Abstract
A panel of six imidazo[1,2-a]pyridine-3-carboxamides (IAPs) were shown to have low-micromolar activity against Mycobacterium avium strains. Compound ND-10885 (compound 2) showed significant activity in the lung, spleen, and liver in a mouse M. avium infection model. A combined regimen consisting of ND-10885 (compound 2) and rifampin was additive in its anti-M. avium activity in the lung. Our data indicate that IAPs represent a new class of antibiotics that are active against M. avium and could potentially serve as an effective addition to a combined treatment regimen.
TEXT
The incidence of nontuberculous mycobacteria (NTM) infections has been increasing in the United States (1, 2). Mycobacterium avium complex (MAC), which consists of M. avium and M. intracellulare, is an important cause of pulmonary disease in individuals with underlying lung diseases, such as cystic fibrosis and chronic obstructive pulmonary disease, and is an opportunistic pathogen in immunocompromised patients (3, 4). Among the NTM species isolated from U.S. patients, 80% are classified as MAC (5). MAC is ubiquitous within the environment and is found in soil, treated or untreated water, house plumbing systems, and animals (6). MAC infection is difficult to treat and has been shown to be resistant to many of the clinically used antituberculosis agents (7, 8). We previously disclosed a novel family of compounds, imidazo[1,2-a]pyridine-3-carboxamides (IAPs), with potent activity against Mycobacterium tuberculosis (9–12). The mechanism of action and anti-M. tuberculosis in vivo efficacy of this exciting new class has been documented by us and other groups (9, 13–16). Through a hit-to-lead optimization effort aided by the Lilly TB Drug Discovery Initiative (LTBDDI), additional IAP compounds (1 to 6) were generated and found to have encouraging in vivo pharmacokinetics (PK). Herein, we describe the activity of these latest analogs against M. avium both in vitro and in vivo.
Activity of selected compounds in vitro.
Six compounds having diverse PKs were selected and synthesized according to our published methods (9–11). Experimental data and information on all previously uncharacterized compounds (1, 2, and 6) can be found in the supplemental material. MIC studies were performed using a resazurin-based colorimetric assay and CFU quantification, as described previously (17). Screening the IAPs against M. avium strains 101 and 2151 using standard protocols indicated that they had moderate potency (Table 1). The activities of these compounds against M. avium (2.6 to 27.8 μM, two strains) were limited relative to M. tuberculosis (see Table S1 in the supplemental material) but comparable to positive controls of clarithromycin and azithromycin (1.4 and 13.4 μM, respectively). These compounds also had a good therapeutic window when screened against Vero cells (9) (Table S1).
TABLE 1.
Screening of compounds 1 to 6 against two strains of M. avium
| Compound | Molecular mass (kDa) | cLogPa | MIC (μM) |
|
|---|---|---|---|---|
| MAC 101 (serotype 1) | MAC 2151 (serotype 2) | |||
| ND-9873 (1) | 363.34 | 4.6 | 2.8 | 27.5 |
| ND-10885 (2) | 321.38 | 3.6 | 1.6 | 15.6 |
| ND-9758 (3) | 389.43 | 5.8 | 1.28 | 2.57 |
| ND-9759 (4) | 405.88 | 6.4 | 0.31 | 1.2 |
| ND-9903 (5) | 425.41 | 6.4 | >23.5 | >23.5 |
| ND-10890 (6) | 382.44 | 3.4 | 2.6 | 13.1 |
| Rifampin | 822.95 | 6.04 | 0.077 | 0.158 |
| Ethambutol | 204.31 | 0.12 | 48.9 | >48.9 |
| Clarithromycin | 747.95 | 2.82 | 1.4 | 1.4 |
| Azithromycin | 748.88 | 2.28 | 13.4 | >13.4 |
cLogP, log of partition coefficient of a compound, was calculated by using ChemBioDraw Ultra 14 (PerkinElmer).
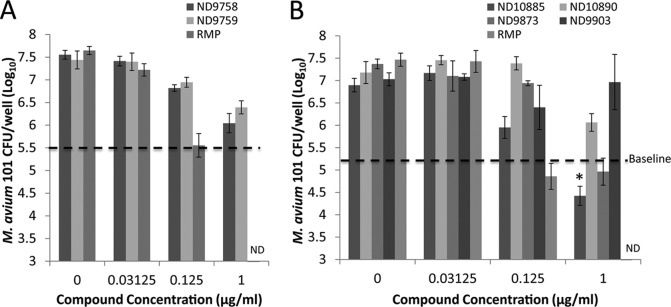
All six compounds were evaluated for their ability to kill or inhibit M. avium replication in vitro. Five out of six compounds were bactericidal or bacteriostatic (Fig. 1). Consistent with its MIC, ND-9903 (compound 5) showed no activity against M. avium 101 at the highest concentration tested. Separate studies of ND-9758 (compound 3), ND-9759 (compound 4), and ND-10890 (compound 6) at 1.0 μg/ml each indicated that they were bacteriostatic, as the bacterial counts were maintained near those of the original inoculum. ND-10885 (compound 2) and ND-9873 (compound 1) had bactericidal activity against M. avium 101 at 1.0 μg/ml, as bacterial numbers decreased by 1 log10 and 0.5 log10, respectively, compared to the original inoculum. Rifampin was the positive control and showed the best bactericidal activity against M. avium 101. Except for ND-9903 (compound 9), all compounds showed dose-dependent activity against M. avium. ND-10885 (compound 2) also showed activity against various other M. avium clinical isolates of different serotypes, although again, it varied 10-fold or more between strains.
FIG 1.
Anti-M. avium activities of imidazo[1,2-a]pyridine-3-carboxyamides in vitro, as measured by CFU counts. M. avium was treated with compounds at various concentrations as shown, and then bacterial CFU were determined on Middlebrook 7H10 agar plates. Baseline, M. avium CFU at the beginning of treatment. The results are representative of the results from three independent experiments. *, P < 0.05 compared to the baseline (one-way analysis of variance [ANOVA] with Tukey posttest). Error bars indicate standard deviations from duplicate infections with or without drug treatment from a single experiment.
Pharmacokinetics.
Single-dose pharmacokinetics of compounds 1 to 6 were determined in uninfected 8-week-old male BALB/c mice. Mice received by oral gavage a single dose of compound 1 (at 10 and 100 mg/kg), compound 2 (at 100 mg/kg), compound 3 (at 10 mg/kg), compound 4 (at 30 mg/kg), compound 5 (at 100 mg/kg), and compound 6 (at 10 and 100 mg/kg). Compounds were analyzed as previously described (10). Calculated parameters include clearance (CL), area under the concentration-time curve (AUC), half-life (t1/2), maximum serum concentration (Cmax), time to Cmax (Tmax), bioavailability (%F), and percent drug fraction unbound in plasma (%fplasma).
As shown in Table 2, all of these compounds had high plasma exposure at their respective doses, but there was a large range in half-lives observed (from 2.3 to >24 h). In our drug development paradigm, we selected the maximum free drug concentration per dose (fCmax) as the most meaningful pharmacokinetic property to use for compound advancement, and ND-10885 (compound 2) and ND-10890 (compound 6) had the highest values (2,795 and 4,232 nM, respectively). The free drug concentrations for those two compounds were high enough above their MICs that they were anticipated to be effective against MAC 101 in vivo. The PK parameters for compounds 1, 2, and 6 by intravenous (i.v.) dose can be found in Table S3 in the supplemental material.
TABLE 2.
Pharmacokinetic parameters of compounds 1 to 6a
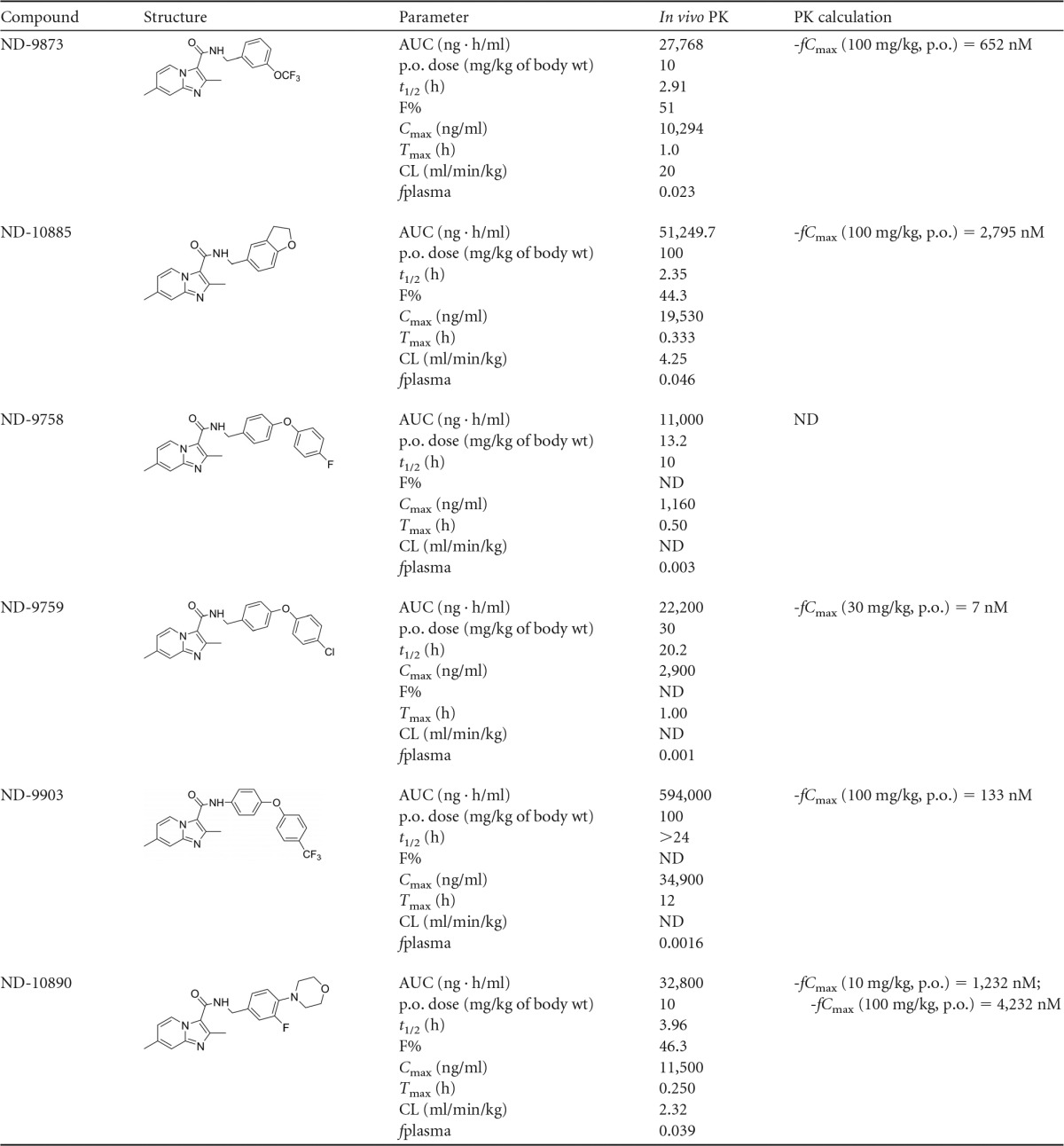
p.o., per os; ND, not determined.
Maximum tolerated dose.
Five compounds were evaluated in mice to determine their maximum tolerated dose, as previously described (13). Compounds ND-9873 (compound 1) and ND-10885 (compound 2) were tolerated at the highest concentration of 250 mg/kg for 1 week, ND-10890 (compound 6) was well-tolerated at 100 mg/kg for 1 week, and as previously reported, ND-9759 (compound 5) was tolerated at 30 mg/kg for 28 days (13). Mice treated with ND-9758 (compound 3) did not tolerate the drug even at the lowest concentration (30 mg/kg).
Efficacy of ND-10885 in MAC-infected mice.
We chose compound ND-10885 (compound 2) for the in vivo studies in wild-type BALB/c mice based on its relatively good in vitro bactericidal activity against M. avium, its PK profile, and its low toxicity in mice. Wild-type BALB/c mice (n = 3) were retro-orbitally infected with M. avium MAC 101 at a dose of 107 CFU in 50 μl of phosphate-buffered saline (PBS) (18). One week after the infection, mice were treated by oral gavage with ND-10885 (compound 2) dissolved in 80% (vol/vol) propylene glycol once daily 6 days a week for 2 weeks. After the final dosing, each mouse was sacrificed, and the mycobacterial burden was determined as described previously (13). The bacterial numbers were quantified by visually counting bacterial colonies. At the beginning of treatment, a group of mice (n = 3) were sacrificed to measure the mycobacterial input in the lung, spleen, and liver. As a negative control, a group of mice (n = 3) were treated with the vehicle, 80% propylene glycol, only.
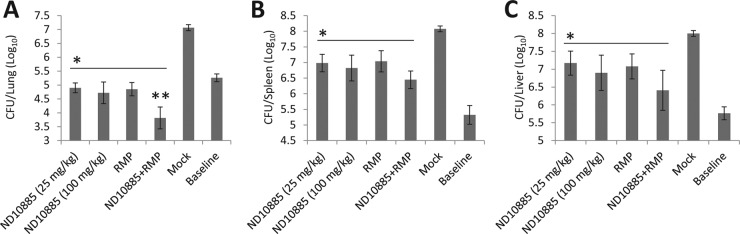
ND-10885 (compound 2) significantly inhibited M. avium growth in the lungs, spleens, and livers compared to the vehicle-treated M. avium-infected mice (Fig. 2). The inhibitory activity of ND-10885 (compound 2) was comparable to that of rifampin in all three organs. In addition, compared to single-compound/drug regimens, mycobacterial counts were lower in all three organs when M. avium-infected mice were treated with a combined regimen of ND-10885 (compound 2) and rifampin, although this was statistically significant in the lung only. Compared with baseline mycobacterial counts at the initiation of treatment, the combined regimen showed bactericidal activity, with the CFU(log10) count decreasing 1.5-fold in the lung.
FIG 2.
Efficacy of ND-10885 (compound 6) against M. avium infection in the BALB/c mouse model. M. avium-infected mice were treated with compounds once daily, 6 day per week, for 2 weeks. Bacterial burden in the lung, spleen, and liver was determined. Mock, mice treated with vehicle alone; baseline, bacterial burden at the beginning of treatment; RMP, rifampin. The results are representative of the results from two independent experiments. *, P < 0.05 compared to the mock; **, P < 0.05 compared to ND10885 (at 100 mg/kg) (one-way ANOVA with Tukey posttest). Error bars indicate standard deviations of M. tuberculosis CFU from individual mouse infections (n = 3) with or without drug treatment.
In conclusion, we show that IAPs are active against M. avium clinical isolates. One compound, ND-10885 (compound 2), has significant activity against M. avium in mice, reducing bacterial burden in the lung, spleen, and liver compared to the untreated group. Most interestingly, ND-10885 (compound 2) has activity comparable to that of rifampin, a critical component in the combined regimen used to treat MAC infections (8), and a combined regimen consisting of ND-10885 (compound 2) and rifampin showed enhanced bactericidal activity in the lung. Our data suggest that IAPs should be pursued as a new class of compounds to treat infections caused by M. avium and perhaps other NTM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Phil Hipskind of the Eli Lilly TB Drug Discovery Initiative for efforts in the M. tuberculosis program. We thank Jennifer DuBois, Lowell Markley, Jed Fisher, Jaroslav Zajicek, Patricia Miller, Ute Mölleman, and Helena Boshoff for meaningful and lasting scientific discussions. We also thank Delphi Chatterjee and Pat Brennan (Colorado State) for the M. avium strains used in this study.
The analytical data were obtained in the Mass Spectrometry and Proteomics Facility at the University of Notre Dame (Bill Boggess and Michelle Joyce), which is supported by grant CHE-0741793 from the NSF. This work was supported in part by NIH grant (R01AI054193) and Hsiri Therapeutics.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00618-16.
REFERENCES
- 1.O'Brien RJ, Geiter LJ, Snider DE Jr. 1987. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis 135:1007–1014. [DOI] [PubMed] [Google Scholar]
- 2.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maekura R, Okuda Y, Hirotani A, Kitada S, Hiraga T, Yoshimura K, Yano I, Kobayashi K, Ito M. 2005. Clinical and prognostic importance of serotyping Mycobacterium avium-Mycobacterium intracellulare complex isolates in human immunodeficiency virus-negative patients. J Clin Microbiol 43:3150–3158. doi: 10.1128/JCM.43.7.3150-3158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowman S, Loebinger M. Nontuberculous mycobacterial pulmonary disease. Clin Pulm Med 22:8–14. [Google Scholar]
- 5.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, Olivier KN. 2010. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstrom S, Price P, Thomson R. 2015. Review: environmental mycobacteria as a cause of human infection. Int J Mycobacteriol 4:81–91. doi: 10.1016/j.ijmyco.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Wagner D, Young LS. 2004. Nontuberculous mycobacterial infections: a clinical review. Infection 32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Moraski GC, Markley LD, Hipskind PA, Boshoff H, Cho S, Franzblau SG, Miller MJ. 2011. Advent of imidazo[1,2-a]pyridine-3-carboxamides with potent multi- and extended drug resistant antituberculosis activity. ACS Med Chem Lett 2:466–470. doi: 10.1021/ml200036r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraski GC, Markley LD, Cramer J, Hipskind PA, Boshoff H, Bailey M, Alling T, Ollinger J, Parish T, Miller MJ. 2013. Advancement of imidazo[1,2-a]pyridines with improved pharmacokinetics and nanomolar activity against Mycobacterium tuberculosis. ACS Med Chem Lett 4:675–679. doi: 10.1021/ml400088y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moraski GC, Miller PA, Bailey MA, Ollinger J, Parish T, Boshoff HI, Cho S, Anderson JR, Mulugeta S, Franzblau SG, Miller MJ. 2015. Putting tuberculosis (TB) to rest: transformation of the sleep aid, Ambien, and “anagrams” generated potent antituberculosis agents. ACS Infect Dis 1:85–90. doi: 10.1021/id500008t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ollinger J, Bailey MA, Moraski GC, Casey A, Florio S, Alling T, Miller MJ, Parish T. 2013. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS One 8:e60531. doi: 10.1371/journal.pone.0060531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y, Moraski GC, Cramer J, Miller MJ, Schorey JS. 2014. Bactericidal activity of an Imidazo[1, 2-a]pyridine using a mouse M. tuberculosis infection model. PLoS One 9:e87483. doi: 10.1371/journal.pone.0087483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrahams KA, Cox JAG, Spivey VL, Loman NJ, Pallen MJ, Constantinidou C, Fernández R, Alemparte C, Remuiñán MJ, Barros D, Ballell L, Besra GS. 2012. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS One 7:e52951. doi: 10.1371/journal.pone.0052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak PA, Rao SPS, Ping Tan M, Lin X, Chyba J, Tay J, Ng SH, Tan BH, Cherian J, Duraiswamy J, Bifani P, Lim V, Lee BH, Ling Ma N, Beer D, Thayalan P, Kuhen K, Chatterjee A, Supek F, Glynne R, Zheng J, Boshoff HI, Barry CE III, Dick T, Pethe K, Camacho LR. 2012. A high-throughput screen to identify inhibitors of ATP homeostasis in non-replicating Mycobacterium tuberculosis. ACS Chem Biol 7:1190–1197. doi: 10.1021/cb2004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim S-A, Nam J, Kang H, Kwon H, Oh C-T, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SPS, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han S-J, No Z, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 17.Palomino J-C, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. 2011. Retro-orbital injections in mice. Lab Anim (NY) 40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.