Abstract
Ivermectin and moxidectin are the most widely administered anthelmintic macrocyclic lactones (MLs) to treat human and animal nematode infections. Their widespread and frequent use has led to a high level of resistance to these drugs. Although they have the same mode of action, differences in terms of selection for drug resistance have been reported. Our objective was to study and compare changes occurring upon ivermectin or moxidectin selection in the model nematode Caenorhabditis elegans. C. elegans worms were submitted to stepwise exposure to increasing doses of moxidectin. The sensitivity of moxidectin-selected worms to MLs was determined in a larval development assay and compared with those of wild-type and ivermectin-selected strains. Selection with either ivermectin or moxidectin led to acquired tolerance to ivermectin, moxidectin, and eprinomectin. Importantly, moxidectin was the most potent ML in both ivermectin- and moxidectin-selected strains. Interestingly, this order of potency was also observed in a resistant Haemonchus contortus isolate. In addition, ivermectin- and moxidectin-selected strains displayed constitutive overexpression of several genes involved in xenobiotic metabolism and transport. Moreover, verapamil potentiated sensitivity to ivermectin and moxidectin, demonstrating that ABC transporters play a role in ML sensitivity in ML-selected C. elegans strains. Finally, both ivermectin- and moxidectin-selected strains displayed a dye-filling-defective phenotype. Overall, this work demonstrated that selection with ivermectin or moxidectin led to cross-resistance to several MLs in nematodes and that the induction of detoxification systems and defects in the integrity of amphidial neurons are two mechanisms that appear to affect the responsiveness of worms to both ivermectin and moxidectin.
INTRODUCTION
The broad-spectrum anthelmintic macrocyclic lactones (MLs) are most commonly used in veterinary medicine to treat diseases caused by gastrointestinal nematodes and external parasites in livestock (1, 2). Ivermectin (IVM) was the first ML approved for use in animals and remains today the sole ML registered for use in humans, mainly to treat onchocerciasis through mass chemotherapy. Another ML, moxidectin (MOX), was subsequently commercialized for the veterinary market and is currently being evaluated for possible use against human onchocerciasis (3). Inevitably, the intensive use of these compounds has led to the emergence of resistance in small ruminant, cattle, and some human nematode parasites (4–7). Discovering the mechanisms by which resistance to MLs occurs remains an important challenge today.
There is consistent evidence that ATP-binding-cassette (ABC) transporters such as P-glycoproteins (Pgps) play an important role in multidrug resistance (MDR) in many organisms, including several nematode species. Gene expression levels of ABC transporters or allele frequencies were modified after ML selection (8–13), and they are involved in the tolerance of Caenorhabditis elegans (9, 14–16) and parasitic nematodes such as Haemonchus contortus or Cooperia oncophora (13, 17–20) to MLs. In addition, mutation of the dyf-7 gene was associated with an IVM resistance phenotype in C. elegans and in H. contortus, leading to an abnormal dendritic morphology of amphid sensory neurons, as revealed by a dye-filling-defective phenotype (21).
Despite a common ML structure and similar modes of action on glutamate-gated chloride channels (GluCls), there are significant differences between IVM and MOX in terms of pharmacokinetics, pharmacodynamics, and toxicity to the host (see reference 2 for a review). In addition, many reports described differences in the emergence of resistance (22, 23). Indeed, MOX seems to select less strongly for resistance than IVM, and resistance to IVM in various species of strongyles is much more widespread than is resistance to MOX (24–26). Moreover, while there is some degree of cross-resistance between IVM and MOX, MOX remains more effective than IVM against various resistant isolates of nematodes in sheep, goats, cattle, horses, and dogs (2, 27–32). The molecular basis for these differences between MOX and IVM in selection for resistance and the mechanisms of cross-resistance still needs to be determined.
In this context, the objective of this study was to perform a comparative in vitro analysis of acquired tolerance to the macrocyclic lactones IVM and MOX, using C. elegans as model nematode organism. For this, a MOX-selected strain of C. elegans was generated by stepwise exposure. The MOX-selected strain was then compared with the wild-type unselected Bristol N2 strain and the previously described IVM-selected strain IVR10 (11) in terms of (i) ML susceptibility and phenotype of cross-resistance against other anthelmintics, (ii) the impact of verapamil (a competitive inhibitor that blocks the function of mammalian ABC transporters) on drug susceptibility, (iii) transcriptional profiles of the detoxification system of C. elegans, and (iv) staining of amphid neurons. This study points out differences and similarities in the mechanisms of adaptation to IVM and MOX, which could help in the design of optimal anthelmintic treatment when IVM resistance is present.
MATERIALS AND METHODS
Materials.
IVM, dimethyl sulfoxide (DMSO), sodium hypochlorite, cholesterol, verapamil monohydrochloride monohydrate (VP), levamisole (LEV), triclabendazole sulfoxide (TCBZ So), and albendazole sulfoxide (ALB So) were purchased from Sigma-Aldrich (St Quentin Fallavier, France). MOX and eprinomectin (EPR) were generous gifts from Fort Dodge International (Fort Dodge, IA) and Merial France (Lyon, France), respectively. DiIC12(3) (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) was obtained from Invitrogen/Life Technology (Cergy Pontoise, France). A 2-mg/ml stock solution of DilC12(3) was prepared in DMSO. Culture plates were supplied by Sarstedt (Orsay, France). All other chemicals were obtained from Sigma-Aldrich, unless otherwise stated. For all experiments, IVM and MOX were dissolved in DMSO, and the maximal concentration of DMSO was 0.3% in all assays.
Ethics statement.
All animal experiments were approved by the French Ministry of Teaching and Research and the regional Val de Loire ethics committee (no. 19) as a protocol registered under no. 00219.02 in the experimental installations (agreement no. C371753).
C. elegans nematode strains and culturing.
Wild-type C. elegans Bristol strain N2 was obtained from the Caenorhabditis elegans Genetics Center (CGC; University of Minnesota, Minneapolis, MN, USA). The IVR10 strain, selected from the wild-type strain with IVM and phenotypically resistant to IVM, was kindly provided by C. E. James (11).
All strains were cultured and handled according to procedures described previously (33). Briefly, nematodes were cultured at 21°C on nematode growth medium (NGM) agar plates (1.7% Bacto agar, 0.2% Bacto peptone, 50 mM NaCl, 5 mg/liter cholesterol, 1 mM CaCl2, 1 mM MgSO4, and 25 mM KPO4 buffer) seeded with Escherichia coli strain OP50 as a food source. ML-containing NGM plates were prepared as follows: stock solutions of IVM and MOX in DMSO were diluted in NGM at an adequate concentration before plates were poured. IVM-selected strains (IVR10 and IVM11R) were cultured on NGM plates containing 11.4 nM (10 ng/ml) IVM, and the MOX-selected strain (MOX5R) was cultured on NGM plates containing 4.6 nM (3 ng/ml) MOX.
Nematodes were synchronized through egg preparation with sodium hypochlorite. Briefly, asynchronous populations with a majority of gravid adults and eggs were collected by washing the bottom of the NGM plates with M9 buffer (3 g KH2PO4, 6g Na2HPO4, 5 g NaCl, and 0.25 g MgSO4·7H2O in 1 liter of water) and centrifuged at 1,200 × g for 1 min. All larval stages except eggs were lysed with a bleaching mixture (5 M NaOH and 1% hypochloride). Three washes with M9 buffer were done to retire the toxic bleaching mixture. C. elegans eggs were then hatched overnight at 21°C in M9 solution without bacteria to obtain a synchronized first-stage larval (L1) population.
Parasite isolates.
The H. contortus isolate tested was Kokstad (HcR-KOK), a line resistant against the three main anthelmintic classes, i.e., levamisole, MLs, and benzimidazole (34), originally obtained from a farm in South Africa and maintained in the INRA laboratory since 2000. The isolate was passaged every 2 months in a 3-month-old sheep (infected with 6,000 infective larvae [L3]). Sheep carrying this resistant isolate of H. contortus were treated with IVM (0.2 mg/kg of body weight) at 35 days postinfection.
Development of acquired tolerance to IVM and MOX in C. elegans Bristol strain N2.
Culture conditions for the development of ML-resistant C. elegans strains following stepwise exposure to MLs were adapted from those described previously by James and Davey (11). Briefly, at week 0, a Bristol N2 worm population was transferred onto NGM plates containing either 0.57 nM IVM or MOX, corresponding to 0.5 ng/ml and 0.37 ng/ml of IVM and MOX, respectively. These concentrations, determined in a preliminary assay (data not shown), correspond to the highest concentrations allowing 100% development to the adult stage. Each week, worms were transferred onto new NGM plates. When worms survived and reproduced, they were transferred onto plates containing higher doses of MLs. The equimolar ML concentrations used to create both IVM- and MOX-selected strains were 0.57, 1.14, 2.29, 3.43, 4.57, 5.71, 6.86, 8.00, 9.14, 10.29, and 11.43 nM, corresponding to 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 ng/ml of IVM and 0.37, 0.73, 1.46, 2.19, 2.92, 3.66, 4.39, 5.12, 5.85, 6.58, and 7.31 ng/ml of MOX, respectively. After 40 weeks, worms were able to survive on 11.4 nM (10 ng/ml) IVM and 5.7 nM (3.7 ng/ml) MOX.
Note that, unless otherwise stated, the IVM-selected strain used for subsequent studies was the IVR10 strain, generated by C. E. James, which was previously characterized (11, 16, 21), cultivated on 11.4 nM IVM, while the MOX-selected strain (MOX5R), which was generated by stepwise exposure in our laboratory, was cultivated on 4.6 nM MOX.
Larval development assay.
The susceptibility of the C. elegans strains and H. contortus isolates to MLs and other anthelmintics was determined in a larval development assay (LDA) as described previously (35).
(i) LDA on C. elegans strains.
The LDA on C. elegans strains measures the potency of anthelmintics in inhibiting the development of C. elegans nematodes from eggs to the young adult stage. Approximately 30 synchronized L1 larvae were added in every well of a 12-well plate poured with NGM containing increasing concentrations of the compound of interest and seeded with OP50 bacteria. DMSO was used as a control at a maximal concentration of 0.3%. At this concentration, no harmful effects of the vehicle on C. elegans were observed. Plates were then incubated at 21°C in the dark during a time period of 52 to 55 h, in which L1 larvae of the negative control were developed into late L4/young adult worms. L1, L2, and L3 larvae were scored as being inhibited in their development, and the late L4 and young adult worms were classified as being developed. Development was calculated as a percentage of late L4 larvae and young adults in the presence of compounds of interest normalized to the untreated control. Every concentration was set up in triplicates, and all experiments were repeated at least three independent times. Curve fitting of data from the larval development assay (sigmoidal dose-response curve with a variable slope) was performed by using GraphPad Prism 6 software (GraphPad, San Diego, CA, USA) and allowed calculation of the effective concentration for a 50% effect (EC50).
(ii) LDA on the H. contortus isolate.
The LDA on the H. contortus isolate measures the potency of anthelmintics in inhibiting the development of trichostrongyle nematodes from eggs to infective L3 larvae. Nematode eggs were recovered from fresh fecal matter by using a standard procedure described previously (36). Briefly, eggs were incubated with 30 μg of inactivated E. coli bacteria, and 10 μg of amphotericin B (Fungizone; Squibb) was added per ml of egg suspension to avoid proliferation of fungi during larval development. Tubes were incubated at 23°C for 48 h. By this time, eggs had hatched and developed to the L1 or L2 stage. After 48 h, larvae were supplemented with nutrient medium, Earle's balanced salt solution, yeast extract (1 g of yeast extract/90 ml of saline solution [pH 7]), and anthelmintics and then incubated for 7 days at 23°C. The proportion of developed L3 compared to the total number of larvae (L3 plus undeveloped stages [L1 plus L2]) present under each condition was calculated and expressed as a percentage, with the mean number of developed larvae under control conditions being fixed at 100.
C. elegans dye-filling assay (Dil staining of amphids).
To visualize the amphid dendrites of the C. elegans wild-type Bristol N2, IVM-selected, and MOX-selected strains, worms were synchronized at late L4. The larvae were then incubated in a dye solution containing 10 ng/ml of DilC12(3) in M9 broth with gentle shaking for 2 h at 21°C. After a recovery period of 2 h on NGM plates, worms were paralyzed by using levamisole (40 mM), and dye-filled L4 larvae were observed by using a Nikon Eclipse 50i microscope equipped with a Luca S camera and analyzed by using Nikon ACT-1 software.
Total RNA isolation and qRT-PCR analysis. (i) Isolation of RNA and cDNA synthesis.
Changes in gene expression profiles of IVM-selected and MOX-selected C. elegans strains were analyzed by using quantitative reverse transcription PCR (qRT-PCR) and compared to those of the wild-type Bristol N2 strain. Synchronized L1 larvae were added on control NGM plates. After 55 h of incubation at 21°C, synchronized young adults were collected by using M9 buffer. After five washes with M9 buffer, sedimented worms were added to 1 ml TRIzol reagent (Invitrogen, Cergy Pontoise, France), frozen in liquid nitrogen, and stored at −80°C. Frozen samples were then homogenized twice for 10 s at 6 m/s in a FastPrep-24 instrument (MP-Biomedicals, NY, USA), and total RNA was extracted according to the manufacturer's instructions. Each independent replicate was performed on a different day. Total RNA was quantified by using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). RNA purity was checked by measurement of the A260/A280 ratio, which was routinely in the range of 1.8 to 2.0, and RNA quality control was carried out by using an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). cDNA was synthesized from 2 μg of total RNA by using the High-Capacity cDNA reverse transcription kit (Applied Biosystems, Life Technologies, Courtaboeuf, France).
(ii) Quantification of mRNA expression by qRT-PCR.
qRT-PCR was performed by using the ViiA7 sequence detection system instrument and software (Applied Biosystems, Life Technologies, Courtaboeuf, France). Gene-specific primers for SYBR green assays were designed according to the genome sequence of C. elegans (http://www.wormbase.org/), using Primer Express software version 2.0 (Applied Biosystems), and synthesized by Invitrogen (Cergy Pontoise, France). All primers were entered into the NCBI BLAST program to ensure specificity. Results were expressed by using the comparative threshold cycle (CT) method as described in User Bulletin 2 (Applied Biosystems). Briefly, the ΔCT values were calculated for every sample for each gene of interest as CT gene of interest − CT reporter gene, with cell division cycle protein 42 (CDC42) as the reporter gene. The relative expression levels of the target genes were calculated by using the comparative 2−ΔΔCT method (37). A dissociation curve allowed us to verify the specificity of amplification.
Statistical analysis.
All experiments were conducted at least in triplicate, and results are expressed as means ± standard deviations (SD). Statistical analysis was performed by using the unpaired t test (individual comparisons between pairs of data) or one-way analysis of variance (ANOVA) with a Tukey posttest (multiple comparisons) (GraphPad Instat, San Diego, CA, USA). Differences with P values of <0.05 were considered to be statistically significant.
RESULTS
C. elegans is able to acquire tolerance to MOX.
In order to compare the adaptations of the nematode C. elegans to IVM and MOX, a MOX-selected strain was generated through stepwise exposure to increasing MOX concentrations and compared to IVM-selected strain IVR10 described previously by James and Davey (11). After 40 weeks, worms were able to survive on a MOX concentration of 4.6 nM (data not shown). The establishment of this MOX-selected strain allowed us to assess the adaptation of C. elegans worms after selection pressure with either IVM or MOX by comparing the susceptibilities of IVM-selected and MOX-selected populations to increasing concentrations of either IVM or MOX to that of the nonexposed control Bristol N2 strain.
MOX is more potent than IVM in both IVM-selected (IVR10) and MOX-selected C. elegans strains.
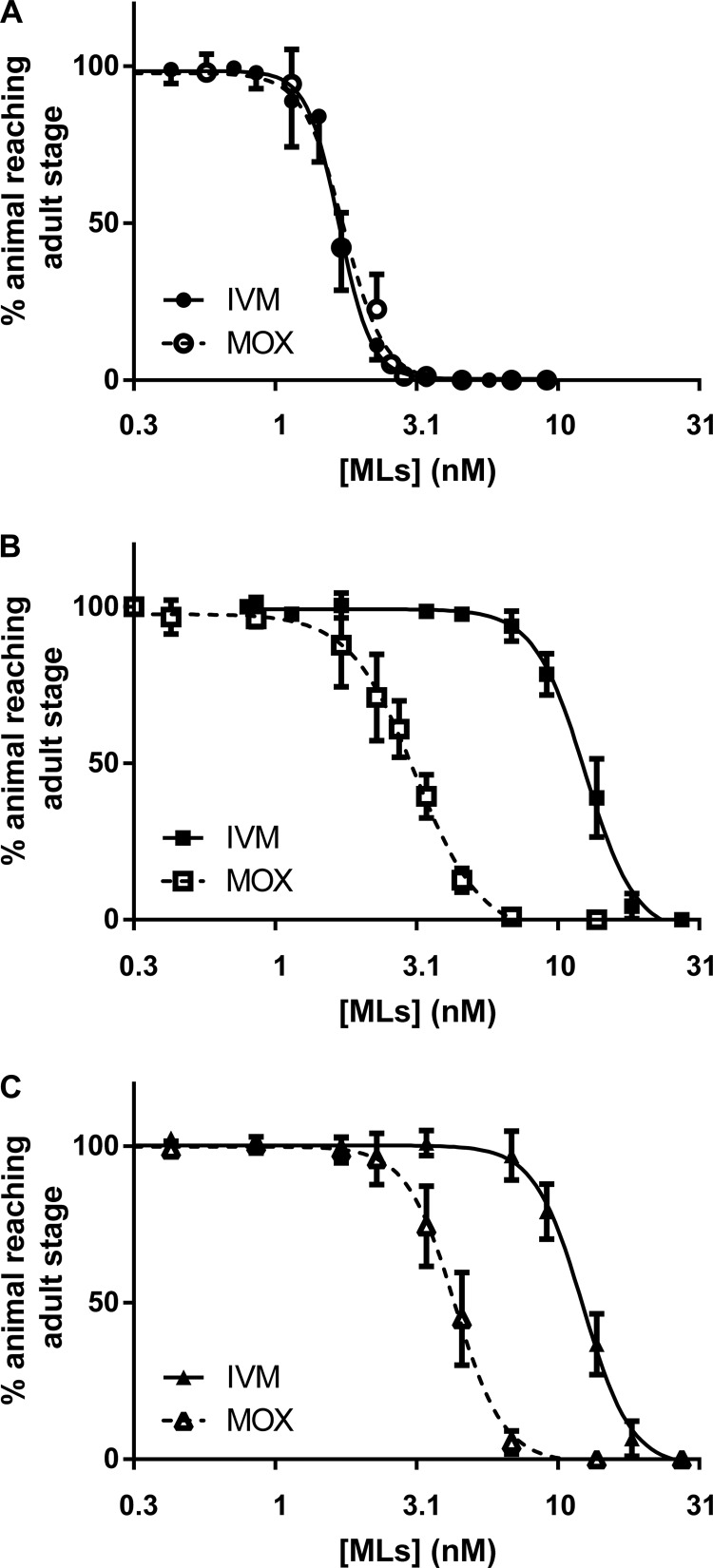
Dose-response curves for IVM and MOX toward development in the adult stage of the three strains are presented in Fig. 1. EC50s, i.e., the concentrations of compound at which 50% of the animals fail to reach the adult stage; resistance factor (RF) values, reflecting differences in the EC50s compared with those of the wild-type strain; as well as the IVM/MOX ratio, reflecting differences in efficiencies between IVM and MOX, are shown in Table 1.
FIG 1.
Profiles of susceptibility of the wild-type Bristol N2 (A), IVM-selected (IVR10) (B), and MOX-selected (C) C. elegans strains to IVM and MOX in a larval development assay. Values represent the percentages of L1 larvae reaching the young adult stage after 55 h of incubation at 21°C in the presence of increasing doses of IVM or MOX. Data are means ± SD from 7 to 15 independent experiments.
TABLE 1.
Susceptibilities of wild-type, IVM-selected, and MOX-selected C. elegans strains to IVM and MOXd
| Treatment | Mean EC50 (nM) ± SD for wild-type Bristol N2 (no. of expts) | IVM-selected strain (IVR10) |
MOX-selected strain |
||
|---|---|---|---|---|---|
| Mean EC50 (nM) ± SD (no. of expts) | RF | Mean EC50 (nM) ± SD (no. of expts) | RF | ||
| IVM | 1.69 ± 0.30 (9) | 12.43 ± 1.65 (7)a | 7.34 | 12.46 ± 0.96 (8)a | 7.35 |
| MOX | 1.77 ± 0.25 (9) | 3.06 ± 0.51 (11)a,b,c | 1.74 | 4.24 ± 0.58 (9)a,b,c | 2.39 |
P < 0.001 versus the wild type.
P < 0.001 versus IVM.
P < 0.001 for IVM-selected versus MOX-selected strains.
The EC50 was calculated from LDA data. RF (resistance factor) is the fold resistance relative to Bristol N2, equal to the EC50 for ML-selected strains/EC50 for N2B. The ratios of the EC50 of IVM to the EC50 of MOX were 0.96 for the Bristol N2 strain, 4.03 for the IVM-selected strain, and 2.94 for the MOX-selected strain.
In the wild-type strain (Fig. 1A), IVM and MOX displayed similar potencies in affecting the development of C. elegans larvae, with EC50s of 1.69 ± 0.30 and 1.77 ± 0.25 nM for IVM and MOX, respectively (Table 1). As expected, both ML-selected strains showed a decrease in susceptibility to the drug used for the selection process, as revealed by a significant shift to the right (high EC50) of the dose-response curves for IVM in the IVM-selected IVR10 subline (Fig. 1B) and for MOX in the MOX-selected subline (Fig. 1C) compared with that for the wild-type unselected strain. As a result, the IVM-selected IVR10 strain was 7.3-fold less sensitive to IVM (EC50 of 12.43 ± 1.65 nM; P < 0.001 versus the wild type) (Table 1) while the MOX-selected strain was 2.4-fold less sensitive to MOX (EC50 of 4.24 ± 0.58 nM; P < 0.001 versus the wild type) (Table 1) than the parental unselected strain.
Interestingly, each of the ML-selected strains showed cross-resistance to the other drug, which had not been used during the selection process. The IVM-selected IVR10 subline was slightly less susceptible to MOX (1.7-fold; EC50 of 3.06 ± 0.51 nM; P < 0.001 versus the wild type) (Table 1), while, more surprisingly, the MOX-selected subline showed a highly significant decrease in IVM susceptibility (7.4-fold; EC50 of 12.46 ± 0.96 nM; P < 0.001 versus the wild type) (Table 1) compared with that of the wild-type unselected strain. As a result, both strains were resistant to both IVM and MOX and displayed highly similar profiles of susceptibility to IVM and MOX, with MOX having higher efficiency in both IVM-selected (4-fold) and MOX-selected (3-fold) sublines and IVM being less potent.
A Pgp inhibitor increases IVM and MOX susceptibility in wild-type and ML-selected strains.
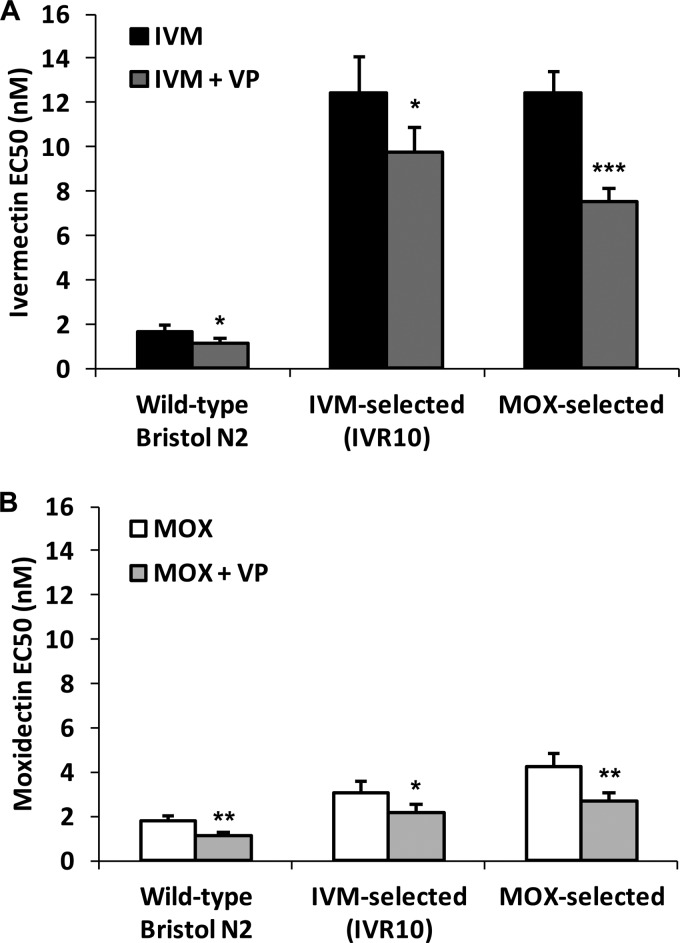
Given the important role of Pgps in effluxing IVM and MOX, thereby protecting the worm, we coadministered the two drugs with the Pgp inhibitor verapamil. Figure 2 shows that verapamil significantly increased IVM and MOX susceptibilities of the wild-type, IVM-selected IVR10, and MOX-selected strains, with the EC50s of both drugs being reduced by ∼30% under each condition. These results suggest that C. elegans ABC transporters are similarly involved in IVM and MOX tolerance in the wild-type and ML-selected strains. Nevertheless, verapamil failed to totally restore wild-type susceptibility in ML-selected strains, showing that the susceptibility of these strains to the two MLs was only partially dependent on Pgp-mediated drug efflux and that other mechanisms determined drug susceptibility in ML-resistant worms.
FIG 2.
Modulation of ML susceptibility by the transporter inhibitor verapamil. Susceptibilities of the wild-type Bristol N2, IVM-selected (IVR10), and MOX-selected strains to IVM and MOX were evaluated with or without the addition of the ABC transporter inhibitor verapamil (VP) (50 μM) in a larval development assay. EC50s in the presence or absence of verapamil for IVM (A) and MOX (B) were calculated as described in Materials and Methods. The bars represent the means ± SD from 3 experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus the absence of verapamil).
IVM- and MOX-selected C. elegans strains display a high level of cross-resistance to EPR.
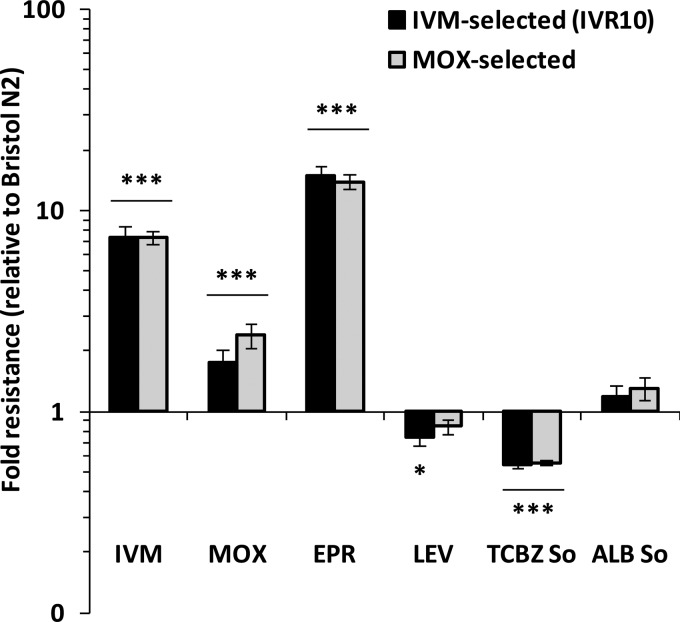
We then investigated the multidrug-resistant phenotype of the IVM-selected IVR10 and MOX-selected strains against other anthelmintics. Figure 3 shows that both ML-selected strains were highly resistant to another ML (EPR) compared with the wild-type strain. The IVM- and MOX-selected strains were 16.5-fold and 15.3-fold less sensitive to EPR (EC50s of 17.82 ± 1.87 nM and 16.49 ± 1.37 nM, respectively; P < 0.001 versus the wild type) (Table 2) than the parental unselected strain. IVM- and MOX-selected strains did not display a resistant phenotype against levamisole, triclabendazole sulfoxide, and albendazole sulfoxide. In contrast, the IVM-selected strain displayed significantly higher sensitivity to levamisole (1.3-fold; P < 0.05 versus the wild type) and to triclabendazole sulfoxide (1.9-fold; P < 0.001 versus the wild type), while the MOX-selected strain was more susceptible to triclabendazole sulfoxide (1.8-fold; P < 0.001 versus the wild type). Susceptibility to the benzimidazole anthelmintic albendazole sulfoxide was unchanged in both ML-selected strains compared to the parental wild-type strain (Fig. 3 and Table 2). This clearly shows that IVM- and MOX-selected strains displayed similar phenotypes regarding drug susceptibility, with EPR being the least potent drug after either IVM or MOX selection pressure.
FIG 3.
Cross-resistance of wild-type Bristol N2, IVM-selected (IVR10), and MOX-selected C. elegans strains to other anthelmintics. A larval development assay was performed on the wild-type Bristol N2, IVM-selected (IVR10), and MOX-selected strains with increasing doses of IVM, MOX, eprinomectin (EPR), levamisole (LEV), triclabendazole sulfoxide (TCBZ So), and albendazole sulfoxide (ALB So). Fold resistances of IVM- and MOX-selected strains relative to the Bristol N2 strain were calculated as ratios of EC50s obtained from the larval development dose-response curves. Data are means ± SD from 3 independent experiments. *, P < 0.05; ***, P < 0.001 (versus the wild type).
TABLE 2.
Susceptibilities of the wild-type Bristol N2, IVM-selected, and MOX-selected C. elegans strains to other anthelminticsa
| Strain | Mean EC50 ± SD |
|||
|---|---|---|---|---|
| EPR (nM) | LEV (μM) | TCBZ So (μM) | ALB So (μM) | |
| Wild-type Bristol N2 | 1.19 ± 0.61 | 16.63 ± 1.19 | 71.41 ± 1.57 | 6.72 ± 1.10 |
| IVM-selected (IVR10) | 17.82 ± 1.87*** | 12.47 ± 1.12* | 38.7 ± 1.16*** | 7.97 ± 1.13 |
| MOX-selected | 16.49 ± 1.37*** | 14.13 ± 1.23 | 39.87 ± 1.12*** | 8.81 ± 1.10 |
EC50 values were calculated from LDA data. *, P < 0.05; ***, P < 0.001 (versus the wild type) (n = 3).
A resistant Haemonchus contortus isolate displays a profile of susceptibility to MLs similar to those of ML-selected C. elegans strains.
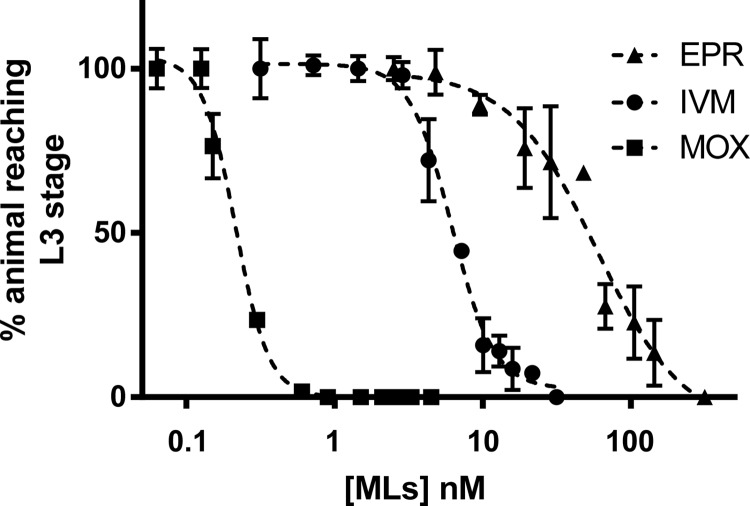
In order to compare the ML susceptibilities of C. elegans with those of parasitic nematodes, we performed an LDA on a resistant H. contortus isolate. The results of dose-response experiments with IVM, MOX, and EPR against H. contortus larvae are shown in Fig. 4 and Table 3. The results show that MOX was the most potent drug against the resistant H. contortus isolate, with EC50s that were 29-fold lower than those of IVM and 280-fold lower than those of EPR. In addition, EPR was the least potent compared with the two other drugs, while IVM displayed intermediate potency. Overall, these results show similar patterns of susceptibility profiles between the resistant H. contortus isolate and ML-selected C. elegans isolates.
FIG 4.
Susceptibility profiles of a resistant Haemonchus contortus isolate in a larval development assay. Values represent the percentages of L1 larvae reaching the L3 stage after 7 days of incubation at 23°C in the presence of increasing concentrations of IVM, MOX, or EPR. Data are means ± SD (n = 3).
TABLE 3.
Susceptibilities of the resistant Haemonchus contortus (HcR-KOK) isolate to IVM, MOX, and EPRa
| Drug | Mean EC50 (nM) ± SD for HcR-KOK isolate | Fold change relative to MOX |
|---|---|---|
| MOX | 0.21 ± 0.02 | 1 |
| IVM | 6.18 ± 0.50 | 29.4 |
| EPR | 58.85 ± 16.25 | 280.2 |
EC50 values were calculated from LDA data. The fold change relative to MOX was calculated as the ratio of the EC50 of IVM or EPR/EC50 of MOX.
The transcriptional profile of the xenobiotic metabolism system is modulated in ML-selected C. elegans strains.
The final concentration of a drug in the parasite is a key determinant for its efficacy and strongly depends on efflux by ABC transporters and on its biotransformation by phase I and phase II enzymes such as cytochrome P450 oxidases, γ-glutamylcysteine synthetase (GCS), and glutathione S-transferases (GSTs). We therefore assessed the impact of IVM and MOX selection pressure on the transcriptional profiles of genes typically involved in metabolism and transport of xenobiotics (Table 4). Interestingly, similar inductions of mRNA expression were observed in both the IVM-selected IVR10 and MOX-selected strains for several ABC transporters, including Pgp1, Pgp2, Pgp3, Pgp5, Pgp6, Pgp9, Pgp11, Pgp12, Pgp14, Mrp3, Mrp6, Haf4, Haf9, Pmp4, and Pmp5; some phase I cytochromes P450, including Cyp14A2 and Cyp14A5; and some phase II detoxification enzymes, including gst4 and gst5. The most substantial changes were observed with Pgp1 (2.2- and 3.0-fold), Pgp6 (4.8- and 3.2-fold), Pgp14 (4.3- and 2.1-fold), Cyp14A2 (3.3- and 2.3-fold), and Cyp14A5 (3.1- and 2.0-fold) in IVM- and MOX-selected strains, respectively. In parallel, the expression levels of Pgp10, Mrp1, Mrp8, and Cyp37B1 were increased only in the IVM-selected strain, while the expressions of Pgp8 and Cyp35A1 were upregulated only in the MOX-selected strain. Beside these changes, expression levels of Pgp4, Pgp7, Pgp13, Mrp2, Mrp4, Mrp5, Mrp7, Haf1, Haf2, Haf3, Haf6, Haf7, Haf8, Pmp1, Pmp2, Pmp3, gcs1, gst1, gst2, gst7, gst10, Cyp13A1, Cyp14A1, Cyp25A1, Cyp25A2, Cyp25A3, Cyp35A2, Cyp35A5, and Cyp35C1 were not affected in either the IVM- or MOX-selected strain. Data from this transcriptomic analysis suggest that the modulated genes in ML-selected C. elegans strains are involved in the xenobiotic metabolism of IVM and MOX and in the production of tolerance against these drugs.
TABLE 4.
Relative constitutive expression levels of genes associated with xenobiotic metabolism and transport in IVM-selected (IVR10) and MOX-selected C. elegans strains compared with the wild-type Bristol N2 strainc
| Category | Gene | Mean fold change in constitutive gene expression relative to wild-type Bristol N2 ± SD |
|
|---|---|---|---|
| IVM-selected strain (IVR10) | MOX-selected strain | ||
| P-glycoproteins (ABCB subfamily) | Cel-pgp1 | 2.16 ± 0.15a | 2.96 ± 0.43a,b |
| Cel-pgp2 | 1.59 ± 0.07a | 1.25 ± 0.11a,b | |
| Cel-pgp3 | 1.90 ± 0.28a | 1.90 ± 0.15a | |
| Cel-pgp4 | 0.86 ± 0.05 | 0.78 ± 0.03 | |
| Cel-pgp5 | 1.74 ± 0.20a | 1.66 ± 0.11a | |
| Cel-pgp6 | 4.77 ± 0.69a | 3.20 ± 0.41a,b | |
| Cel-pgp7 | 1.08 ± 0.09 | 1.00 ± 0.11 | |
| Cel-pgp8 | 1.26 ± 0.14 | 1.51 ± 0.25a | |
| Cel-pgp9 | 2.13 ± 0.13a | 2.06 ± 0.21a | |
| Cel-pgp10 | 1.37 ± 0.13a | 1.24 ± 0.08 | |
| Cel-pgp11 | 1.43 ± 0.13a | 1.40 ± 0.08a | |
| Cel-pgp12 | 1.76 ± 0.31a | 1.78 ± 0.18a | |
| Cel-pgp13 | 1.36 ± 0.21 | 1.22 ± 0.11 | |
| Cel-pgp14 | 4.26 ± 0.35a | 2.06 ± 0.23a,b | |
| Multidrug resistance-associated proteins (MRPs) (ABCC subfamily) | Cel-mrp1 | 1.41 ± 0.13a | 1.12 ± 0.17 |
| Cel-mrp2 | 1.46 ± 0.29 | 1.23 ± 0.13 | |
| Cel-mrp3 | 2.21 ± 0.40a | 1.77 ± 0.12a | |
| Cel-mrp4 | 1.05 ± 0.14 | 1.00 ± 0.01 | |
| Cel-mrp5 | 1.09 ± 0.13 | 1.06 ± 0.07 | |
| Cel-mrp6 | 1.79 ± 0.14a | 1.48 ± 0.03a | |
| Cel-mrp7 | 1.18 ± 0.20 | 1.22 ± 0.04 | |
| Cel-mrp8 | 1.41 ± 0.12a | 1.17 ± 0.04b | |
| Mitochondrial half-molecular ABC transporters | Cel-haf1 | 0.99 ± 0.05 | 1.01 ± 0.08 |
| Cel-haf2 | 1.08 ± 0.17 | 0.97 ± 0.05 | |
| Cel-haf3 | 1.22 ± 0.20 | 1.09 ± 0.02 | |
| Cel-haf4 | 2.12 ± 0.29a | 1.60 ± 0.02a,b | |
| Cel-haf6 | 1.35 ± 0.20 | 1.14 ± 0.11 | |
| Cel-haf7 | 1.39 ± 0.26 | 1.36 ± 0.15 | |
| Cel-haf8 | 1.08 ± 0.12 | 0.91 ± 0.13 | |
| Cel-haf9 | 2.00 ± 0.12a | 1.70 ± 0.10a | |
| Peroxisomal membrane protein related (putative ABCD transporter subfamily) | Cel-pmp1 | 1.10 ± 0.09 | 1.26 ± 0.15 |
| Cel-pmp2 | 1.07 ± 0.04 | 1.14 ± 0.06 | |
| Cel-pmp3 | 0.89 ± 0.02 | 0.98 ± 0.07 | |
| Cel-pmp4 | 1.57 ± 0.08a | 1.34 ± 0.20a | |
| Cel-pmp5 | 1.73 ± 0.13a | 1.73 ± 0.09a | |
| Detoxification enzymes (glutamate-cysteine ligase and glutathione S-transferases) | Cel-gcs1 | 1.05 ± 0.23 | 0.81 ± 0.09 |
| Cel-gst1 | 1.23 ± 0.21 | 0.92 ± 0.11 | |
| Cel-gst2 | 1.52 ± 0.31 | 1.45 ± 0.16 | |
| Cel-gst4 | 2.14 ± 0.27a | 1.70 ± 0.57 | |
| Cel-gst5 | 2.03 ± 0.36a | 1.73 ± 0.52 | |
| Cel-gst7 | 1.07 ± 0.19 | 0.94 ± 0.07 | |
| Cel-gst10 | 1.54 ± 0.25 | 1.70 ± 0.47 | |
| Cytochromes P450 | Cel-cyp13A1 | 2.60 ± 1.00 | 2.10 ± 0.61 |
| Cel-cyp14A1 | 1.66 ± 0.47 | 1.50 ± 0.19 | |
| Cel-cyp14A2 | 3.26 ± 0.42a | 2.31 ± 0.39a,b | |
| Cel-cyp14A5 | 3.06 ± 0.39a | 1.96 ± 0.38a,b | |
| Cel-cyp25A1 | 0.74 ± 0.23 | 0.98 ± 0.38 | |
| Cel-cyp25A2 | 1.19 ± 0.20 | 1.29 ± 0.07 | |
| Cel-cyp25A3 | 1.46 ± 0.57 | 1.03 ± 0.06 | |
| Cel-cyp35A1 | 1.77 ± 0.49 | 4.78 ± 0.26a,b | |
| Cel-cyp35A2 | 1.33 ± 0.34 | 1.49 ± 0.42 | |
| Cel-cyp35A5 | 0.68 ± 0.12 | 1.02 ± 0.19 | |
| Cel-cyp35C1 | 0.78 ± 0.08 | 1.08 ± 0.16 | |
| Cel-cyp37B1 | 5.69 ± 0.75a | 1.89 ± 0.46b | |
Significantly different from the wild-type Bristol N2 strain (P < 0.05).
Significantly different from the IVM-selected strain (P < 0.05).
Data are expressed as fold changes relative to the wild-type Bristol N2 strain and are reported as the means ± SD of data from two to four independent experiments. Boldface type indicates upregulated genes.
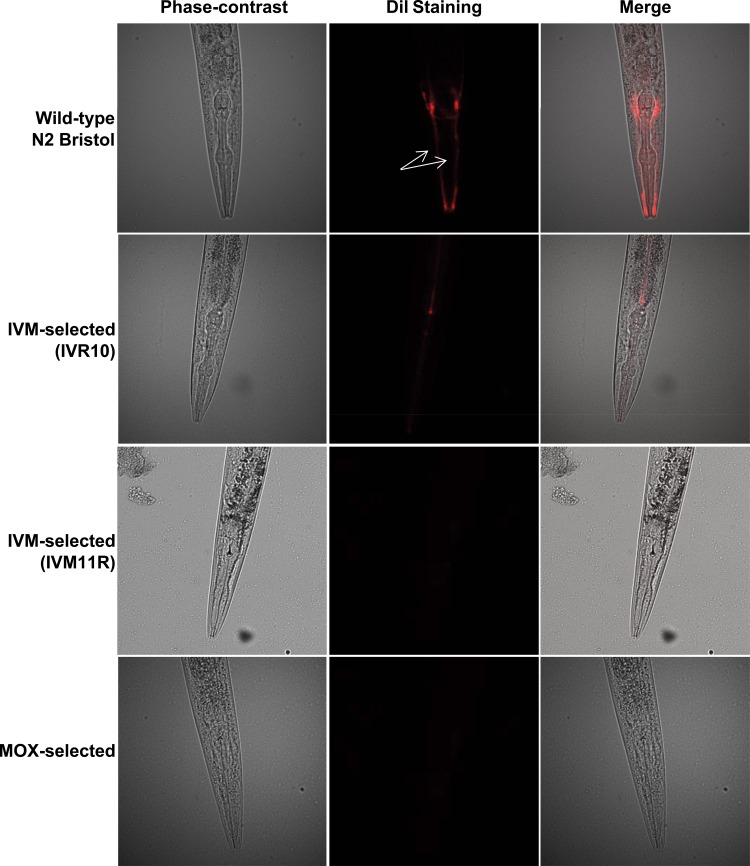
IVM- and MOX-selected C. elegans strains are both dye-filling defective.
The dye-filling phenotype in nematodes relates to the capacity of the worm to take up fluorescent dye that specifically labels the amphids, the principal chemosensory organs of nematodes. The dye-filling-defective phenotype is known to be associated with the IVM resistance phenotype (38, 39), which was shown to be linked to a mutation on the dyf-7 gene in C. elegans and resistant H. contortus worms (21). We therefore evaluated the dye-filling phenotype of IVM- and MOX-selected strains.
We first confirmed that the wild-type Bristol N2 strain displayed a normal morphology of both amphid neurons, while IVM selection in C. elegans led to a dye-filling-defective phenotype. This was observed previously in IVM-selected strain IVR10 by James and Davey (11) but also in another IVM-selected strain, IVM11R, independently generated in our laboratory (Fig. 5). More interestingly, our results show that drug pressure under MOX exposure also selects for worms with a dye-filling-defective phenotype (Fig. 5). Altogether, these results show that ML selection pressure in C. elegans with either IVM or MOX will select for close mechanisms of acquired tolerance, with both involving a defect in the integrity of chemosensory neurons.
FIG 5.
Dye filling of amphid neurons in the Bristol N2, IVM-selected, and MOX-selected strains. Young adult C. elegans worms from the wild-type Bristol N2, IVM-selected (IVR10 [11] and IVM11R [independently generated in our laboratory]), and MOX-selected strains were examined by fluorescence microscopy to visualize the dye filling of the amphid dendrites after staining with the fluorescent dye DiIC12(3) (DiI). Arrows indicate the amphid dendrites.
DISCUSSION
Data from natural populations of nematodes, and from artificial drug selection, suggest that MOX resistance generally develops more slowly than IVM resistance and that MOX efficacy is maintained at higher levels than is IVM efficacy as ML resistance develops (24–28, 40–43). In order to compare the impacts of drug selection with either IVM or MOX on the development of tolerance in nematodes, we generated a MOX-selected strain of the model nematode C. elegans, which was compared with an IVM-selected C. elegans strain described previously (11).
Both IVM and MOX selection led to acquired tolerance to the two drugs. Interestingly, MOX was significantly more potent than IVM in both strains selected under IVM or MOX pressure. It was surprising that the MOX-selected strain developed a relatively high-resistance phenotype against IVM, despite the worms never having been exposed to the drug. Similarly, despite the worms never having been exposed to EPR, selection pressure with IVM and MOX led to high levels of acquired tolerance to EPR (RF of ∼15). Interestingly, EPR was far less potent against either IVM- or MOX-selected strains. As a result, both IVM- and MOX-selected strains displayed the same levels of tolerance against each ML, showing that the relative potency of IVM, MOX, and EPR was independent of the ML compound used for selection pressure. This suggests that the mechanism(s) of acquired tolerance will be similar whatever the ML used for selection and that it will alter EPR to a greater extent than IVM and MOX. In addition, this is, to our knowledge, the first study demonstrating that after selection using subtherapeutic levels of MOX, the selected strain developed a higher degree of tolerance to EPR and IVM than to MOX.
Importantly, in this study, the degree of resistance to MLs and the order of potency of MLs observed for ML-selected C. elegans strains were comparable to those observed for the resistant H. contortus Kokstad isolate, with MOX being much more potent than IVM and EPR. These results are in full agreement with data from previous in vivo studies, which showed that MOX efficacy is maintained at higher levels than is IVM efficacy as ML resistance develops, as well as in vitro studies on drug-resistant parasitic nematodes, where MOX was more potent than IVM, while EPR was the least potent drug, displaying the highest resistance ratio compared to IVM and MOX (35, 43–51). These similarities with the ML-selected C. elegans strains clearly show that the model of drug selection pressure in C. elegans can be relevant to studies of the adaptation of parasitic nematodes to ML treatment in the field. Knowing that very few studies have been carried out on the experimental evolution of ML resistance in parasitic nematodes, primarily because of the difficulty in establishing a resistant subline derived from a susceptible isolate by multiple passages in sheep and challenge with drug treatment at each generation, our study demonstrates that experimental evolution in replicate C. elegans worm populations exposed to MLs can contribute to the understanding of the evolutionary fate of sublethal effects caused by these anthelmintics.
Our study therefore suggests that IVM resistance and, to a greater extent, EPR resistance are easier to select, under either IVM or MOX drug pressure, than is MOX resistance. In the context where EPR is widely used in lactating animals and in a long-acting formulation (52) and is suggested to be an alternative to IVM for malaria parasite transmission control (53), these results have several important implications. Indeed, EPR efficacy may be limited in ML-resistant nematodes and could not be used as an alternative to IVM or MOX when IVM or MOX resistance occurs.
Interestingly, both IVM- and MOX-selected worms displayed higher susceptibility to levamisole and triclabendazole sulfoxide, a benzimidazole routinely used for the treatment of trematode infections such as fascioliasis. Negative cross-resistance between IVM and levamisole in H. contortus was described previously (29, 35). Since levamisole and IVM bind different kinds of gated ion channels, operating on excitatory and inhibitory circuits, respectively, it is therefore possible that resistance to one of these drugs will increase susceptibility to the other, entailing an environmental adaptation cost in the case of levamisole susceptibility.
Several studies have shown a correlation between ABC transporter expression (25, 54, 55), drug metabolism (56), and ML resistance in nematodes. In our study, C. elegans adapted to the drug selection pressure by upregulating constitutively genes involved in xenobiotic metabolism and transport. The gene expression levels of a number of P-glycoproteins were increased in both IVM- and MOX-selected C. elegans strains, suggesting that the efflux pumps contribute to the observed drug tolerance. Particularly, Pgp14 and Pgp6 were the most highly overexpressed genes in both IVM- and MOX-selected strains. Interestingly, Pgp14 is the most important Pgp involved in IVM susceptibility in C. elegans (14), while Pgp6 plays a great role in protecting C. elegans from MOX toxicity (9, 57). Since PGP6 is expressed in the amphids, its overexpression could help protect the nematodes from the effects of ML on extrapharyngeal neurons associated with the amphids. In addition, our results show the constitutive overexpression of Pgp2, Pgp9, and Pgp11 after both IVM and MOX selection, in accordance with the association of their homologs in ML resistance in H. contortus, Teladorsagia circumcincta, and Parascaris equorum (13, 43, 44, 58, 59). Generally, a similar pattern of expression of the regulated genes was found in the IVM-selected and the MOX-selected strains, in agreement with the similar transcriptional profiles of the multidrug resistance-associated proteins (MRPs) in C. elegans (8) and ABC transporters in C. oncophora (10) following exposure to IVM and MOX. Overall, the significantly increased expression levels of Pgp genes after repeated selection pressure with either IVM or MOX suggest that the increased transporter activity in tolerant worms resulted in an increased ability to transport MLs and therefore that these drugs might be substrates for the Pgps of the worm. In addition, some cytochrome P450 (CYP) and GST genes were also constitutively overexpressed in ML-selected strains. Interestingly, cytochrome P450 enzymes were previously implicated in the metabolism of MLs in H. contortus (60), suggesting that their increased expression levels can reduce ML concentrations in the worm and, consequently, ML efficacy. However, it is noteworthy that the expression levels of some genes were increased specifically in the IVM-selected strain (Pgp10, Mrp1, Mrp8, gst4, gst5, and Cyp37B1) or in the MOX-selected strain (Pgp8 and Cyp35A1). Our results clearly demonstrate that ABC transporters influence, at least partly, worm susceptibility to MLs, in agreement with data from other studies performed with susceptible or multiresistant parasitic nematodes (11, 17, 61). Accordingly, the contribution of Pgps to the effects of IVM and MOX was supported by the potentiation of ML efficacy by verapamil in both IVM- and MOX-selected strains. This was also observed for the wild-type unselected strain, revealing that Pgps are factors determining the responsiveness of susceptible C. elegans strains to MLs without any history of selection for resistance. Overall, the constitutive overexpression of several ABC transporters, CYP, and GSTs occurring in response to IVM and MOX drug selection pressure may contribute to metabolize and extrude the drugs more efficiently and to protect worms against their pharmacological action, thus playing a role in acquired tolerance development under ML selection.
The cross-resistance phenotype observed in ML-selected strains is certainly based, at least partly, on the overexpression of genes encoding multidrug ABC transporters. Given that the different MLs are structurally related and that they all interact with Pgps of parasitic nematodes (62–65), it is expected that the overexpression of Pgps in worms will lead to cross-resistance to IVM, MOX, EPR, and eventually other MLs and drugs that are Pgp substrates. Interestingly, MOX interacts weakly with nematode Pgps (62–66), and this is in agreement with its higher level of toxicity in ML-resistant strains. Moreover, the cross-resistance phenotype between ML compounds in parasitic nematodes was associated with the overexpression of several Pgps (10, 13, 43, 44). Given all the similarities between various different isolates with C. elegans, this model nematode clearly represents a relevant tool to study the mechanism of adaptation of parasitic nematodes to drugs.
One important point that remains is the higher potency of MOX than of IVM in ML-resistant worms, which could be explained in several ways. First, IVM and MOX have different physicochemical properties, with MOX having higher lipophilicity (100 times higher than that of IVM), resulting in different biodispositions in the host and certainly in the target body. This may be responsible for differences in the interactions of MOX with the nematode receptors and transporters compared with those of IVM and could have implications for differences in resistance selection. Moreover, the higher lipophilicity of MOX could allow the compound to enter differentially into the worm. However, a recent study demonstrated a higher level of efficacy for MOX than for IVM associated with lower MOX concentrations recovered within adult H. contortus worms than IVM (43), suggesting that the activity of an ML compound against resistant nematodes is not strictly related to its ability to enter and accumulate in the target. Second, differential patterns of interaction of IVM and MOX at the GluCl receptors of nematodes (2, 22) but also at the mammalian GABA receptors (67) were reported previously. Knowing that there is a large diversity of ligand-gated chloride channels in nematodes, it is possible that the two drugs may have different affinities for different channels and that some ligand-gated chloride channels may be affected to different extents and may be under different selection by IVM and MOX. In addition, it has been suggested that different subunits from GluCls were important for the effects of IVM and MOX (22, 57). Third, IVM and MOX differentially interact with Pgps and other multidrug resistance (MDR) transporters. Indeed, it was clearly demonstrated that the interaction of MOX with mammalian Pgp (68), but also with H. contortus Pgp-2, Pgp-9, and Pgp-16 (62–64); Cylicocylus elongatus Pgp-9 (65); and Dirofilaria immitis Pgp-11 (66), is much weaker than that of IVM. Moreover, it was suggested that the MRPs may play less of a role in protecting C. elegans from MOX toxicity than they do in protecting the nematode from IVM toxicity (22). Since Pgps were recently shown to be directly involved in ML sensitivity in C. elegans (14, 15), it can be hypothesized that the same specific transporters may not be involved to the same extent with each ML and that IVM and MOX will be transported and removed differentially from the site of action. Fourth, in addition to ABC transporters, it cannot be ruled out that metabolism through the C. elegans detoxification network will differ from IVM to MOX, leading to different drug concentrations at the site of action. Finally, studies on T. circumcincta and H. contortus suggested a genetic basis for differences between resistance to IVM and resistance to MOX, with IVM resistance being dominant and MOX resistance being incompletely dominant or recessive (23, 69), therefore suggesting that different or additional genetic mechanisms are involved in MOX resistance compared with IVM resistance.
In this study, we show that MOX selection pressure will select for a dye-filling-defective phenotype, similarly to IVM selection (21). Data from this study, combined with previously reported results indicating that IVM resistance is a general feature of mutants with dye-filling defects, constitute compelling evidence that changes to the anatomy and/or function of amphid sensory endings are associated with ML susceptibility in general. Knowing that high levels of IVM resistance in C. elegans can be achieved only by a triple mutation of avr-14, avr-15, and glc-1, encoding GluCl a-type subunits (38), it is understandable that no drug-target-specific changes have been observed in ML-resistant nematodes in the field. In contrast, our results show that ML drug pressure will select for a defect in amphid neuron integrity, leading to a decrease in ML susceptibility.
In conclusion, by comparing the development of acquired tolerance to MLs under IVM and MOX selection pressure in C. elegans, we have highlighted the various degrees to which the resistance mechanisms are able to act on the potency of different MLs. Even if the mechanisms of acquired tolerance remain to be elucidated, we hypothesize a biphasic pattern in adaptation to MLs, involving (i) pharmacokinetic-mediated tolerance based on increases in the activities of drug transporters and biotransformation enzymes, leading to a decreased quantity of drug reaching the target, conferring low levels of resistance, and allowing the more tolerant individuals to survive the anthelmintic therapy, therefore causing gradual selection which may end up in the development of a resistant strain, and (ii) pharmacodynamic-mediated tolerance based on an altered amphid neuronal structure after both IVM and MOX selection. Since GluCls are located in extrapharyngeal neurons, which connect to the amphids, a defect in neuron integrity may reduce the GluCl receptor density and response to the drug, leading to higher levels of resistance. However, one important remaining point is that the impact on drug efficacy will be similar whatever the ML used for selection, showing that alteration of drug potency will depend intrinsically on the pharmacochemical properties of each ML.
Overall, these findings can be regarded as a warning that stepwise exposure to sublethal doses of IVM or MOX will lead to acquired tolerance to the anthelmintic macrocyclic lactone family. In addition, our study reveals two key mechanisms that affect the responsiveness of worms to MLs: the induction of the detoxification system and a defect in amphid neuron integrity. The similarities with parasite nematodes are of concern and highlight the use of drug-selected strains of C. elegans as a relevant model organism for research on ML resistance in nematodes.
ACKNOWLEDGMENTS
We thank Jean-François Sutra (Membrane Transporters and Resistance, INRA UMR1331) for high-performance liquid chromatography analysis of macrocyclic lactone stock solutions. We also acknowledge Yannick Lippi and Claire Naylies from the Technological Platform on Transcriptomics GeT-TRiX (Transcriptomic Impact of Xenobiotics, INRA UMR1331) for access and expertise on transcriptomic studies.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Geary TG. 2005. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol 21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Prichard R, Menez C, Lespine A. 2012. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist 2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awadzi K, Opoku NO, Attah SK, Lazdins-Helds J, Kuesel AC. 2014. A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in Onchocerca volvulus infection. PLoS Negl Trop Dis 8:e2953. doi: 10.1371/journal.pntd.0002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilleard JS, Beech RN. 2007. Population genetics of anthelmintic resistance in parasitic nematodes. Parasitology 134:1133–1147. doi: 10.1017/S0031182007000066. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RM. 2004. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Osei-Atweneboana MY, Eng JK, Boakye DA, Gyapong JO, Prichard RK. 2007. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet 369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- 7.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. 2004. Drug resistance in veterinary helminths. Trends Parasitol 20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Ardelli BF, Prichard R. 2008. Effects of ivermectin and moxidectin on the transcription of genes coding for multidrug resistance associated proteins and behaviour in Caenorhabditis elegans. J Nematol 40:290–298. [Google Scholar]
- 9.Ardelli BF, Prichard RK. 2013. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Vet Parasitol 191:264–275. doi: 10.1016/j.vetpar.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 10.De Graef J, Demeler J, Skuce P, Mitreva M, von Samson-Himmelstjerna G, Vercruysse J, Claerebout E, Geldhof P. 2013. Gene expression analysis of ABC transporters in a resistant Cooperia oncophora isolate following in vivo and in vitro exposure to macrocyclic lactones. Parasitology 140:499–508. doi: 10.1017/S0031182012001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James CE, Davey MW. 2009. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int J Parasitol 39:213–220. doi: 10.1016/j.ijpara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Tompkins JB, Stitt LE, Morrissette AM, Ardelli BF. 2011. The role of Brugia malayi ATP-binding cassette (ABC) transporters in potentiating drug sensitivity. Parasitol Res 109:1311–1322. doi: 10.1007/s00436-011-2378-4. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, Molento M, Blackhall W, Ribeiro P, Beech R, Prichard R. 1998. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol Biochem Parasitol 91:327–335. doi: 10.1016/S0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 14.Janssen IJ, Krucken J, Demeler J, von Samson-Himmelstjerna G. 2013. Caenorhabditis elegans: modest increase of susceptibility to ivermectin in individual P-glycoprotein loss-of-function strains. Exp Parasitol 134:171–177. doi: 10.1016/j.exppara.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Janssen IJ, Krucken J, Demeler J, von Samson-Himmelstjerna G. 2015. Transgenically expressed Parascaris P-glycoprotein-11 can modulate ivermectin susceptibility in Caenorhabditis elegans. Int J Parasitol Drugs Drug Resist 5:44–47. doi: 10.1016/j.ijpddr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan R, Urdaneta-Marquez L, Keller K, James CE, Davey MW, Prichard RK. 2012. The role of several ABC transporter genes in ivermectin resistance in Caenorhabditis elegans. Vet Parasitol 190:519–529. doi: 10.1016/j.vetpar.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Bartley DJ, McAllister H, Bartley Y, Dupuy J, Menez C, Alvinerie M, Jackson F, Lespine A. 2009. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology 136:1081–1088. doi: 10.1017/S0031182009990345. [DOI] [PubMed] [Google Scholar]
- 18.Demeler J, Krucken J, AlGusbi S, Ramunke S, De Graef J, Kerboeuf D, Geldhof P, Pomroy WE, von Samson-Himmelstjerna G. 2013. Potential contribution of P-glycoproteins to macrocyclic lactone resistance in the cattle parasitic nematode Cooperia oncophora. Mol Biochem Parasitol 188:10–19. doi: 10.1016/j.molbiopara.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Lifschitz A, Suarez VH, Sallovitz J, Cristel SL, Imperiale F, Ahoussou S, Schiavi C, Lanusse C. 2010. Cattle nematodes resistant to macrocyclic lactones: comparative effects of P-glycoprotein modulation on the efficacy and disposition kinetics of ivermectin and moxidectin. Exp Parasitol 125:172–178. doi: 10.1016/j.exppara.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Raza A, Kopp SR, Jabbar A, Kotze AC. 2015. Effects of third generation P-glycoprotein inhibitors on the sensitivity of drug-resistant and -susceptible isolates of Haemonchus contortus to anthelmintics in vitro. Vet Parasitol 211:80–88. doi: 10.1016/j.vetpar.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Urdaneta-Marquez L, Bae SH, Janukavicius P, Beech R, Dent J, Prichard R. 2014. A dyf-7 haplotype causes sensory neuron defects and is associated with macrocyclic lactone resistance worldwide in the nematode parasite Haemonchus contortus. Int J Parasitol 44:1063–1071. doi: 10.1016/j.ijpara.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Ardelli BF, Stitt LE, Tompkins JB, Prichard RK. 2009. A comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet Parasitol 165:96–108. doi: 10.1016/j.vetpar.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland IA, Leathwick DM, Moen IC, Bisset SA. 2002. Resistance to therapeutic treatment with macrocyclic lactone anthelmintics in Ostertagia circumcincta. Vet Parasitol 109:91–99. doi: 10.1016/S0304-4017(02)00247-9. [DOI] [PubMed] [Google Scholar]
- 24.Milillo P, Boeckh A, Cobb R, Otranto D, Lia RP, Perrucci S, di Regalbono AF, Beraldo P, von Samson-Himmelstjerna G, Demeler J, Bartolini R, Traversa D. 2009. Faecal cyathostomin egg count distribution and efficacy of anthelmintics against cyathostomins in Italy: a matter of geography? Parasit Vectors 2(Suppl 2):S4. doi: 10.1186/1756-3305-2-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prichard RK, Roulet A. 2007. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology 134:1123–1132. doi: 10.1017/S0031182007000091. [DOI] [PubMed] [Google Scholar]
- 26.Ranjan S, Wang GT, Hirschlein C, Simkins KL. 2002. Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet Parasitol 103:109–117. doi: 10.1016/S0304-4017(01)00551-9. [DOI] [PubMed] [Google Scholar]
- 27.Almeida GD, Feliz DC, Heckler RP, Borges DG, Onizuka MK, Tavares LE, Paiva F, Borges FA. 2013. Ivermectin and moxidectin resistance characterization by larval migration inhibition test in field isolates of Cooperia spp. in beef cattle, Mato Grosso do Sul, Brazil. Vet Parasitol 191:59–65. doi: 10.1016/j.vetpar.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Craig TM, Hatfield TA, Pankavich JA, Wang GT. 1992. Efficacy of moxidectin against an ivermectin-resistant strain of Haemonchus contortus in sheep. Vet Parasitol 41:329–333. doi: 10.1016/0304-4017(92)90090-V. [DOI] [PubMed] [Google Scholar]
- 29.Le Jambre LF, Gill JH, Lenane IJ, Lacey E. 1995. Characterisation of an avermectin resistant strain of Australian Haemonchus contortus. Int J Parasitol 25:691–698. doi: 10.1016/0020-7519(94)00200-8. [DOI] [PubMed] [Google Scholar]
- 30.Pankavich JA, Berger H, Simkins KL. 1992. Efficacy of moxidectin, nemadectin and ivermectin against an ivermectin-resistant strain of Haemonchus contortus in sheep. Vet Rec 130:241–243. doi: 10.1136/vr.130.12.241. [DOI] [PubMed] [Google Scholar]
- 31.Pomroy WE, Whelan NC. 1993. Efficacy of moxidectin against an ivermectin-resistant strain of Ostertagia circumcincta in young sheep. Vet Rec 132:416. doi: 10.1136/vr.132.16.416. [DOI] [PubMed] [Google Scholar]
- 32.Varady M, Praslicka J, Corba J. 1995. Efficacy of moxidectin against multiple resistant Ostertagia spp. in lambs. N Z Vet J 43:89–90. doi: 10.1080/00480169.1995.35859. [DOI] [PubMed] [Google Scholar]
- 33.Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neveu C, Charvet C, Fauvin A, Cortet J, Castagnone-Sereno P, Cabaret J. 2007. Identification of levamisole resistance markers in the parasitic nematode Haemonchus contortus using a cDNA-AFLP approach. Parasitology 134:1105–1110. doi: 10.1017/S0031182007000030. [DOI] [PubMed] [Google Scholar]
- 35.Gill JH, Redwin JM, van Wyk JA, Lacey E. 1995. Avermectin inhibition of larval development in Haemonchus contortus—effects of ivermectin resistance. Int J Parasitol 25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- 36.Rossanigo CE, Gruner L. 1991. Accuracy of two methods for counting eggs of sheep nematode parasites. Vet Parasitol 39:115–121. doi: 10.1016/0304-4017(91)90067-6. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Dent JA, Smith MM, Vassilatis DK, Avery L. 2000. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc Natl Acad Sci U S A 97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman AS, Nghiem C, Li J, Ashton FT, Guerrero J, Shoop WL, Schad GA. 2003. Amphidial structure of ivermectin-resistant and susceptible laboratory and field strains of Haemonchus contortus. Vet Parasitol 110:217–226. doi: 10.1016/S0304-4017(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 40.Bartley DJ, Jackson E, Sargison N, Jackson F. 2005. Further characterisation of a triple resistant field isolate of Teladorsagia from a Scottish lowland sheep farm. Vet Parasitol 134:261–266. doi: 10.1016/j.vetpar.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Coles GC, Rhodes AC, Wolstenholme AJ. 2005. Rapid selection for ivermectin resistance in Haemonchus contortus. Vet Parasitol 129:345–347. doi: 10.1016/j.vetpar.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Kieran PJ. 1994. Moxidectin against ivermectin-resistant nematodes—a global view. Aust Vet J 71:18–20. doi: 10.1111/j.1751-0813.1994.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 43.Lloberas M, Alvarez L, Entrocasso C, Virkel G, Ballent M, Mate L, Lanusse C, Lifschitz A. 2013. Comparative tissue pharmacokinetics and efficacy of moxidectin, abamectin and ivermectin in lambs infected with resistant nematodes: impact of drug treatments on parasite P-glycoprotein expression. Int J Parasitol Drugs Drug Resist 3:20–27. doi: 10.1016/j.ijpddr.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackhall WJ, Liu HY, Xu M, Prichard RK, Beech RN. 1998. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol Biochem Parasitol 95:193–201. doi: 10.1016/S0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 45.Demeler J, Gill JH, von Samson-Himmelstjerna G, Sangster NC. 2013. The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int J Parasitol Drugs Drug Resist 3:109–118. doi: 10.1016/j.ijpddr.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolinska M, Konigova A, Letkova V, Molnar L, Varady M. 2013. Detection of ivermectin resistance by a larval development test—back to the past or a step forward? Vet Parasitol 198:154–158. doi: 10.1016/j.vetpar.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 47.Gill JH, Lacey E. 1998. Avermectin/milbemycin resistance in trichostrongyloid nematodes. Int J Parasitol 28:863–877. doi: 10.1016/S0020-7519(98)00068-X. [DOI] [PubMed] [Google Scholar]
- 48.Kotze AC, Le Jambre LF, O'Grady J. 2006. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet Parasitol 137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Kotze AC, Ruffell AP, Knox MR, Kelly GA. 2014. Relative potency of macrocyclic lactones in in vitro assays with larvae of susceptible and drug-resistant Australian isolates of Haemonchus contortus and H. placei. Vet Parasitol 203:294–302. doi: 10.1016/j.vetpar.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Molento MB, Wang GT, Prichard RK. 1999. Decreased ivermectin and moxidectin sensitivity in Haemonchus contortus selected with moxidectin over 14 generations. Vet Parasitol 86:77–81. doi: 10.1016/S0304-4017(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 51.Paiement JP, Leger C, Ribeiro P, Prichard RK. 1999. Haemonchus contortus: effects of glutamate, ivermectin, and moxidectin on inulin uptake activity in unselected and ivermectin-selected adults. Exp Parasitol 92:193–198. doi: 10.1006/expr.1999.4413. [DOI] [PubMed] [Google Scholar]
- 52.Forbes AB. 2013. LongRange (eprinomectin 5%) extended-release injection parasiticide and the utility of extended-activity antiparasitics in cattle. Vet Parasitol 192:308–312. doi: 10.1016/j.vetpar.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 53.Butters MP, Kobylinski KC, Deus KM, da Silva IM, Gray M, Sylla M, Foy BD. 2012. Comparative evaluation of systemic drugs for their effects against Anopheles gambiae. Acta Trop 121:34–43. doi: 10.1016/j.actatropica.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beech RN, Skuce P, Bartley DJ, Martin RJ, Prichard RK, Gilleard JS. 2011. Anthelmintic resistance: markers for resistance, or susceptibility? Parasitology 138:160–174. doi: 10.1017/S0031182010001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolstenholme AJ, Kaplan RM. 2012. Resistance to macrocyclic lactones. Curr Pharm Biotechnol 13:873–887. doi: 10.2174/138920112800399239. [DOI] [PubMed] [Google Scholar]
- 56.Kudzi W, Dodoo AN, Mills JJ. 2010. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans? BMC Med Genet 11:111. doi: 10.1186/1471-2350-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bygarski EE, Prichard RK, Ardelli BF. 2014. Resistance to the macrocyclic lactone moxidectin is mediated in part by membrane transporter P-glycoproteins: implications for control of drug resistant parasitic nematodes. Int J Parasitol Drugs Drug Resist 4:143–151. doi: 10.1016/j.ijpddr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dicker AJ, Nisbet AJ, Skuce PJ. 2011. Gene expression changes in a P-glycoprotein (Tci-pgp-9) putatively associated with ivermectin resistance in Teladorsagia circumcincta. Int J Parasitol 41:935–942. doi: 10.1016/j.ijpara.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Janssen IJ, Krucken J, Demeler J, Basiaga M, Kornas S, von Samson-Himmelstjerna G. 2013. Genetic variants and increased expression of Parascaris equorum P-glycoprotein-11 in populations with decreased ivermectin susceptibility. PLoS One 8:e61635. doi: 10.1371/journal.pone.0061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvinerie M, Dupuy J, Eeckhoutte C, Sutra JF, Kerboeuf D. 2001. In vitro metabolism of moxidectin in Haemonchus contortus adult stages. Parasitol Res 87:702–704. doi: 10.1007/s004360100408. [DOI] [PubMed] [Google Scholar]
- 61.Stitt LE, Tompkins JB, Dooley LA, Ardelli BF. 2011. ABC transporters influence sensitivity of Brugia malayi to moxidectin and have potential roles in drug resistance. Exp Parasitol 129:137–144. doi: 10.1016/j.exppara.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Godoy P, Che H, Beech RN, Prichard RK. 2016. Characterisation of P-glycoprotein-9.1 in Haemonchus contortus. Parasit Vectors 9:52. doi: 10.1186/s13071-016-1317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Godoy P, Che H, Beech RN, Prichard RK. 2015. Characterization of Haemonchus contortus P-glycoprotein-16 and its interaction with the macrocyclic lactone anthelmintics. Mol Biochem Parasitol 204:11–15. doi: 10.1016/j.molbiopara.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Godoy P, Lian J, Beech RN, Prichard RK. 2015. Haemonchus contortus P-glycoprotein-2: in situ localisation and characterisation of macrocyclic lactone transport. Int J Parasitol 45:85–93. doi: 10.1016/j.ijpara.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Kaschny M, Demeler J, Janssen IJ, Kuzmina TA, Besognet B, Kanellos T, Kerboeuf D, von Samson-Himmelstjerna G, Krucken J. 2015. Macrocyclic lactones differ in interaction with recombinant P-glycoprotein 9 of the parasitic nematode Cylicocylus elongatus and ketoconazole in a yeast growth assay. PLoS Pathog 11:e1004781. doi: 10.1371/journal.ppat.1004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mani T, Bourguinat C, Keller K, Ashraf S, Blagburn B, Prichard RK. Interaction of macrocyclic lactones with a Dirofilaria immitis P-glycoprotein. Int J Parasitol, in press. [DOI] [PubMed] [Google Scholar]
- 67.Ménez C, Sutra JF, Prichard R, Lespine A. 2012. Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab(−/−) mice and effects on mammalian GABA(A) channel activity. PLoS Negl Trop Dis 6:e1883. doi: 10.1371/journal.pntd.0001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Orlowski S, Alvinerie M. 2007. Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship. Eur J Pharm Sci 30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Le Jambre LF, Geoghegan J, Lyndal-Murphy M. 2005. Characterization of moxidectin resistant Trichostrongylus colubriformis and Haemonchus contortus. Vet Parasitol 128:83–90. doi: 10.1016/j.vetpar.2004.10.019. [DOI] [PubMed] [Google Scholar]