Abstract
Tedizolid, a novel oxazolidinone, exhibits bacteriostatic activity through inhibition of protein synthesis. The efficacies of tedizolid, linezolid, and vancomycin were compared in a murine catheter-related biofilm infection caused by methicillin-susceptible and -resistant Staphylococcus aureus (MSSA and MRSA, respectively) strains engineered for bioluminescence. We observed significantly improved efficacy in terms of decreased S. aureus densities and bioluminescent signals in the tedizolid-treated group versus the linezolid- and vancomycin-treated groups in the model of infection caused by the MSSA and MRSA strains.
TEXT
Staphylococcus aureus is a leading cause of skin and skin structure infections and is particularly associated with intravenous (i.v.) catheters (1–4). Despite the current use of newer antibiotics, infections due to S. aureus remain a significant problem. The emergence of methicillin-resistant S. aureus (MRSA) and high rates of vancomycin clinical failures emphasize this public health threat (5, 6). Therefore, potential alternative strategies for the treatment of such infections are urgently needed.
Tedizolid is a novel oxazolidinone derivative that has potent activity against staphylococci and enterococci (7, 8). It exerts bacteriostatic microbial activity through inhibition of protein synthesis by binding to the 50S ribosomal subunit of the bacteria. It has been reported that tedizolid is more active against staphylococci and enterococci than linezolid is in vitro (9, 10).
In this investigation, we evaluated (i) the MICs (11) and in vitro killing activities (12, 13) of tedizolid, linezolid, and vancomycin against lux mutant methicillin-susceptible S. aureus (MSSA) (Xen29) and MRSA (Xen30) strains (4, 14, 15), (ii) the influence of these antibiotics on biofilm formation (16, 17), and (iii) the real-time in vivo efficacy of tedizolid versus that of linezolid and vancomycin in a well-characterized model of murine subcutaneous catheter-related infection (18) caused by the MSSA or MRSA strain by using a novel bioluminescence in vivo imaging system (IVIS).
The IVIS was developed to provide a sensitive and noninvasive technique for rapid and real-time monitoring of therapeutic efficacy (4, 14, 19–21). In the murine subcutaneous catheter-related infection model, BALB/c mice (female, 18 to 22 g; Jackson Laboratory) were infected by implanting a precolonized Teflon catheter segment (1 cm) inoculated with the bioluminescent strains at 1 × 106 CFU/catheter. At 3 days after catheter implantation, animals were randomized to receive (i) control treatment with the vehicle, (ii) tedizolid phosphate at 10 mg/kg i.v. twice a day (bid), (iii) linezolid at 80 mg/kg i.v. bid, or (iv) vancomycin at 110 mg/kg subcutaneously (s.c.) bid. These antibiotic doses were chosen to simulate pharmacokinetic values similar to those achieved by the recommended dosing of humans, i.e., 200 mg of tedizolid i.v. once daily (22), 600 mg of linezolid i.v. bid (23), and 1 g of vancomycin i.v. bid (24). Treatments lasted 3 and 6 days for MSSA and MRSA infections, respectively. At 24 h after the last antibiotic dose, half of the untreated and tedizolid-treated animals were sacrificed for evaluation of antibiotic efficacy. The other half of the survivors were left untreated for an additional 3 days for assessment of relapse. At sacrifice, catheters were quantitatively cultured by using standard assays of CFU counts per catheter (25). In addition, animals were serially imaged daily after infected-catheter implantation for bioluminescent signals (BLS) with the IVIS (Caliper Life Sciences). BLS were expressed by using a pseudocolor scale, with red representing the most intense luminescence and blue representing the least intense luminescence (21).
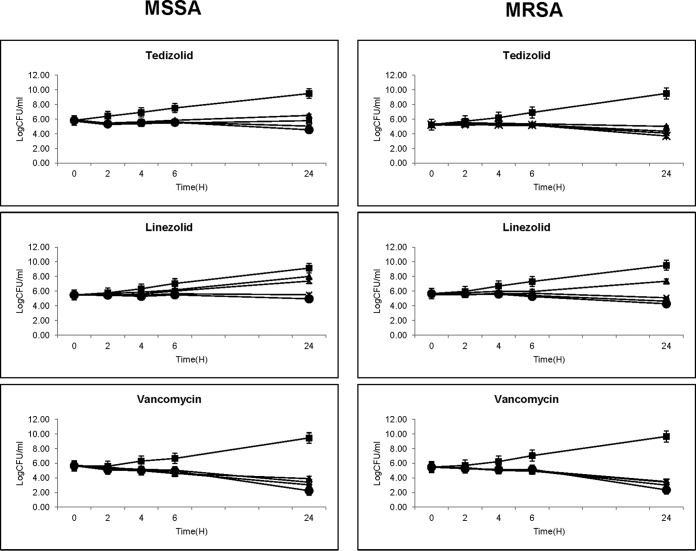
The MICs of tedizolid, linezolid, and vancomycin for study strains Xen29 and Xen30 were 0.25 and 0.25, 1.0 and 2.0, and 1.0 and 1.0 μg/ml, respectively. Tedizolid prevented substantial regrowth between 6 and 24 h of incubation (Fig. 1). However, linezolid could not prevent the regrowth of both MSSA and MRSA strains at the MIC or 2× MIC. Vancomycin exhibited time-dependent bactericidal activity against both study strains. No significant differences in the in vitro activities of tedizolid, linezolid, and vancomycin were observed between the study MSSA and MRSA strains (Fig. 1).
FIG 1.
Tedizolid, linezolid, and vancomycin in vitro MSSA (Xen29) and MRSA (Xen30) time-kill curves. Symbols: ■, control; ▲, 1× MIC; X, 2× MIC; *, 5× MIC; ●, 10× MIC.
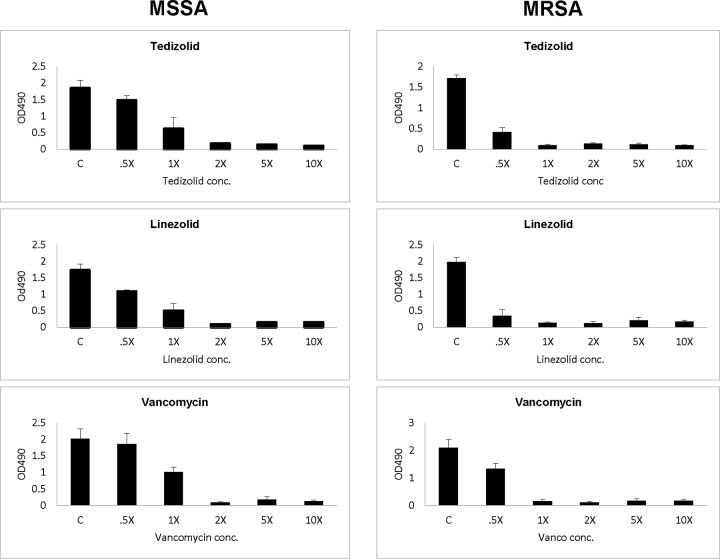
The two study MSSA and MRSA strains formed good biofilm (range of optical densities at 490 nm, 1.7 to 2.1) (Fig. 2). Interestingly, sublethal levels (0.5× MIC) of tedizolid, linezolid, or vancomycin did not induce biofilm formation in either study strain. Of note, linezolid is more effective than tedizolid or vancomycin against MSSA biofilm formation, and the ozaxolidinones are more active than vancomycin against MRSA strain biofilm formation. Importantly, all three antibiotics at the MIC to 10× MIC significantly reduced biofilm formation in both MSSA and MRSA strains.
FIG 2.
Impact of tedizolid, linezolid, or vancomycin (from 0.5× MIC to 10× MIC) on MSSA (Xen29) and MRSA (Xen30) biofilm formation. OD490, optical density at 490 nm; C, control.
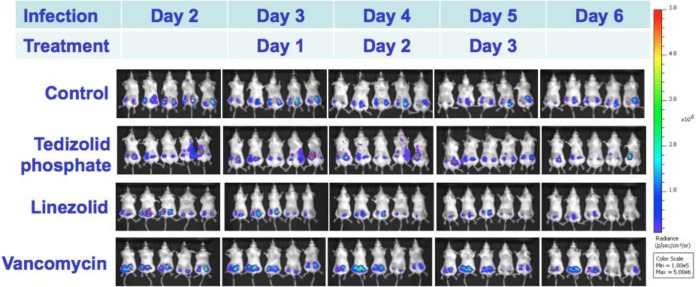
The burden of organisms in catheters in the untreated controls, different therapies, and relapse groups in the murine model are shown in Tables 1 and 2. At the end of treatment, tedizolid phosphate therapy (10 mg/kg i.v. bid) produced significantly lower densities of both MSSA and MRSA bacteria in catheters than those found in untreated controls or vancomycin- and linezolid-treated animals. In addition, vancomycin and linezolid had no therapeutic efficacy in reducing MSSA densities, while linezolid showed a significant reduction of MRSA densities versus untreated controls in the model. Of importance, tedizolid treatment had no significant relapse after discontinuation of therapy. The BLS from animals treated with tedizolid showed a progressive reduction compared to that from linezolid- and vancomycin-treated groups (Fig. 3).
TABLE 1.
Efficacies of tedizolid, vancomycin, and linezolid in a murine subcutaneous catheter-related infection due to MSSA strain Xen29
| Regimen (no. of samples) | Mean log10 no. of CFU/catheter ± SD | P value(s) |
|---|---|---|
| Treatementa | ||
| Control (20) | 7.50 ± 0.33 | |
| Tedizolid phosphate, 10 mg/kg i.v. bid (22) | 5.49 ± 0.62 | <1.37E−07 vs control, <2.74E−07 vs linezolid, <3.61E−09 vs vancomycin |
| Linezolid, 80 mg/kg i.v. bid (20) | 7.41 ± 0.48 | <0.56 vs control |
| Vancomycin, 110 mg/kg s.c. bid (20) | 7.46 ± 0.27 | <0.79 vs control |
| Relapse | ||
| Control (20) | 7.13 ± 0.42 | |
| Tedizolid phosphate, 10 mg/kg i.v. bid (20) | 5.77 ± 0.66 | <6.88E−09 vs control relapse |
Treatment lasted 3 days.
TABLE 2.
Efficacies of tedizolid, vancomycin, and linezolid in a murine subcutaneous catheter-related infection due to MRSA strain Xen30
| Regimen (no. of samples) | Mean log10 no. of CFU/catheter ± SD | P value(s) |
|---|---|---|
| Treatmenta | ||
| Control (20) | 7.14 ± 0.37 | |
| Tedizolid phosphate, 10 mg/kg i.v. bid (20) | 5.70 ± 0.53 | <3.07E−06 vs control, <0.03 vs linezolid, <0.002 vs vancomycin |
| Linezolid, 80 mg/kg i.v. bid (20) | 6.47 ± 0.57 | <0.05 vs control |
| Vancomycin, 110 mg/kg s.c. bid (20) | 6.64 ± 0.83 | <0.07 vs control |
| Relapse | ||
| Control (20) | 6.63 ± 0.53 | |
| Tedizolid phosphate, 10 mg/kg i.v. bid (20) | 5.34 ± 0.73 | <0.0002 vs control relapse |
Treatment lasted 6 days.
FIG 3.
Real-time monitoring of the efficacy of tedizolid, linezolid, and vancomycin in a murine subcutaneous catheter-related MSSA biofilm model.
Our in vitro results demonstrated that the study MSSA and MRSA strains were susceptible to tedizolid (MICs of 0.25 μg/ml), linezolid (MICs of ≤2.0 μg/ml), and vancomycin (MICs of 1.0 μg/ml). In addition to these MIC data, in vitro time-kill studies showed that tedizolid had better anti-S. aureus activity than linezolid in that it prevented regrowth at 24 h of incubation. The in vitro findings in this study mirror those in previously published investigations of tedizolid and linezolid activity against S. aureus (9, 26). In the present study, we further evaluated the impact of tedizolid versus that of linezolid and vancomycin on biofilm formation in S. aureus and found that exposure of these antibiotics at the MIC to 10× MIC significantly decreased S. aureus biofilm formation. These findings differ from those of a previous study that showed that tedizolid had no activity when staphylococcal organisms were in a biofilm state (26).
Importantly, the present study translated the above-described in vitro outcomes into a clinically relevant catheter-related biofilm infection model in mice. Our results demonstrated that tedizolid had significantly better efficacy than linezolid and vancomycin treatments in reducing MSSA and MRSA densities in the murine subcutaneous catheter-related biofilm infection model. In addition, it is notable that tedizolid had significant efficacy in the experimental model of MSSA infection, even with short-course (3 days) therapy, and there was no substantial relapse after 3 days without treatment. On the other hand, neither linezolid nor vancomycin was efficacious in producing lower MSSA densities than those in the control group in the model. These results suggest that tedizolid had significantly better efficacy in decreasing S. aureus densities in this model of catheter-related biofilm infection caused by MSSA and MRSA strains.
ACKNOWLEDGMENTS
This study was supported by research grants from Merck to Y.Q.X. and Trius Therapeutics to A.S.B.
REFERENCES
- 1.Archer GL. 1998. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis 26:1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, Tonthat GT, Rude TH, Barriere SL, Corey R, Fowler VG Jr. 2008. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol 46:678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, Hennessey JP Jr, Fu Y, Schmidt CS, Edwards JE Jr, Xiong YQ, Ibrahim AS. 2014. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci U S A 111:E5555–E5563. doi: 10.1073/pnas.1415610111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, Bayer AS. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother 52:269–278. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 340:493–501. [DOI] [PubMed] [Google Scholar]
- 7.Chahine EB, Sucher AJ, Knutsen SD. 2015. Tedizolid: a new oxazolidinone antibiotic for skin and soft tissue infections. Consult Pharm 30:386–394. doi: 10.4140/TCP.n.2015.386. [DOI] [PubMed] [Google Scholar]
- 8.Zhanel GG, Love R, Adam H, Golden A, Zelenitsky S, Schweizer F, Gorityala B, Lagace-Wiens PR, Rubinstein E, Walkty A, Gin AS, Gilmour M, Hoban DJ, Lynch JP III, Karlowsky JA. 2015. Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant Gram-positive pathogens. Drugs 75:253–270. doi: 10.1007/s40265-015-0352-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen KH, Huang YT, Liao CH, Sheng WH, Hsueh PR. 2015. In vitro activities of tedizolid and linezolid against Gram-positive cocci associated with acute bacterial skin and skin structure infections and pneumonia. Antimicrob Agents Chemother 59:6262–6265. doi: 10.1128/AAC.00390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson KS, Goering RV. 2013. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant Staphylococcus aureus strains of diverse epidemiological origins. Antimicrob Agents Chemother 57:2892–2895. doi: 10.1128/AAC.00274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing, 18th information supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Xiong YQ, Hady WA, Bayer AS, Chen L, Kreiswirth BN, Yang SJ. 2012. Telavancin in therapy of experimental aortic valve endocarditis in rabbits due to daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:5528–5533. doi: 10.1128/AAC.00922-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong YQ, Hady WA, Deslandes A, Rey A, Fraisse L, Kristensen HH, Yeaman MR, Bayer AS. 2011. Efficacy of NZ2114, a novel plectasin-derived cationic antimicrobial peptide antibiotic, in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 55:5325–5330. doi: 10.1128/AAC.00453-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, Rubin M, Bellinger-Kawahara C, Parr TR Jr, Contag PR. 2003. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun 71:882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. 2005. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother 49:380–387. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelhady W, Bayer AS, Seidl K, Moormeier DE, Bayles KW, Cheung A, Yeaman MR, Xiong YQ. 2014. Impact of vancomycin on sarA-mediated biofilm formation: role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis 209:1231–1240. doi: 10.1093/infdis/jiu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidl K, Bayer AS, Fowler VG Jr, McKinnell JA, Abdel Hady W, Sakoulas G, Yeaman MR, Xiong YQ. 2011. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother 55:575–582. doi: 10.1128/AAC.01028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. 2008. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J Infect Dis 198:258–261. doi: 10.1086/589307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. 2000. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun 68:3594–3600. doi: 10.1128/IAI.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadurugamuwa JL, Sin LV, Yu J, Francis KP, Kimura R, Purchio T, Contag PR. 2003. Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrob Agents Chemother 47:3130–3137. doi: 10.1128/AAC.47.10.3130-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong YQ, Li Y, Francis KP, Kadurugamuwa JL, Bayer AS. 2005. Real-time in vivo bioluminescent profiling of Staphylococcus aureus (SA) for monitoring virulence gene expression in an experimental endocarditis (IE) model, abstr B-2001. 45th Intersci Conf Antimicrob Agents Chemother, Washington, DC, 16 to 19 December 2005. [Google Scholar]
- 22.Lodise TP, Drusano GL. 2014. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin Infect Dis 58(Suppl 1):S28–S34. doi: 10.1093/cid/cit615. [DOI] [PubMed] [Google Scholar]
- 23.Slatter JG, Adams LA, Bush EC, Chiba K, Daley-Yates PT, Feenstra KL, Koike S, Ozawa N, Peng GW, Sams JP, Schuette MR, Yamazaki S. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907–924. doi: 10.1080/00498250210158249. [DOI] [PubMed] [Google Scholar]
- 24.Hegde SS, Reyes N, Skinner R, Difuntorum S. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother 61:169–172. [DOI] [PubMed] [Google Scholar]
- 25.Abdelhady W, Bayer AS, Seidl K, Nast CC, Kiedrowski MR, Horswill AR, Yeaman MR, Xiong YQ. 2013. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:1447–1454. doi: 10.1128/AAC.02073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt-Malan SM, Greenwood Quaintance KE, Karau MJ, Patel R. 2016. In vitro activity of tedizolid against staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis 85:77–79. doi: 10.1016/j.diagmicrobio.2016.01.008. [DOI] [PubMed] [Google Scholar]