Abstract
We assessed the pharmacokinetic profile of eravacycline, a novel antibiotic of the tetracycline class, and determined the dose in an immunocompetent murine thigh infection model that would provide free-drug exposure similar to that observed in humans after the administration of 1 mg/kg intravenously (i.v.) every 12 h (q12h). Eravacycline demonstrated a nonlinear protein-binding profile. The 2.5-mg/kg i.v. q12h dose in mice resulted in an area under the concentration-time curve for the free, unbound fraction of the drug of 1.64 mg · h/liter, which closely resembles the human exposure level.
TEXT
The understanding of the pharmacokinetic (PK) profile and its correlation to drug efficacy, as defined by the pharmacodynamic (PD) profile, is considered a cornerstone of antimicrobial drug development (1, 2). The desired PK profile of an agent can be used to construct a dosing regimen(s) that provides humanized exposures to allow the assessment of the compound's efficacy across a wide range of genotypic or phenotypic resistance profiles prior to clinical studies. The assessment of the free, non-protein-bound portion of the drug in plasma is necessary when estimating the appropriate dose (i.e., exposure) of the antimicrobial agent since it is the free, biologically active drug that is responsible for the antibacterial effect (3). While the extent of protein binding remains constant across the dose range for some antimicrobial classes, the magnitude of protein binding of tetracyclines follows a pattern (4). In other words, protein binding of tigecycline increases with increased total drug concentrations (5, 6).
Eravacycline, a novel member of the tetracycline class, has a broad spectrum of activity against Gram-negative and Gram-positive organisms (7). This study aimed to develop a human-simulated dose in mice based on data from studies with humans. A phase 1 study of eravacycline in healthy human volunteers has shown that a 1-mg/kg intravenous (i.v.) dose every 12 h (q12h) achieves an area under the concentration-time curve for the free, unbound fraction of the drug from 0 to 24 h (fAUC0–24) of 1.54 ± 0.28 mg · h/liter (8). This study also aimed to assess the protein-binding profile of eravacycline in mice and to define a dose in this species that would provide human exposure (i.e., fAUC0–24) for use in the immunocompetent murine thigh infection model.
PK studies.
Eravacycline (lot B110342; Tetraphase Pharmaceuticals, Inc., Watertown, MA) provided in 52.5-mg current good manufacturing practice vials was used throughout these experiments. Immunocompetent, specific-pathogen-free ICR (CD-1) female mice (Envigo RMS, Inc., Indianapolis, IN) were used in these studies. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT. Mice were infected in the thighs with Klebsiella pneumoniae 404 (eravacycline MIC of 0.25 μg/ml, SHV genotype). Two hours postinfection, eravacycline was administered via the i.v. route at single doses of 2.5, 5, 6, 7.5, and 10 mg/kg. Terminal blood samples were collected at eight postdose time points (0.083, 0.25, 0.5, 1, 2, 4, 8, and 12 h). Plasma was separated by centrifugation for 15 min at 4°C at a relative centrifugal force (RCF) of 3,000.
To determine protein binding, an aliquot (approximately 0.9 ml) of plasma was transferred to Centrifree Ultrafiltration devices (Millipore Corporation, Billerica, MA) with a 30,000 molecular weight cutoff and centrifuged for 45 min at 4°C at an RCF of 2,000.
Nonspecific binding to the ultrafiltration membrane was measured in triplicate at multiple eravacycline concentrations. All plasma and aqueous samples were stored at −80°C until shipped for analysis.
Eravacycline concentration determination.
Plasma drug concentrations were determined by Frontage Laboratories, Inc. (Exton, PA), by a verified liquid chromatography-tandem mass spectrophotometry method. The mean concentration per time point was calculated for each dose studied. Since the fAUC/MIC ratio is the PK/PD parameter that best correlates with the efficacy of tetracyclines (3), both the AUC0–12 and fAUC0–12 of eravacycline were calculated from total and free drug concentrations, respectively, by using the linear trapezoidal rule.
Results.
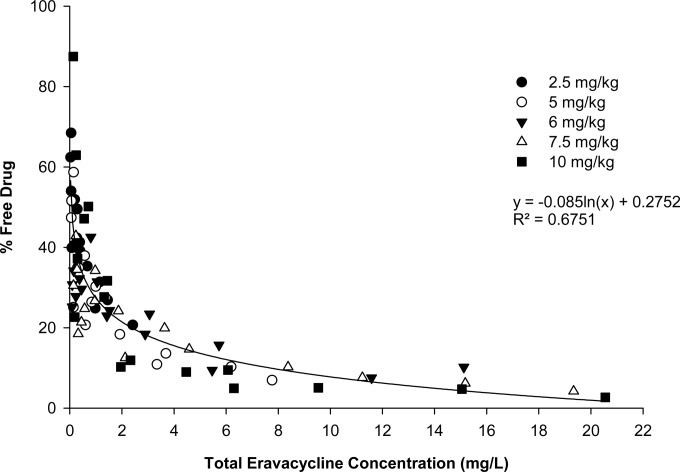
The protein binding of eravacycline in these studies ranged from 12.5 to 97.3%, with a mean ± standard deviation of 71.4% ± 17.1%, and showed a nonlinear, concentration-dependent relationship to the total drug concentration (Fig. 1). Namely, the protein binding of eravacycline increased nonlinearly as the total drug concentration increased. A nonspecific-binding study showed negligible binding of eravacycline to the membrane of the ultrafiltration device. Therefore, no correction for nonspecific binding was undertaken.
FIG 1.
Total plasma eravacycline concentrations and percentages of the free drug after i.v. administration of eravacycline in the immunocompetent murine thigh infection model.
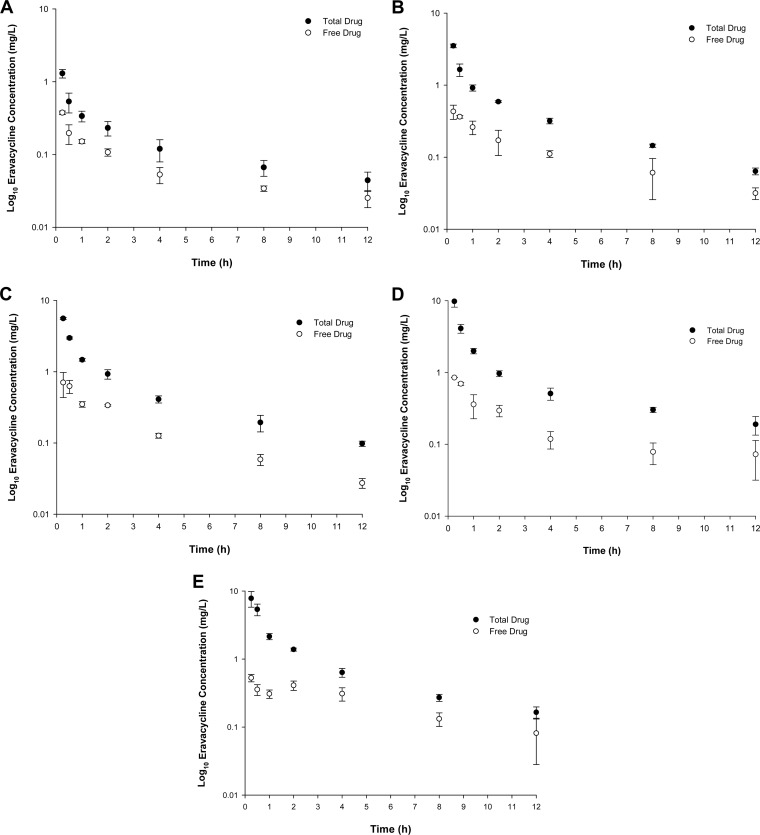
The total and free eravacycline concentrations in plasma are graphically displayed in Fig. 2A to E. The AUC0–12 and fAUC0–12 of eravacycline achieved by 2.5- to 10-mg/kg i.v. doses are shown in Table 1. None of the doses studied, if administered q24h, yielded a fAUC0–24 equivalent to the human target exposure. However, if 2.5 mg/kg, which has a fAUC0–12 of 0.82 mg · h/liter, was administered q12h, it yielded a predicted fAUC0–24 of 1.64 mg · h/liter, which closely approximates the human exposure from a 1-mg/kg q12h i.v. dose.
FIG 2.
Total and free eravacycline concentrations in plasma in the immunocompetent murine thigh infection model following the administration of a single i.v. eravacycline dose of 2.5 mg/kg (A), 5 mg/kg (B), 6 mg/kg (C), 7.5 mg/kg (D), or 10 mg/kg (E).
TABLE 1.
AUC0–12 and fAUC0–12 of eravacycline in the immunocompetent murine thigh infection model
| Dose (mg/kg) | AUC0–12 (mg · h/liter) | fAUC0–12 (mg · h/liter) |
|---|---|---|
| 2.5 | 2.00 | 0.82 |
| 5 | 5.47 | 1.40 |
| 6 | 8.66 | 1.97 |
| 7.5 | 11.82 | 2.07 |
| 10 | 12.87 | 2.79 |
Discussion.
The fixed protein-binding profile of most antimicrobial agents, such as β-lactams and fluoroquinolones, makes prediction of the free drug concentration profile easy by applying the percentage of the free fraction of the drug to the measured total concentration in serum and thus makes the simulation of the human dose in a mouse a simple process. Conversely, the tetracycline class has been known for having a variable protein-binding profile (4).
This pattern is more prominent with the newer members of the class, such as tigecycline and eravacycline (9, 10). In this profile, the total plasma drug concentration is inversely proportional to the percentage of the free fraction of the drug (i.e., a nonlinear relationship). Under such conditions, a fixed protein-binding value does not exist; therefore, direct measurement of the free unbound drug for a given dose is a necessity when correlating the fAUC/MIC ratio with the antibacterial effects. The pattern seen in the current in vivo studies and in a previous study using the microdialysis method for protein binding determination largely resembles the profile seen in humans (11).This current series of studies used a novel in vivo approach in estimating the protein-binding profile of eravacycline in mice similar to that commonly done in humans to eliminate bias associated with in vitro studies to assess protein binding. This profile was used to develop a humanized regimen to be used in murine efficacy studies using a target fAUC0–24 of 1.54 ± 0.28 mg · h/liter based on the exposures measured in a phase 1 study of humans given a 1-mg/kg i.v. dose q12h (8).
We conclude that an eravacycline regimen of 2.5 mg/kg i.v. q12h in mice yields a fAUC0–24 that approximates the exposure seen in healthy volunteers given a 1-mg/kg i.v. dose q12h, after accounting for the nonlinear protein-binding profile. The results of this study advance our knowledge of the protein binding of tetracyclines and provide insight into mouse-based studies of eravacycline efficacy.
ACKNOWLEDGMENTS
This project was funded in part by Tetraphase Pharmaceuticals, Inc., and in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract HHSO100201200002C.
D.P.N. is a member of the scientific board of Tetraphase Pharmaceuticals, Inc., and has received research support from Tetraphase Pharmaceuticals, Inc. The other authors have nothing to disclose.
We thank Jared Crandon, Lucinda Lamb, Jennifer Tabor-Rennie, Sara Robinson, Debora Santini, Christina Sutherland, Kimelyn Greenwood, Yukihiro Hamada, Islam Ghazi, Wonhee So, and Jaime Verastegui from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance with the conduct of this study and Jack Wang and Abdul Mutlib from Frontage Laboratories, Inc., Exton, PA, for analysis of eravacycline concentrations.
Funding Statement
This project has been funded in part by Tetraphase Pharmaceuticals, Inc., and in part with federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under contract no. HHSO100201200002C.
REFERENCES
- 1.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 2.Levison ME, Levison JH. 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 23:791–815. doi: 10.1016/j.idc.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 4.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 5.Bulik CC, Wiskirchen DE, Shepard A, Sutherland CA, Kuti JL, Nicolau DP. 2010. Tissue penetration and pharmacokinetics of tigecycline in diabetic patients with chronic wound infections described by using in vivo microdialysis. Antimicrob Agents Chemother 54:5209–5213. doi: 10.1128/AAC.01051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandon JL, Banevicius MA, Nicolau DP. 2009. Pharmacodynamics of tigecycline against phenotypically diverse Staphylococcus aureus isolates in a murine thigh model. Antimicrob Agents Chemother 53:1165–1169. doi: 10.1128/AAC.00647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors KP, Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, Redican S, Nicolau DP. 2014. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother 58:2113–2118. doi: 10.1128/AAC.02036-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 49:220–229. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi P, Esposito C, Koroma J, Cannon EP, Tanaka S. 2003. In vitro assessment of plasma protein binding and metabolic stability of PTK 0796, abstr 2675, poster F-760. 43rd Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 11.Singh RS, Falcao NM, Sutcliffe J, Derendorf H. 2013. Plasma protein binding of eravacycline in mouse, rat, rabbit, cynomolgus monkey, African green monkey and human using microdialysis, abstr A-015. 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]