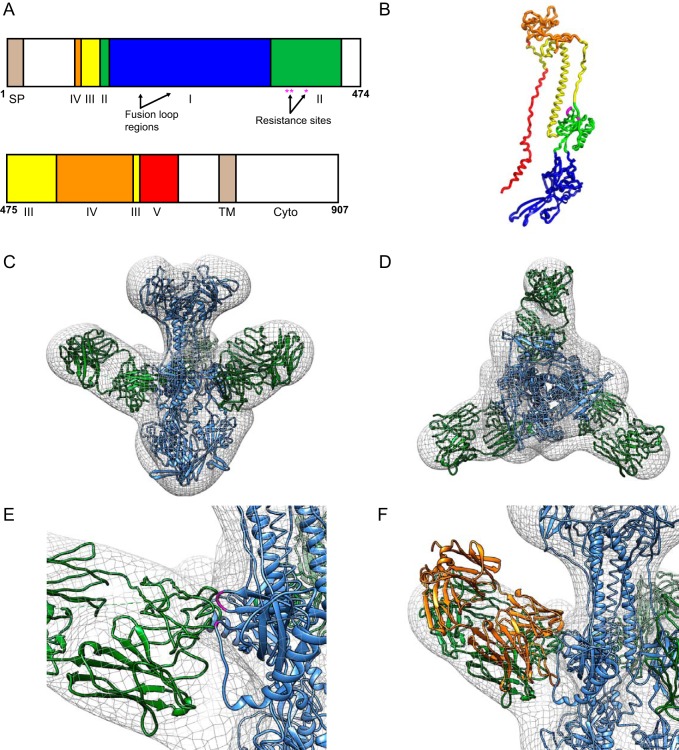
To determine the architecture of the gB complex bound to LJP538, we used negative staining electron microscopy and single-particle analysis. A stoichiometric gB-LJP538 complex was reconstituted by incubating a molar excess of neutralizing Fab with truncated gB. Purified gB-LJP538 was then imaged by negative staining electron microscopy, and ∼15,000 particles were isolated and used to determine the structure with a resolution of ∼15Å (Fig. 5). Comparative models of gB and LJP538 were obtained in silico with the Modeler software package by using respective starting models herpes simplex virus gB (Protein Data Bank [PDB] code 2GUM; 30% sequence identity to gB) and HCMV-specific neutralizing antibody SM5 (PDB code 4OT1). Both models could be placed with high confidence into the electron density of gB-LJP538 by using the fit in map algorithm of the Chimera software package (Fig. 5).
FIG 5.
Structure and epitope mapping of the gB-LJP538 complex. (A) Domain map of full-length gB based on reference 22. Arabic numbers indicate residue positions. Abbreviations: SP, signal peptide; TM, transmembrane; Cyto, cytoplasmic domain. (B) Model of gB monomer colored in accordance with panel A. (C, D) Negative-staining reconstruction and comparative modeling of LJP538 (green) in complex with gB (blue), shown in different orientations. (E) Closeup of resistance-associated residues E361, D362, and E381 (magenta) in the gB structure. (F) Superposition of neutralizing antibody SM5 (orange) over the LJP538 component of the electron microscopic reconstruction.
The locations of the residues associated with in vitro resistance mutations (E361, D362, and E381; Table 5) were distributed across two separate loops at the interface between gB and LJP538 (Fig. 5). The positions of these mutations suggest that the wild-type residues help mediate the interaction between gB and LJP538 and that the mutations interfere with binding and allow escape from neutralization. Interestingly, superposition of the electron microscopy reconstruction and a recently determined X-ray structure of the SM5 antibody bound to a portion of HCMV gB (PDB code 4OSU) (21) showed that SM5 and LJP538 bind the same region of gB and likely neutralize HCMV infection by using a similar mechanism (Fig. 5). While this report was under review, the structure of HCMV gB was determined through X-ray crystallography (22). Following superposition of this structure (PDB code 5C6T) and the model described in this study, equivalent C-α coordinates differed by a 0.89 Å root mean square deviation. Notably, the locations of the resistance-associated residues were also equivalent. These results demonstrate the accuracy of the model and support the conclusion that viral resistance is mediated by unfavorable physical interactions between gB residues and LJP538 following mutation.
DISCUSSION
Here we characterized the in vitro activities of two human monoclonal antibodies, LJP538 and LJP539, in a combination known as CSJ148 that is currently being evaluated for safety and efficacy in clinical trials of HCMV infection in stem cell transplant patients. In vitro, LJP538 and LJP539 potently inhibited HCMV infection in five epithelial and three endothelial cell lines; LJP538 also blocked the infection of fibroblasts. These cell lines may be relevant to pathogenesis in HCMV retinitis patients, kidney transplant patients, and transmission from mother to fetus. While we are unable to test all of the cell types that may be important to disease, these results suggest that the antibodies may be effective across multiple tissue types in vivo. LJP538 and LJP539 were also capable of neutralizing >20 clinical isolates. In all of the strains and cell types tested, LJP538 and LJP539 were more potent at neutralizing HCMV than hyperimmune globulin was. Clinical trials using HCMV hyperimmune globulin in pregnant women have shown a trend toward efficacy, suggesting that a more potent immunoglobulin may be beneficial in this population.
LJP538 and LJP539 were also tested in a long-term (28-day) infection experiment. LJP538 was capable of inhibiting infection at both 1× and 10× EC90 over the course of the experiment. While full inhibition was observed with LJP539 at 10× EC90, breakthrough occurred with this antibody at 1× EC90. Previously published data demonstrate that HCMV infection in endothelial and epithelial cell cultures is primarily focal, strictly cell associated, and highly dependent on the pentameric complex (23, 24). It is therefore possible that LJP539 is less effective at inhibiting the transmission of endothelial cell-tropic virus because of the stoichiometry of the pentameric complex, yet-to-be-understood biological differences in this transmission process, or limitation of antibody access. Since LJP538 is capable of inhibiting infection at the lower concentration, however, antibody access is unlikely to be an issue, consistent with a previous report (23). Further analysis is needed to better understand this phenomenon.
The ability of LJP538 and LJP539 to control cell-to-cell spread, as measured by syncytium formation, was examined by using adenoviral expression to mimic fusion from within. Although EC50s were higher in the syncytium formation assay than in the neutralization assay, both antibodies were able to inhibit syncytium formation at EC50s with 2- to 4-log greater potency than hyperimmunoglobulin. Previously published data indicated that gB, gH, and gL together are sufficient to induce cell-cell fusion (25). However, we observed that expression of gB/gH/gL was not sufficient for fusion to occur. Although similar adenovirus expression systems were used, this difference may result from the larger amount of vector transduced here (we used adenovirus at an MOI of 100 versus 25 in the previous study). This finding further stresses the complexity of the fusion process based on stoichiometry, as well as geometry, of complex formation. In addition, a recent paper using a plaque reduction assay format concluded that LJP539 (previously designated 4I22) was unable to inhibit cell-to-cell spread at an antibody concentration of 10× EC50 (26). In our studies, we observed a 76-fold shift in the EC50 of LJP539 between the neutralization and syncytium formation assays, demonstrating that significantly larger amounts of antibody are needed to inhibit cell-cell spread. Thus, it is possible that higher antibody concentrations are similarly needed to inhibit cell-cell spread in the overlay method.
Both LJP538 and LJP539 are fully human IgG1 antibodies containing an unaltered Fc region. Key immunoglobulin features potentially important to the inhibition of HCMV infection are therefore expected to be preserved, including a long half-life, the ability to cross the placenta, and antibody effector functions, such as ADCC. We examined the abilities of LJP538 and LJP539 to induce ADCC by using PBMC derived from uninfected donors. Through ADCC, NK cells and other effectors mediate cytotoxicity of antibody-coated cells or microorganisms. The NK cell is thought to bind to the immunoglobulin-coated cell through its Fc receptor (CD16), and this interaction can activate both resting and noneducated forms of the immune cells (27). As a counterdefense, HCMV expresses proteins on the surface of infected cells that can also function as Fc-gamma receptors (28). These proteins may capture circulating antibodies and limit the extent of ADCC. Our in vitro data show that LJP538 exhibits a low level of ADCC, while LJP539 did not induce this activity. Thus, in addition to the ability of LJP538 to inhibit HCMV entry, it may stimulate the immune system to kill infected cells.
HCMV isolates with lowered sensitivity to LJP538 or LJP539 can be selected after serial passage of virus in the presence of either antibody alone. When introduced individually into the HCMV genome, two of the gB mutations observed on selection with LJP538 were found to result in decreased susceptibility to the antibody (E361K and E381 deletion). In contrast, the single D362N mutation conferred no significant resistance. However, a combination of the E361K and D362N mutations resulted in further reduced susceptibility to LJP538 than the E361K mutation alone. When introduced into the HCMV genome, a UL130 mutation (Q191K) resulted in a virus with decreased susceptibility to LJP539. Notably, in fibroblasts, LJP538 did not display a mechanism of resistance similar to that of the clinically unsuccessful candidate MSL-109. Importantly, when HCMV was propagated in the presence of LJP538 and LJP539 in combination, virus replication was severely hampered. In addition, even after propagation for 439 days, the virus did not appear to acquire any of the mutations found after selection in the presence of individual antibodies. This suggests that the combination of antibodies will have a higher barrier to the development of resistance than either antibody alone when administered to patients.
Importantly, a survey of unique gB and UL130 sequences indicated that none of the amino acid changes associated with significant levels of LJP538 or LJP539 resistance were highly prevalent in circulating strains. Two isolates had a D362E change, but both demonstrated a susceptibility to LJP538 similar to that of VR1814. Furthermore, we have confirmed experimentally that both LJP538 and LJP539 neutralize >20 different geographically and temporally diverse HCMV clinical isolates in vitro with potencies higher than that of HCMV hyperimmune globulin. These antibodies therefore have the potential for in vivo activity against a broad spectrum of naturally occurring HCMV strains.
Cross-resistance studies indicated that LJP539 can neutralize HCMV with reduced susceptibility to LJP538, while LJP538 can neutralize HCMV with reduced susceptibility to LJP539 at concentrations similar to those required to inhibit the wild-type virus. Although these data were not unexpected, considering that the antibodies target distinct glycoprotein complexes, the lack of cross-resistance is of clinical importance. Furthermore, each antibody remained effective against ganciclovir-resistant strains, indicating that the antibody combination may be a useful therapy for ganciclovir-resistant HCMV infections.
These antibodies will be administered in combination to patients, which is important for multiple reasons. Disease pathogenesis appears to require HCMV to infect different cell types. Infection of endothelial and hematopoietic cells may facilitate the systemic spread of the virus, while infection of fibroblasts may contribute to high-level replication (6). While earlier studies suggested that epitopes within gB made up half of the neutralizing activity of human sera (10), more recent studies show that naturally infected individuals make antibodies that inhibit epithelial epitopes at a significantly higher titer than those inhibiting fibroblast epitopes (29). Analysis of HCMV hyperimmune globulin showed that most the of neutralizing activity contained therein was directed against the pentameric complex, further highlighting the importance of antipentamer antibodies in controlling HCMV infection (12). While these data suggest that antibodies directed against the pentameric complex may be essential to viral control, the anti-gB antibody LJP538 has the added benefit of inducing some level of ADCC in vitro. Furthermore, animal models of congenital transmission of CMV (guinea pig, rhesus macaque), as well as human vaccine studies, demonstrated that inhibition of only one cell entry pathway is not sufficient to provide efficient protection (30, 31, 32). Neutralization of the two major cell entry pathways may therefore be more effective in reducing the HCMV load. In addition, in vitro studies suggest that the combination of antibodies may have a significantly higher barrier to resistance than each antibody alone. Taken together, these data support the continued development of LJP538 and LJP539 in combination for the prevention and treatment of HCMV infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Peter Pertel for critical review of the manuscript. We thank Catherine Jones for writing assistance. We thank Thomas Shenk, Mark Prichard, Thomas Mertens, and Peter Barry for their generous contributions of reagents.
This work was supported by Novartis.
All of us are, or were at the time the work described here was conducted, employees of Novartis and/or shareholders of Novartis stock. A.C. is a shareholder of GSK stock.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00382-16.
REFERENCES
- 1.Torres-Madriz G, Boucher HW. 2008. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis 47:702–711. doi: 10.1086/590934. [DOI] [PubMed] [Google Scholar]
- 2.Boeckh M, Ljungman P. 2009. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 4.Polilli E, Parruti G, D'Arcangelo F, Tracanna E, Clerico L, Savini V, D'Antonio F, Rosati M, Manzoli L, D'Antonio D, Nigro G. 2012. Preliminary evaluation of the safety and efficacy of standard intravenous immunoglobulins in pregnant women with primary cytomegalovirus infection. Clin Vaccine Immunol 19:1991–1993. doi: 10.1128/CVI.00509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, CHIP Study Group . 2014. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 6.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. [DOI] [PubMed] [Google Scholar]
- 7.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou M, Lanchy JM, Ryckman BJ. 2015. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types, whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J Virol 89:8999–9009. doi: 10.1128/JVI.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall GS, Rabalais GP, Stout GG, Waldeyer SL. 1992. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis 165:381–384. doi: 10.1093/infdis/165.2.381. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Li F, Freed DC, Finnefrock AC, Tang A, Grimes SN, Casimiro DR, Fu TM. 2011. Quantitative analysis of neutralizing antibody response to human cytomegalovirus in natural infection. Vaccine 29:9075–9080. doi: 10.1016/j.vaccine.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 12.Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 86:7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. 1995. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1:1311–1318. [PubMed] [Google Scholar]
- 15.Nokta M, Tolpin MD, Nadler PI, Pollard RB. 1994. Human monoclonal anti-cytomegalovirus (CMV) antibody (MSL 109): enhancement of in vitro foscarnet- and ganciclovir-induced inhibition of CMV replication. Antiviral Res 24:17–26. doi: 10.1016/0166-3542(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 16.Manley K, Anderson J, Yang F, Szustakowski J, Oakeley EJ, Compton T, Feire AL. 2011. Human cytomegalovirus escapes a naturally occurring neutralizing antibody by incorporating it into assembling virions. Cell Host Microbe 10:197–209. doi: 10.1016/j.chom.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Ibig-Rehm Y, Gotte M, Gabriel D, Woodhall D, Shea A, Brown NE, Compton T, Feire AL. 2011. High-content screening to distinguish between attachment and post-attachment steps of human cytomegalovirus entry into fibroblasts and epithelial cells. Antiviral Res 89:246–256. doi: 10.1016/j.antiviral.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Chou SW, Dennison KM. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis 163:1229–1234. doi: 10.1093/infdis/163.6.1229. [DOI] [PubMed] [Google Scholar]
- 19.Chou S. 1992. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J Infect Dis 166:604–607. doi: 10.1093/infdis/166.3.604. [DOI] [PubMed] [Google Scholar]
- 20.Boeckh M, Bowden RA, Storer B, Chao NJ, Spielberger R, Tierney DK, Gallez-Hawkins G, Cunningham T, Blume KG, Levitt D, Zaia JA. 2001. Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 7:343–351. doi: 10.1016/S1083-8791(01)80005-7. [DOI] [PubMed] [Google Scholar]
- 21.Spindler N, Diestel U, Stump JD, Wiegers AK, Winkler TH, Sticht H, Mach M, Muller YA. 2014. Structural basis for the recognition of human cytomegalovirus glycoprotein B by a neutralizing human antibody. PLoS Pathog 10:e1004377. doi: 10.1371/journal.ppat.1004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandramouli S, Ciferri C, Nikitin PA, Calo S, Gerrein R, Balabanis K, Monroe J, Hebner C, Lilja AE, Settembre EC, Carfi A. 2015. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat Commun 6:8176. doi: 10.1038/ncomms9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scrivano L, Sinzger C, Nitschko H, Koszinowski UH, Adler B. 2011. HCMV spread and cell tropism are determined by distinct virus populations. PLoS Pathog 7:e1001256. doi: 10.1371/journal.ppat.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, Gallina A, Baldanti F, Revello MG. 2008. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 89:853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 25.Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J Virol 82:11837–11850. doi: 10.1128/JVI.01623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob CL, Lamorte L, Sepulveda E, Lorenz IC, Gauthier A, Franti M. 2013. Neutralizing antibodies are unable to inhibit direct viral cell-to-cell spread of human cytomegalovirus. Virology 444:140–147. doi: 10.1016/j.virol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Watzl C. 2014. How to trigger a killer: modulation of natural killer cell reactivity on many levels. Adv Immunol 124:137–170. doi: 10.1016/B978-0-12-800147-9.00005-4. [DOI] [PubMed] [Google Scholar]
- 28.Atalay R, Zimmermann A, Wagner M, Borst E, Benz C, Messerle M, Hengel H. 2002. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcgamma receptor homologs. J Virol 76:8596–8608. doi: 10.1128/JVI.76.17.8596-8608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Meza BP, Adler SP, McVoy MA. 2008. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine 26:5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auerbach MR, Yan D, Vij R, Hongo JA, Nakamura G, Vernes JM, Meng YG, Lein S, Chan P, Ross J, Carano R, Deng R, Lewin-Koh N, Xu M, Feierbach B. 2014. A neutralizing anti-gH/gL monoclonal antibody is protective in the guinea pig model of congenital CMV infection. PLoS Pathog 10:e1004060. doi: 10.1371/journal.ppat.1004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleiss MR, Buus R, Choi KY, McGregor A. 2013. An attenuated CMV vaccine with a deletion in tegument protein GP83 (pp65 homolog) protects against placental infection and improves pregnancy outcome in a guinea pig challenge model. Future Virol 8:1151–1160. doi: 10.2217/fvl.13.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prichard MN, Aseltine KR, Shipman CJ. 1993. MacSynergy II manual. University of Alabama at Birmingham, Birmingham, Alabama: http://www.uab.edu/images/pediatrics/ID/MacSynergy.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.