Abstract
The clinical development of antibiotics with a new mode of action combined with efficient pulmonary drug delivery is a priority against untreatable Pseudomonas aeruginosa lung infections. POL7001 is a macrocycle antibiotic belonging to the novel class of protein epitope mimetic (PEM) molecules with selective and potent activity against P. aeruginosa. We investigated ventilator-associated pneumonia (VAP) and cystic fibrosis (CF) as indications of the clinical potential of POL7001 to treat P. aeruginosa pulmonary infections. MICs of POL7001 and comparators were measured for reference and clinical P. aeruginosa strains. The therapeutic efficacy of POL7001 given by pulmonary administration was evaluated in murine models of P. aeruginosa acute and chronic pneumonia. POL7001 showed potent in vitro activity against a large panel of P. aeruginosa isolates from CF patients, including multidrug-resistant (MDR) isolates with adaptive phenotypes such as mucoid or hypermutable phenotypes. The efficacy of POL7001 was demonstrated in both wild-type and CF mice. In addition to a reduced bacterial burden in the lung, POL7001-treated mice showed progressive body weight recovery and reduced levels of inflammatory markers, indicating an improvement in general condition. Pharmacokinetic studies indicated that POL7001 reached significant concentrations in the lung after pulmonary administration, with low systemic exposure. These results support the further evaluation of POL7001 as a novel therapeutic agent for the treatment of P. aeruginosa pulmonary infections.
INTRODUCTION
Pseudomonas aeruginosa is a difficult-to-treat human pathogen causing a wide range of infections, especially in the respiratory tract. These infections, such as ventilator-associated pneumonia (VAP), are often life-threatening. Cystic fibrosis (CF) is another disease where P. aeruginosa lung infections are associated with worse outcomes (1). The high prevalence of P. aeruginosa infections, in nearly 80% of CF patients >18 years of age (2), is partially due to the propensity of this species to form biofilms and cause chronic infection.
Frequently observed inefficacy of available treatments is due to intrinsic or acquired resistance of P. aeruginosa and/or limited penetration of antibiotics into biofilms (3). However, despite the need for new drugs, only few novel antipseudomonal drugs or modifications of existing molecules are currently in the late stage of preclinical or clinical development (4). A new family of potent protein epitope mimetic (PEM) antibiotics has recently been described. These molecules show selective activity against P. aeruginosa by inhibiting the transport of the lipopolysaccharide to the outer membrane (5). Among these antibiotics, we showed that POL7001 provided protection against lethal P. aeruginosa infection in a mouse septicemia model (5).
In this work, to investigate the efficacy of POL7001 in models relevant for VAP or CF, we used murine P. aeruginosa acute and chronic pneumonia, including in CF mice. We report that pulmonary delivery of POL7001 is effective against P. aeruginosa infections with efficacy similar to that of ciprofloxacin (CIP), one of the currently available treatments. In addition, we demonstrate that the antibacterial activity of POL7001 is associated with a positive impact on the inflammatory profile of the host.
MATERIALS AND METHODS
Ethics statement.
Animal studies were conducted according to protocols approved by San Raffaele Scientific Institute (Milan, Italy) Institutional Animal Care and Use Committee (IACUC) and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals. Survival endpoints were not allowed for animal studies. Research on bacterial isolates from CF patients has been approved by the CF Center of Hannover Medical School, Hanover, Germany. Patients gave informed consent before the sample collection. Approval for storing of biological materials was obtained by the Hannover Medical School, Hanover, Germany.
Bacterial strains.
Clinical and laboratory P. aeruginosa strains used in this study included PAO1 (6), PA14 (7), LESB58 (8), and clone TB (9) and 40 sequential P. aeruginosa strains from CF patients of the CF clinic in Hanover (10, 11). Among the latter, P. aeruginosa strains from the first positive cultures were designated “early” isolates, whereas “intermediate” isolates were collected 1 to 5 years thereafter and “late” isolates were collected 7 to 16 years after colonization or prior to death or lung transplantation (10). In particular, the MDR-RP73 strain, used for murine models of infection, was isolated 16.9 years after the onset of chronic colonization of the CF patient's airways with P. aeruginosa (12). P. aeruginosa was cultured in Trypticase soy agar plates at 37°C for 24 h.
Pharmacokinetic (PK) measurements.
POL7001 was administered to C57BL/6NCr mice by parenteral and local delivery. At specific time points, lungs were recovered and homogenized with an Ultraturax Pro200 blender after addition of water. Plasma samples were processed without further treatment. Briefly, after addition of an internal standard, lung homogenates or plasma samples were extracted with acetonitrile acidified with formic acid. Supernatants were evaporated to dryness under a stream of nitrogen, reconstituted in an appropriate solvent, and then analyzed by reverse-phase chromatography (Acquity BEH C18 column [Waters]; 100 by 2.1 mm, 1.7-μm pore size), using an acidified water/acetonitrile gradient elution (ultraperformance liquid chromatography [UPLC]; Waters). The detection and quantification were performed by mass spectrometry, with an electrospray interface in positive mode and selective fragmentation of analytes (AB Sciex 4000 Q Trap mass spectrometer). Standards, quality controls, and samples were extracted and assayed using the same procedure.
Animals, infection, and measurements.
Immunocompetent C57Bl/6NCrlBR male mice (8 to 10 weeks of age) from Charles River and gut-corrected CF transmembrane conductance regulator (CFTR)-deficient male C57BL/6 Cftrtm1UNCTgN(FABPCFTR)#Jaw mice and the corresponding congenic wild-type (wt) mice (11 to 18 weeks of age) (12), obtained from Case Western Reserve University and maintained at San Raffaele Scientific Institute (Milan, Italy), were used. Mice were infected with 1 × 107 CFU of the planktonic multidrug-resistant (MDR)-RP73 strain for acute infection or 1 × 106 CFU of the strain embedded in agar beads for chronic infection, as previously described (10, 13, 14). After infection, mice were treated with antibiotics or saline solution and monitored for body weight according to the schedule shown in Fig. 1. Mice were monitored twice per day for the following parameters: vocalization, piloerection, attitude, locomotion, breathing, curiosity, nasal secretion, grooming, and dehydration. If mice lost >25% body weight and had evidence of severe clinical disease, such as scruffy coat, inactivity, loss of appetite, poor locomotion, or painful posture, they were sacrificed before the termination of the experiments with an overdose of carbon dioxide.
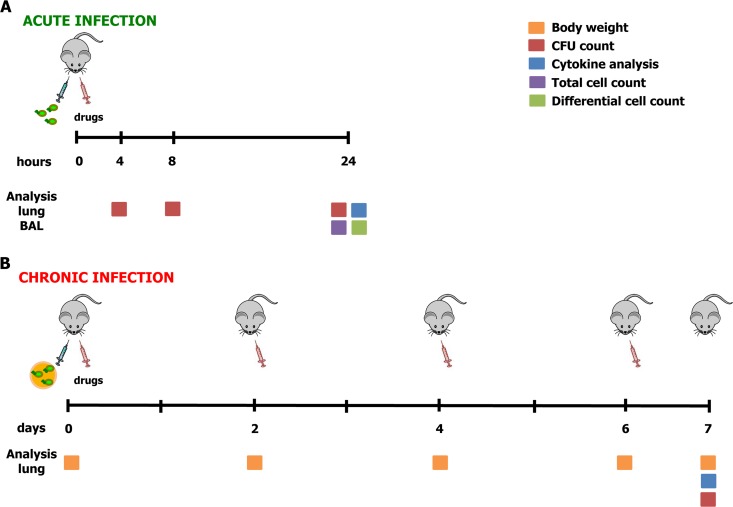
FIG 1.
Treatment schedule with antibiotics in murine models of acute and chronic P. aeruginosa RP73 infection and readouts. At day 0, mice were infected with P. aeruginosa planktonic cells to mimic acute infection (A) or with P. aeruginosa embedded in agar beads to achieve long-term chronic infection (B). The treatment schedule used with the antibiotics was administration of a single dose by the i.t. route for acute infection or administration every 2 days by endotracheal delivery for chronic infection. Readouts of the disease progression were body weight recorded before treatment or sacrifice (for chronic infection), CFU counts (performed at 4, 8, or 24 h for acute infection or after 7 days for chronic infection), total and differential cell counts (performed at 24 h for acute infection), and analysis of cytokines assayed at the time of sacrifice (performed at 24 h for acute infection or after 7 days for chronic infection).
A total of at least two experiments were performed (with at least 8 mice/group) to evaluate weight loss, bacteriology, and inflammatory parameters. The results of these experiments have been combined. In some cases, mortality occurred after surgery due to intratracheal (i.t.) injection, as previously described (15, 16), reducing the final number of mice. Thus, not all outcome measures were the same for each experiment. Lung CFU, counts of cytokines or chemokines, and bronchoalveolar lavage fluid (BALF) cell counts were analyzed as previously described (13, 17).
Antibiotic treatments.
POL7001, synthesized by Polyphor Ltd., and CIP (MPBiomedicals), both in saline solution as a vehicle and saline for the vehicle control group, were administered either i.t. with a 22-g cannula or endotracheally with a MicroSprayer Aerosolizer device (Penn-Century Inc.) under conditions of anesthesia (5% isoflurane–oxygen, running at 4 liters/min) according to established procedures. Treatment by subcutaneous (s.c.) parenteral administration was carried out for PK comparison.
MIC measurement.
MICs of POL7001 and control antibiotics were determined according to CLSI guidelines (18). The antimicrobial agents were prepared using a 1 mg/ml stock solution in Milli-Q water. The stock solutions were then further diluted for testing of the agents in the range of 8 to 0.008 μg/ml. The medium used for the MIC testing was cation-adjusted Mueller-Hinton broth (MH-II broth) (BBL; catalog no. 212322). POL7001 was tested in the presence of polysorbate-80 (P-80) or Tween 80 (Merck, catalog no. 655207) (sterile filtered; 10% solution) at a 0.002% (vol/vol) final concentration to avoid the binding of the compound to the plastic. MIC testing was run in sterile 96-well Microtiter plates (Greiner Bio-One; catalog no. 651161) (polystyrene V shape). The P. aeruginosa ATCC 27853 control strain was included.
Statistical analysis.
The sample size expected to show a standard deviation difference of at least 1.5 between the saline solution-treated mice and the CIP- or POL7001-treated mice has been predicted. According to the prediction, in order to achieve statistical significance, 8 mice per group are required to detect differences between groups with 80% power and an alpha error equal to 0.05. Thus, efficacy was tested starting with groups of 8 mice. Testing of the most promising doses was repeated for confirmation. Two-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison test was used to compare changes in body weight. For CFU determinations, analysis of levels of cells and cytokines or chemokines was performed by one-way ANOVA followed by Dunnett's analysis to compare results from three or more experimental groups and by the Mann-Whitney U test to compare results from two experimental groups. The analyses were performed using Prism (GraphPad). Tests are considered statistically significant when the significance level is ≤0.05.
RESULTS
In vitro activity of POL7001 against a panel of P. aeruginosa CF isolates.
The MICs of POL7001 and comparators were determined against a panel of P. aeruginosa CF strains (Table 1). P. aeruginosa lineages from nine CF patients, longitudinally collected during a period of up to 16 years and comprising 17 “early,” 6 “intermediate,” and 17 “late” isolates, were included in the study (19). Clonal P. aeruginosa isolates of each lineage showed different phenotypes that changed during genetic adaptation in CF lung (10, 20, 21). Additional P. aeruginosa strains were PAO1 and ATCC 27853 reference strains (6), PA14 as one of the most abundant genotypes (7), the highly pathogenic clone TB (22), and the highly transmissible Liverpool epidemic strain (LESB) (8).
TABLE 1.
In vitro activity of POL7001 and comparators against a panel of multidrug-resistant P. aeruginosa CF isolatesa
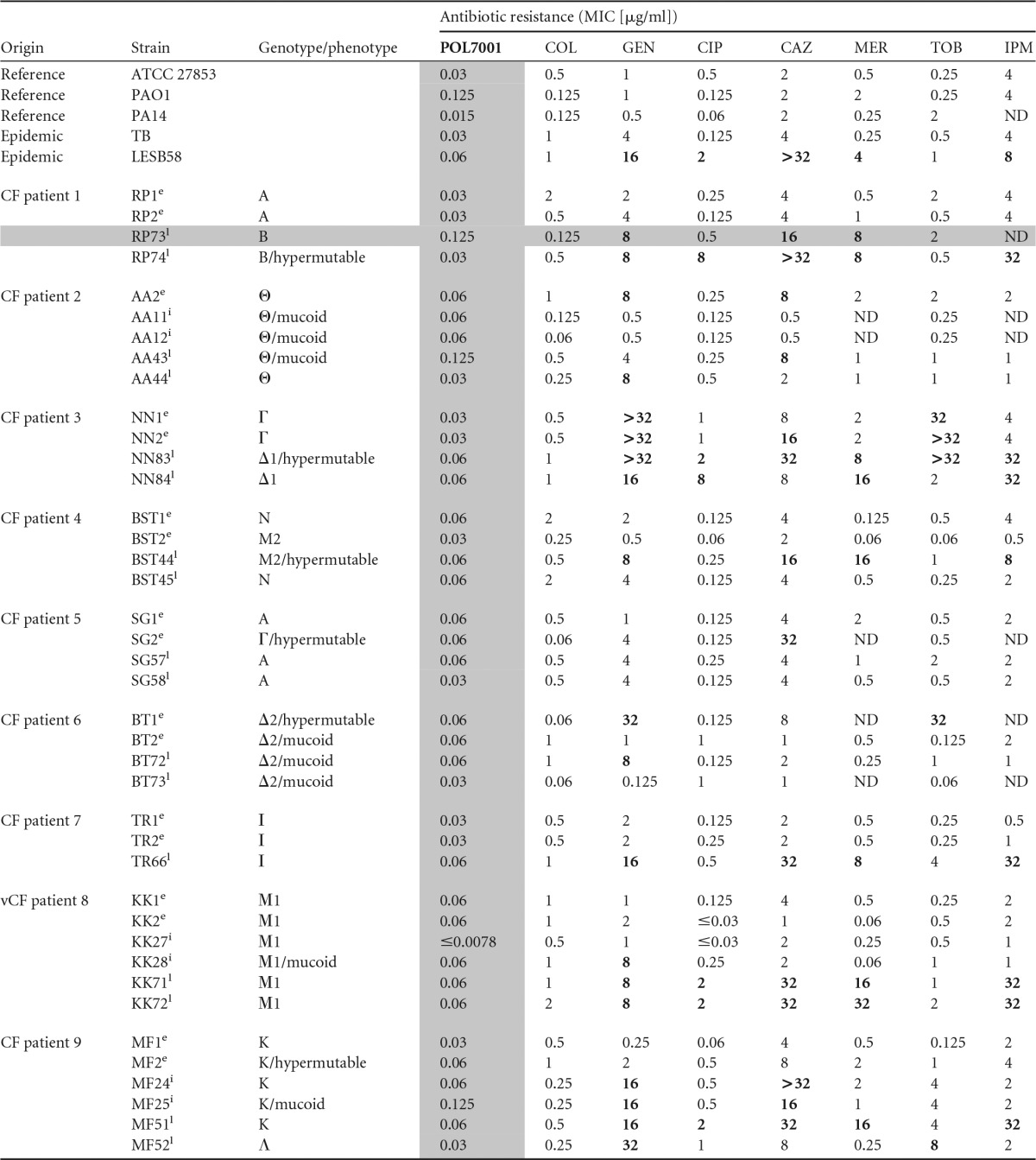
MIC values were determined by the microdilution method in cation-adjusted Mueller-Hinton (MH-II) broth, according to the CLSI guidelines. COL, colistin; GEN, gentamicin; CIP, ciprofloxacin; CAZ, ceftazidime; MER, meropenem; TOB, tobramycin; IMP, imipenem. POL7001 was tested in the presence of 0.002% (vol/vol) P-80 to avoid binding to the plastic. Resistance is indicated with boldface characters (http://www.eucast.org/clinical_breakpoints/). MICs of POL7001 are indicated with gray shading. Times of isolation are indicated as described in Materials and Methods using superscript letters as follows: e, early; i, intermediate; l, late. Genotypes and phenotypes of relevant P. aeruginosa strains are indicated as previously described (10, 11), and the data from the MDR-RP73 strain used for in vivo efficacy studies are indicated with gray shading. ND, not determined.
MIC values of POL7001 ranged from ≤0.0078 to 0.125 μg/ml, with an MIC90 of 0.06 μg/ml for all the isolates. Although many of the P. aeruginosa isolates from the CF patients were resistant to two or more currently used antibiotics, they were sensitive to POL7001. In particular, the P. aeruginosa RP73 strain used in this work was isolated from a patient with CF after long-term chronic infection and showed resistance to meropenem, ceftazidime, and gentamicin but was sensitive to POL7001 and CIP. There was no difference in the levels of activity of POL7001 against mucoid and nonmucoid or hypermutable phenotypes.
Efficacy of lung administration of POL7001 in acute P. aeruginosa respiratory infection.
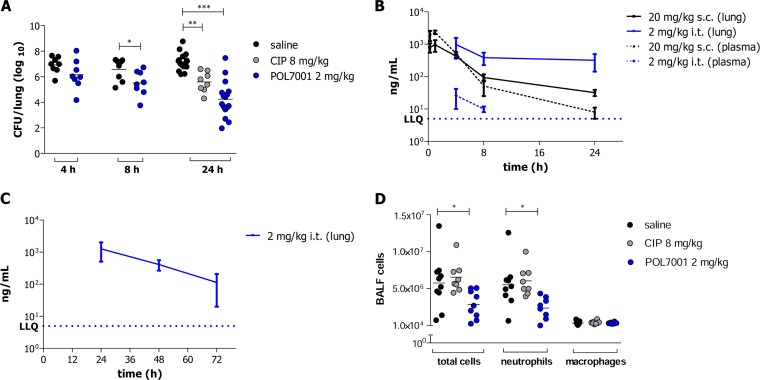
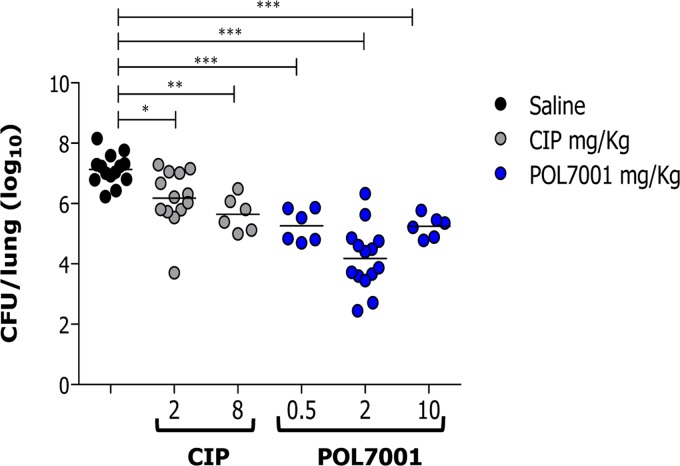
The efficacy of POL7001 was tested in a murine model of acute lung infection against the P. aeruginosa MDR-RP73 strain according to the treatment schedule shown in Fig. 1A. C57BL/6 mice were inoculated i.t. with planktonic P. aeruginosa MDR-RP73 and treated 15 min postinfection with 8 mg CIP/kg of body weight or 2 mg/kg POL7001 or saline solution, by the same route (Fig. 2A and Table 2). The dose of 2 mg/kg of POL7001 was chosen based on dose response (Fig. 3). In order to show treatability of the infection, CIP was dosed at 8 mg/kg to keep the same dose/MIC ratio (Table 1). Based on a rat pneumonia model, an intrapulmonary dose of CIP of 200 μg/kg gives a value of 103 μg · h/ml for the area under the concentration-time curve for the free, unbound fraction of a drug (fAUC) in the lung. For a strain with a drug MIC of 0.5 μg/ml, this dose gives a value for the fAUC over 24 h in the steady state divided by the MIC (fAUC/MIC ratio) of 206, which is larger than the effective fAUC/MIC ratio of >125 that is the common exposure target associated with favorable outcomes of CIP therapy against P. aeruginosa (23). A dose of 8 mg/kg in mice is then expected to largely exceed the target exposure of CIP. The antibacterial effect of POL7001 was significant at 8 h posttreatment, with a 1 log10 CFU reduction compared to the results seen with saline solution-treated mice. At 24 h, both the POL7001-treated mice and the CIP-treated mice had a significantly lower level of CFU in the lungs. POL7001 displayed higher efficacy than CIP, with reductions of 2.9 log10 and 1.6 log10 CFU compared to the results seen with the saline solution-treated mice, respectively (Fig. 2A and Table 2).
FIG 2.
In vivo efficacy and pharmacokinetic of POL7001 against P. aeruginosa RP73 in a mouse model of acute airway infection.(A) C57BL/6 male mice (8 to 10 weeks of age) were infected i.t. with 1 × 107 CFU of planktonic MDR-RP73. After MDR-RP73 infection, 8 mg/kg CIP or 2 mg/kg POL7001 or saline solution was administered via the i.t. route. Mouse lungs were collected after 4, 8, or 24 h, homogenized, and plated on tryptic soy agar (TSA) plates to determine the bacterial load. (B) The concentration of POL7001 at 4, 8, or 24 h postdosing was measured in lung tissue and plasma of infected mice. Data represent mean values ± standard errors of the means (SEM). (C) The concentration of POL7001 at 24, 48, or 72 h after 2 mg/kg i.t. dosing was measured in lung tissue and plasma of healthy (noninfected) C57BL/6 male mice (8 to 10 weeks of age). Data represent mean values ± standard errors of the means (SEM). The concentration of POL7001 in plasma was below the lowest limit of quantification (LLQ) of 5 ng/ml at the 3 time points. (D) BALF was recovered from C57BL/6 male mice (8 to 10 weeks of age) 24 h after i.t. infection with 1 × 107 CFU of planktonic MDR-RP73 and treatment with 8 mg/kg CIP or 2 mg/kg POL7001 or saline solution administered by the i.t. route. Counts of total number of cells, neutrophils, and macrophages were performed. The data are pooled from two or three independent experiments (n = 8 to 16). Statistical significance determined by the Mann-Whitney U test or one-way ANOVA followed by Dunnett's analysis is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 2.
Efficacy of antibiotic treatment in mouse models of acute and chronic P. aeruginosa respiratory infectiona
| Infection category and treatment | Dose (mg/kg) | Time of analysis | No. of mice | CFU/lung (log10) | P value | Total no. of cells | P value | No. of neutrophils | P value | No. of macrophages | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MDR-RP73 acute infection—i.t. treatment | |||||||||||
| Saline solution | 4 h | 8 | 6.929 | ||||||||
| POL7001 | 2 | 4 h | 8 | 6.160 | ns | ||||||
| CIP | 8 | 4 h | |||||||||
| Saline solution | 8 h | 8 | 6.579 | ||||||||
| POL7001 | 2 | 8 h | 8 | 5.514 | <0.05 | ||||||
| CIP | 8 | 8 h | |||||||||
| Saline solution | 24 h | 9–16 | 7.172 | 5,777,000 | 5,534,000 | 352,319 | |||||
| POL7001 | 2 | 24 h | 8–16 | 4.247 | <0.001 | 2,862,000 | <0.05 | 2,376,000 | <0.05 | 335,633 | ns |
| CIP | 8 | 24 h | 8 | 5.597 | <0.01 | 6,552,000 | ns | 6,053,000 | ns | 413,412 | ns |
| MDR-RP73 chronic infection—aerosol treatment | |||||||||||
| Saline solution | 7 days | 9 | 4.883 | ||||||||
| POL7001 | 2 | 7 days | 6 | 3.921 | <0.01 | ||||||
| CIP | 8 | 7 days | 11 | 4.113 | <0.05 |
All P values indicate P < 0.05. ns, not significant.
FIG 3.
Dose response of POL7001 against P. aeruginosa in a mouse model of acute airway infection in comparison with CIP. C57BL/6 male mice (8 to 10 weeks of age) were infected i.t. with 1 × 107 CFU of planktonic MDR-RP73. After MDR-RP73 infection, saline solution, 2 or 8 mg/kg CIP, or different doses of POL7001 (0.5, 2, and 10 mg/kg) were administered 15 min postinfection by the i.t. route. Mouse lungs were collected after 24 h, homogenized, and plated on TSA plates to determine bacterial load. Dots represent measurements from individual mice, and horizontal lines represent the mean values. The data are pooled from two or three independent experiments (n = 6 to 14). Statistical significance determined by one-way ANOVA followed by Dunnett's analysis is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine the absorption and biodistribution of POL7001, the concentration of the compound in lung tissue and plasma was determined after 2 mg/kg local pulmonary administration (i.t.) in comparison to 20 mg/kg parenteral administration (s.c.) in C57BL/6 mice. The time course of the concentrations of POL7001 in lung and plasma is shown in Fig. 2B. POL7001 in the lung was markedly more highly concentrated and had a longer terminal half-life after pulmonary administration than after parenteral administration over 24 h. POL7001 is retained in the lung for up to 48 h and 72 h after a single pulmonary administration (Fig. 2C). The pharmacokinetic profile of POL7001 in plasma compared to lung tissue showed lower systemic exposure and higher concentrations in lung tissue after pulmonary administration than after parenteral administration.
Inflammatory response after treatment with POL7001 in acute P. aeruginosa respiratory infection.
To evaluate the effect of POL7001 on the airway inflammatory response after pulmonary administration, the leukocyte recruitment in the BALF and in a panel of cytokines or chemokines in murine lung homogenate was measured. Twenty-four hours after MDR-RP73 acute infection, total and differential counts of the cells in BALF showed that mice treated with POL7001 had significantly fewer cells in total than the mice in the negative-control group, indicating a reduction of inflammation. In contrast, CIP-treated mice showed no difference in the number of recruited cells compared to the negative-control mice. In particular, mice infected with MDR-RP73 and treated with POL7001 showed a significant decrease in levels of neutrophils, while the amounts of monocytes and macrophages were unchanged (Fig. 2D and Table 2). In mice treated with CIP, the levels of neutrophils were unchanged compared with the levels seen with the saline solution-treated control mice.
CXCL2/MIP-2, CCL2/JE, and CXCL1/KC production in the lungs of infected mice treated with POL7001 was significantly reduced in comparison to the levels seen with saline solution-treated controls at 24 h postinfection (Table 3). Despite showing the lowest mean values, the level of interleukin-1β (IL-1β) was not significantly reduced by treatment with POL7001. The same trend of reduction was observed in lung homogenates of CIP-treated mice.
TABLE 3.
Cytokines and chemokines after P. aeruginosa acute and chronic airway infection and antibiotic treatmenta
| Infection category, treatment, and cytokine or chemokine | Level (mean pg/ml ± SEM) |
P value |
|||
|---|---|---|---|---|---|
| Saline solution | CIP (8 mg/kg) | POL7001 (2 mg/kg) | Saline solution vs CIP | Saline solution vs POL7001 | |
| MDR-RP73 acute infection—i.t. treatment | |||||
| IL-1β | 7,068 ± 1,337 | 4,300 ± 1,137 | 4,203 ± 1,422 | ns | ns |
| CXCL1/KC | 74,255 ± 11,474 | 19,480 ± 4,793 | 22,591 ± 3,790 | <0.001 | <0.001 |
| CXCL2/MIP-2 | 111,456 ± 14,596 | 34,093 ± 8,157 | 31,750 ± 6,019 | <0.001 | <0.001 |
| CCL2/JE | 8,731 ± 1,609 | 3,275 ± 397.7 | 4,085 ± 1,170 | <0.05 | <0.05 |
| MDR-RP73 chronic infection—aerosol treatment | |||||
| IL-1β | 1,034 ± 7.575 | 838.2 ± 70.26 | 586.8 ± 158.5 | ns | <0.01 |
| CXCL1/KC | 33,174 ± 9,638 | 9,186 ± 2,849 | 5,145 ± 1,789 | <0.01 | <0.01 |
| CXCL2/MIP-2 | 14,919 ± 3,351 | 2,859 ± 573.8 | 2,279 ± 414.5 | <0.001 | <0.001 |
| CCL2/JE | 12,269 ± 1,916 | 11,269 ± 1,558 | 8,577 ± 1,160 | ns | ns |
C57BL/6 male mice (8 to 10 weeks old) were infected with MDR-RP73 and treated according to the schedule depicted in Fig. 1. IL-1β, CXCL1/KC, CXCL2/MIP-2, and CCL2/JE levels in lung homogenates were measured by enzyme-linked immunosorbent assay (ELISA) after 24 h or 7 days of P. aeruginosa acute and chronic infection. Data represent mean values ± standard errors of the means (SEM) of results from mice (n = 4 to 11) pooled from two to three independent experiments. Statistical significance as determined by one-way ANOVA followed by Dunnett's analysis is indicated. ns, not significant.
Efficacy of POL7001 in P. aeruginosa chronic lung infection.
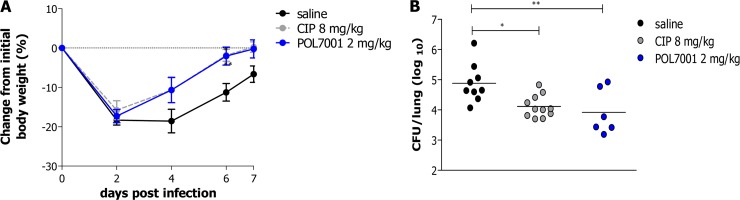
In order to investigate the efficacy of POL7001 in a chronic infection environment similar to the one characteristic of lungs of CF patients, mice were infected with MDR-RP73 embedded in agar beads and inoculated i.t. according to established procedures (13, 24). In order to perform endotracheal drug administration, a MicroSprayer Aerosolizer from Penn-Century was used. Aerosol treatment with either 8 mg/kg CIP or 2 mg/kg POL7001 (to keep the same doses as used in the acute infection model) was started 15 min after infection and repeated every second day for a total of four administrations according to the treatment schedule shown in Fig. 1B. Saline solution was used as a negative control and was administered in the same way as the antibiotics. After an initial weight loss observed in the first 2 days, the groups of POL7001- and CIP-treated mice showed statistically significant increases in body weight that occurred more rapidly than those seen with the saline solution-treated group of mice and had recovered their initial body weight almost completely by day 7 postinfection (Fig. 4). After 7 days of chronic infection and a total of four treatments, mice treated with both CIP and POL7001 showed a significant reduction of the bacterial load in comparison to the saline solution-treated group (Fig. 4B and Table 2). However, POL7001 displayed higher efficacy than CIP, with 1 log10 and 0.7 log10 CFU reductions compared to the results seen with the saline solution-treated group, respectively.
FIG 4.
Efficacy of POL7001 against P. aeruginosa RP73 in a murine model of chronic lung infection after aerosol administration. (A) C57BL/6 male mice (8 to 10 weeks of age) were challenged with 1 × 106 CFU of MDR-RP73, embedded in agar beads, by i.t. inoculation. Aerosol treatment with saline solution, CIP (8 mg/kg), or POL7001 (2 mg/kg) was performed every 2 days by the use of a Penn-Century instrument. Before each administration, mice were weighed and changes from initial body weight were averaged for each group of mice. Data represent mean values ± standard errors of the means (SEM). (B) At day 7, mice were sacrificed and lungs were excised, homogenized, and plated onto TSA plates to determine bacterial load. Dots indicate CFU/lung data representing measurements from individual mice, and horizontal lines represent the mean values (n = 6 to 11). The data are pooled from two or three independent experiments. Statistical significance determined by two-way ANOVA with the Bonferroni posttest is indicated in the body weight curves, and results of one-way ANOVA followed by Dunnett's analysis are indicated in the CFU/lung data. *, P < 0.05; **, P < 0.01.
Cytokine and chemokine profiles of lung tissue from P. aeruginosa-infected mice showed that the levels of CXCL1/KC and CXCL2/MIP-2 were significantly reduced in mice treated with POL7001 or CIP compared to the levels seen in the saline solution-treated mice at day 7 postinfection (Table 3). Levels of IL-1β were significantly reduced only in POL7001-treated mice, while levels of CCL2/JE were reduced but the differences did not reach statistical significance for either of the treatments.
Antibacterial and anti-inflammatory effect of POL7001 in P. aeruginosa chronic lung infection in CFTR-deficient mice.
Chronic infection with MDR-RP73 was established in CF transmembrane conductance regulator (CFTR) knockout C57BL/6 Cftrtm1UNCTgN(FABPCFTR)#Jaw (CF) mice and the corresponding congenic wild-type (wt) (non-CF) mice. Animals were challenged i.t. with MDR-RP73 embedded in agar beads and treated with either 2 mg/kg POL7001 or saline solution by the use of a MicroSprayer Aerosolizer 15 min postinfection and then every second day, for a total of four administrations.
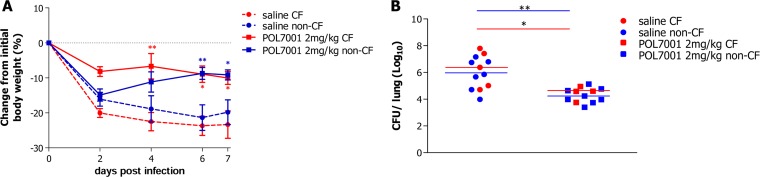
Body weight loss was observed in the first 2 days after infection in both the saline solution-treated and POL7001-treated mice (Fig. 5A). While saline solution-treated mice did not recover weight until day 7, mice treated with POL7001 gained weight from the second day onward, with a significant difference compared to the levels seen with the saline solution-treated mice from day 4. After 7 days of chronic infection, mice treated with POL7001 showed a significant 1.5 to 2 log10 reduction in the bacterial load in comparison to the saline solution-treated group (Fig. 5B and Table 4). No significant differences between CF or non-CF mice in bacterial load have been observed, indicating that the treatment is effective in different genetic backgrounds and is not influenced by the CF environment.
FIG 5.
Efficacy of POL7001 against P. aeruginosa RP73 in a CF murine model of chronic lung infection after aerosol administration. (A) C57BL/6 Cftrtm1UNCTgN(FABPCFTR)#Jaw mice and the corresponding congenic wt mice (11 to 18 weeks old) were challenged with 1 × 106 CFU of P. aeruginosa RP73 (embedded in agar beads) after i.t. inoculation. Aerosol treatment was performed by the use of a MicroSprayer Aerosolizer (Penn-Century) at 15 min postinfection and then every 2 days with either saline solution or POL7001 (2 mg/kg) for a total of four administrations. Before each administration, mice were weighed. Changes from initial body weight were calculated for each group of mice. Data represent mean values ± standard errors of the means (SEM). (B) At day 7, mice were sacrificed and lungs were excised, homogenized, and plated on TSA plates to determine bacterial load. The data are pooled from two independent experiments. Dots represent measurements from individual CF mice (red) (saline solution, n = 5; POL7001, n = 4) or congenic wt mice (blue) (saline solution, n = 7; POL7001, n = 8), and horizontal lines represent the median values. Statistical significance determined by two-way ANOVA with the Bonferroni posttest is indicated in the body weight curves. Results of Mann-Whitney U test analysis are indicated in the CFU/lung data. *, P < 0.05; **, P < 0.01.
TABLE 4.
Efficacy of antibiotic treatment in a CF mouse model of chronic P. aeruginosa respiratory infection
| MDR-RP73 chronic infection—aerosol treatment conditions | Dose (mg/kg) | Time of analysis (no. of days) | No. of mice | CFU/lung (log10) |
P valuea |
|||
|---|---|---|---|---|---|---|---|---|
| Saline solution CF vs non-CF | POL7001 CF vs non-CF | Non-CF POL7001 vs saline solution | CF POL7001 vs saline solution | |||||
| Saline solution (non-CF mice) | 7 | 7 | 5.846 | ns | ||||
| Saline solution (CF mice) | 7 | 5 | 6.263 | ns | ||||
| POL7001 (non-CF mice) | 2 | 7 | 8 | 4.234 | ns | <0.01 | ||
| POL7001 (CF mice) | 2 | 7 | 4 | 4.378 | ns | <0.05 | ||
ns, not significant.
The levels of CXCL2/MIP-2 were significantly reduced in lung homogenates of CF and wt mice treated with POL7001 compared to saline solution-treated mice. IL-1β levels were significantly reduced only in the CF mice and not in the wt mice, while levels of CXCL1/KC and CCL2/JE were reduced but the changes did not reach statistical significance (Table 5).
TABLE 5.
Cytokines and chemokines after P. aeruginosa RP73 chronic airway infection in CF and non-CF mice treated with POL7001 by pulmonary administrationa
| Cytokine or chemokine | Level (under conditions of MDR-RP73 chronic infection—aerosol treatment) (pg/ml [mean ± SEM]) |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| Non-CF—saline solution | CF—saline solution | Non-CF—POL7001 (2 mg/kg) | CF—POL7001 (2 mg/kg) | Non-CF saline solution vs CF saline solution | Non-CF POL vs CF POL | Non-CF saline solution vs non-CF POL | CF saline solution vs CF POL | |
| IL-1β | 1,684 ± 262.5 | 1,691 ± 305.3 | 1,280 ± 231.5 | 598.5 ± 257.3 | ns | ns | ns | <0.05 |
| CXCL1/KC | 19,776 ± 2,460 | 13,210 ± 2,284 | 14,167 ± 1,833 | 11,989 ± 1,442 | ns | ns | ns | ns |
| CXCL2/MIP-2 | 9,577 ± 2,604 | 8,419 ± 2,625 | 1,087 ± 165.4 | 1,282 ± 286.8 | ns | ns | <0.01 | <0.05 |
| CCL2/JE | 2,352 ± 467 | 2,574 ± 795.1 | 1,718 ± 394 | 1,232 ± 252.6 | ns | ns | ns | ns |
C57BL/6 Cftrtm1UNCTgN(FABPCFTR)#Jaw (CF) mice and the corresponding wt congenic mice (non-CF) (11 to 18 weeks of age) were infected with MDR-RP73 embedded in agar beads and treated with saline solution or POL7001 (2 mg/kg) by the use of a Penn-Century device according to the schedule shown in Fig. 1B. Lungs were collected after 7 days of infection, and IL-1β, CXCL1/KC, CXCL2/MIP-2, and CCL2/JE levels were measured by ELISA using supernatants of lung homogenates. Data represent mean values ± SEM. The data are pooled from the results determined for mice from two independent experiments (n = 4 to 8). Statistical significance determined by Mann-Whitney U test analysis is indicated. ns, not significant.
Taken together, these results suggest a possible therapeutic effect of POL7001 in CF mice.
DISCUSSION
Current antibiotic treatments of P. aeruginosa infections are often ineffective, due to increasing rates of MDR strains, and the number of drug candidates in clinical development remains disappointingly low. To address this unmet medical need, PEM technology was applied to identify a new class of macrocycle molecules showing antibacterial activity with a novel mode of action. POL7001 is a potent PEM antibiotic that is selective against a wide range of P. aeruginosa isolates (5). In this work, we demonstrated that POL7001 is active against a panel of P. aeruginosa strains isolated from CF patients at different stages of chronic infection. Many of the P. aeruginosa isolates tested in this study were resistant to two or more antibiotics; however, they were sensitive to POL7001. In particular, this collection included mucoid and hypermutable isolates which evolved during long-term chronic infection and whose phenotype has been associated with antibiotic resistance. In vitro serial passage and spontaneous mutation rate data indicate that POL7001 has a low propensity to develop resistance (data not shown). While mucoid strains produce large amounts of alginate which protect the cells from antibiotic penetration, the capacity of the hypermutable population to generate adaptive mutations enables bacteria to rapidly and efficiently develop antibiotic resistance (25). The presence of mucoid strains has been associated with poor prognosis for CF patients (26), and recent clinical studies suggest that the presence of hypermutable strains is linked to the poorer respiratory function of CF patients compared to patients colonized by nonhypermutable isolates (27). Our results confirm potent in vitro activity of POL7001 against clinically relevant P. aeruginosa phenotypes.
In order to investigate the potential application of POL7001 to treat clinical conditions such as VAP or CF, previously described animal models of acute and chronic P. aeruginosa respiratory infection were used (13, 28). Based on the human clinical response to infection, microbiology and inflammation were chosen as clinically relevant endpoints (29).
In the present study, we investigated the efficacy of POL7001 in a model of acute pneumonia induced by the P. aeruginosa MDR-RP73 clinical isolate. The administration of POL7001 by pulmonary delivery showed significant, although not complete, bacterial load reduction, indicating the difficulty of achieving the complete eradication of P. aeruginosa pathoadaptive strains even with potent drugs. POL7001 and ciprofloxacin were also tested in an acute infection model induced by the reference non-MDR P. aeruginosa PAO1 strain (30). In the PAO1 lung acute infection model, a reduction of at least 4 log10, compared to the levels seen with the vehicle-treated group, was obtained for both POL7001 and CIP (with both tested at 2 mg/ml to keep the same dose/MIC ratio), with the CFU level in the lung tissue of most of the animals treated with POL7001 and CIP below the lowest limit of quantification of 20 CFU/lung. In the P. aeruginosa MDR-RP73 model, treatment with POL7001 was as efficacious as treatment with CIP, indicating the great potential of this new drug candidate. The inflammatory profile in the airways, in particular, the neutrophil burden and the concentrations of chemokines and cytokines such as CXCL1/KC, CXCL2/MIP-2 and CCL2/JE, was reduced in mice treated with POL7001, suggesting a positive impact of this treatment on the pulmonary physiology. The administration of antibiotics by the inhaled route is widely adopted in patients with CF and bronchiectasis (31) and has recently been tested for the treatment of pneumonia, including VAP (32). Advantages of inhaled administration include higher efficacy due to high local concentrations and fewer side effects due to low systemic exposure of the drug. The favorable tissue distribution with low systemic exposure of POL7001 found in pharmacokinetic studies combined with preliminary evidence of significant efficacy following endotracheal delivery supports the pulmonary administration.
The preclinical testing of POL7001 was extended to more-complex models of chronic infection, including CF mice. An in vivo model for long-term severe P. aeruginosa airway infection can be achieved by challenging mice with the MDR-RP73 P. aeruginosa clinical strain embedded in agar beads. We have previously shown that agar beads provide microanaerobic conditions for bacterial growth and biofilm formation comparable to those present in the mucus of CF patients (24, 33, 34). In addition, this model develops lesions similar to the ones found in CF patients and displays certain phenotypes of human CF lung disease (34), providing a valuable tool for the preclinical testing of antibacterial and anti-inflammatory molecules (29, 35). It has been reported that the use of a conventional human-adapted nebulizer resulted in low deposition efficiency of drugs in rodents (36). For this reason, we used a MicroSprayer Aerosolizer (from Penn-Century) for pulmonary administration of POL7001. This is a relatively noninvasive pulmonary aerosol delivery system that ensures a good spread of molecules into the lower airways (37). This technique has been previously used in a preclinical study of the new levofloxacin formulation MP-376 (38).
Treatment of mice with POL7001 administered repeatedly by a MicroSprayer Aerosolizer significantly decreased the P. aeruginosa load compared to treatment with vehicle, indicating clear antibacterial efficacy. In this context, preliminary data suggest that POL7001 is effective in both prevention of biofilm formation and reduction of viable cell numbers within well-established biofilms (data not shown). In addition, POL7001 attenuated the inflammatory response to chronic endobronchial infection by significantly decreasing the concentrations of inflammatory mediators such as IL-1β, CXCL1/KC, CXCL2/MIP-2, and CCL2/JE compared to the results seen with vehicle. The efficacy of POL7001 was also observed in CF mice. CF mice treated with POL7001 showed significantly improved weight gain and reduced bacterial load in the lung compared to animals treated with the vehicle. It has been reported that CF is a difficult environment which may reduce the efficacy of treatments (39). Repeated treatment with POL7001 showed similar beneficial effects in mice with different genetic backgrounds, suggesting that the CF environment does not represent an obstacle for this antibiotic treatment.
Overall, these data support the further evaluation of POL7001 for the treatment of P. aeruginosa infections.
ACKNOWLEDGMENTS
We thank B. Tümmler (Hannover Medical School, Hanover, Germany) for supplying the P. aeruginosa strains from CF patients and M. Cámara (University of Nottingham, United Kingdom) and his group for the biofilm study.
This work is dedicated to the memory of Gerd Döring and to his commitment to CF research.
Funding Statement
This work has been supported by the European Commission (NABATIVI-223670, EU-FP7-HEALTH-2007-B, to Alessandra Bragonzi and Daniel Obrecht) and Italian Cystic Fibrosis Research Foundation (FFC#10/2011 to Alessandra Bragonzi and Daniel Obrecht) with the contribution of the Delegazione FFC di Milano. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Moss RB. 2010. Allergic bronchopulmonary aspergillosis and Aspergillus infection in cystic fibrosis. Curr Opin Pulm Med 16:598–603. doi: 10.1097/MCP.0b013e32833e24a6. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation. 2013. Patient registry. Annual data report 2012. http://www.cff.org/LivingWithCF/QualityImprovement/PatientRegistryReport/.
- 3.Gellatly SL, Hancock RE. 2013. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 4.Projan SJ, Youngman PJ. 2002. Antimicrobials: new solutions badly needed. Curr Opin Microbiol 5:463–465. doi: 10.1016/S1369-5274(02)00364-8. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RL, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Kach A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. 2010. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 6.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 7.Wiehlmann L, Wagner G, Cramer N, Siebert B, Gudowius P, Morales G, Kohler T, van Delden C, Weinel C, Slickers P, Tummler B. 2007. Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ. 2004. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax 59:334–336. doi: 10.1136/thx.2003.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezuidt OK, Klockgether J, Elsen S, Attree I, Davenport CF, Tummler B. 2013. Intraclonal genome diversity of Pseudomonas aeruginosa clones CHA and TB. BMC Genomics 14:416. doi: 10.1186/1471-2164-14-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Doring G, Tummler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 11.Bragonzi A, Wiehlmann L, Klockgether J, Cramer N, Worlitzsch D, Doring G, Tummler B. 2006. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 152:3261–3269. doi: 10.1099/mic.0.29175-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. 1994. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 13.Facchini M, De Fino I, Riva C, Bragonzi A. 17 March 2014. Long term chronic Pseudomonas aeruginosa airway infection in mice. J Vis Exp doi: 10.3791/51019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorè NI, Cigana C, De Fino I, Riva C, Juhas M, Schwager S, Eberl L, Bragonzi A. 2012. Cystic fibrosis-niche adaptation of Pseudomonas aeruginosa reduces virulence in multiple infection hosts. PLoS One 7:e35648. doi: 10.1371/journal.pone.0035648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Heeckeren AM, Schluchter MD. 2002. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 36:291–312. doi: 10.1258/002367702320162405. [DOI] [PubMed] [Google Scholar]
- 16.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, Haxhiu MA, Ferkol TW. 2000. Effect of Pseudomonas aeruginosa infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med 161:271–279. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 17.Kukavica-Ibrulj I, Facchini M, Cigana C, Levesque RC, Bragonzi A. 2014. Assessing Pseudomonas aeruginosa virulence and the host response using murine models of acute and chronic lung infection. Methods Mol Biol 1149:757–771. doi: 10.1007/978-1-4939-0473-0_58. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. 2014. CLSI M100-S24. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 19.Cramer N, Wiehlmann L, Ciofu O, Tamm S, Hoiby N, Tummler B. 2012. Molecular epidemiology of chronic Pseudomonas aeruginosa airway infections in cystic fibrosis. PLoS One 7:e50731. doi: 10.1371/journal.pone.0050731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montanari S, Oliver A, Salerno P, Mena A, Bertoni G, Tummler B, Cariani L, Conese M, Doring G, Bragonzi A. 2007. Biological cost of hypermutation in Pseudomonas aeruginosa strains from patients with cystic fibrosis. Microbiology 153:1445–1454. doi: 10.1099/mic.0.2006/003400-0. [DOI] [PubMed] [Google Scholar]
- 21.Alcalá-Franco B, Montanari S, Cigana C, Bertoni G, Oliver A, Bragonzi A. 2012. Antibiotic pressure compensates the biological cost associated with Pseudomonas aeruginosa hypermutable phenotypes in vitro and in a murine model of chronic airways infection. J Antimicrob Chemother 67:962–969. doi: 10.1093/jac/dkr587. [DOI] [PubMed] [Google Scholar]
- 22.Römling U, Kader A, Sriramulu DD, Simm R, Kronvall G. 2005. Worldwide distribution of Pseudomonas aeruginosa clone C strains in the aquatic environment and cystic fibrosis patients. Environ Microbiol 7:1029–1038. doi: 10.1111/j.1462-2920.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 23.Justo JA, Danziger LH, Gotfried MH. 2013. Efficacy of inhaled ciprofloxacin in the management of non-cystic fibrosis bronchiectasis. Ther Adv Respir Dis 7:272–287. doi: 10.1177/1753465813487412. [DOI] [PubMed] [Google Scholar]
- 24.Bragonzi A, Worlitzsch D, Pier GB, Timpert P, Ulrich M, Hentzer M, Andersen JB, Givskov M, Conese M, Doring G. 2005. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J Infect Dis 192:410–419. doi: 10.1086/431516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 27.Ferroni A, Guillemot D, Moumile K, Bernede C, Le Bourgeois M, Waernessyckle S, Descamps P, Sermet-Gaudelus I, Lenoir G, Berche P, Taddei F. 2009. Effect of mutator P. aeruginosa on antibiotic resistance acquisition and respiratory function in cystic fibrosis. Pediatr Pulmonol 44:820–825. doi: 10.1002/ppul.21076. [DOI] [PubMed] [Google Scholar]
- 28.Bragonzi A. 2010. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int J Med Microbiol 300:584–593. doi: 10.1016/j.ijmm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C. 2011. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol 186:5425–5434. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 30.Yu H, Boucher JC, Hibler NS, Deretic V. 1996. Virulence properties of Pseudomonas aeruginosa lacking the extreme-stress sigma factor AlgU (sigmaE). Infect Immun 64:2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarogoulidis P, Kioumis I, Porpodis K, Spyratos D, Tsakiridis K, Huang H, Li Q, Turner JF, Browning R, Hohenforst-Schmidt W, Zarogoulidis K. 2013. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 7:1115–1134. doi: 10.2147/DDDT.S51303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratjen F, Munck A, Kho P, Angyalosi G. 2010. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 65:286–291. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 33.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Doring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325. doi: 10.1172/JCI0213870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cigana C, Lore NI, Riva C, De Fino I, Spagnuolo L, Sipione B, Rossi G, Nonis A, Cabrini G, Bragonzi A. 2016. Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections. Sci Rep 6:21465. doi: 10.1038/srep21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Döring G, Bragonzi A, Paroni M, Akturk FF, Cigana C, Schmidt A, Gilpin D, Heyder S, Born T, Smaczny C, Kohlhaufl M, Wagner TO, Loebinger MR, Bilton D, Tunney MM, Elborn JS, Pier GB, Konstan MW, Ulrich M. 2014. BIIL 284 reduces neutrophil numbers but increases P. aeruginosa bacteremia and inflammation in mouse lungs. J Cyst Fibros 13:156–163. doi: 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stangl R. 2008. A nebulizer for rodent inhalation studies. Respir Drug Deliv 3:697–700. http://www.rddonline.com/publications/articles/article.php?ArticleID=1218&return=1. [Google Scholar]
- 37.Bivas-Benita M, Zwier R, Junginger HE, Borchard G. 2005. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm 61:214–218. doi: 10.1016/j.ejpb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:3923–3928. doi: 10.1128/AAC.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherrard LJ, Tunney MM, Elborn JS. 2014. Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet 384:703–713. doi: 10.1016/S0140-6736(14)61137-5. [DOI] [PubMed] [Google Scholar]