Abstract
Antimony (Sb) resistance in leishmaniasis chemotherapy has become one of the major challenges to the control of this spreading worldwide public health problem. Since the plasma membrane pore-forming protein aquaglyceroporin 1 (AQP1) is the major route of Sb uptake in Leishmania, functional studies are relevant to characterize drug transport pathways in the parasite. We generated AQP1-overexpressing Leishmania guyanensis and L. braziliensis mutants and investigated their susceptibility to the trivalent form of Sb (SbIII) in the presence of silver and nitrate salts. Both AQP1-overexpressing lines presented 3- to 4-fold increased AQP1 expression levels compared with those of their untransfected counterparts, leading to an increased SbIII susceptibility of about 2-fold. Competition assays using silver nitrate, silver sulfadiazine, or silver acetate prior to SbIII exposure increased parasite growth, especially in AQP1-overexpressing mutants. Surprisingly, SbIII-sodium nitrate or SbIII-potassium nitrate combinations showed significantly enhanced antileishmanial activities compared to those of SbIII alone, especially against AQP1-overexpressing mutants, suggesting a putative nitrate-dependent modulation of AQP1 activity. The intracellular level of antimony quantified by graphite furnace atomic absorption spectrometry showed that the concomitant exposure to SbIII and nitrate favors antimony accumulation in the parasite, increasing the toxicity of the drug and culminating with parasite death. This is the first report showing evidence of AQP1-mediated SbIII susceptibility modulation by silver in Leishmania and suggests the potential antileishmanial activity of the combination of nitrate salts and SbIII.
INTRODUCTION
Leishmaniasis is one of six diseases regarded by the World Health Organization to be major threats to developing countries. This complex disease is caused by different species of protozoan parasites belonging to the genus Leishmania. This disease is endemic in 98 countries, with approximately 350 million people being at risk and an estimated 12 million people being infected worldwide. Its clinical manifestations range from self-healing skin lesions in cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL) to visceral leishmaniasis (VL), which is lethal if left untreated. In the New World, Leishmania (Viannia) guyanensis and L. (Viannia) braziliensis cause both CL and MCL (1, 2). Approximately 0.7 million to 1.2 million cases of CL and 0.2 million to 0.4 million cases of VL are diagnosed each year (3). In the absence of an effective vaccine for humans, chemotherapy is still the main strategy used to control the disease; however, the emergence of drug-resistant Leishmania parasites has become a challenge for antimony-based leishmaniasis chemotherapy. The epicenter of antimony-resistant visceral leishmaniasis has been found to be in India, but antimony-unresponsive cases are reported worldwide, and these include cutaneous cases in the New World (4). Miltefosine (hexadecylphosphocholine) and amphotericin B (AMB), a polyene macrolide, have been chosen as first-line alternative drugs to treat leishmaniasis in the Indian subcontinent, where VL is endemic (5, 6). Although the evidence is scarce, recent evidence of clinical miltefosine unresponsiveness (7), cases of relapse after AMB treatment (8), and the selection of AMB-resistant Leishmania field isolates (9) indicate the current major threats of long-term drug exposure.
Pentavalent antimony-containing compounds (SbV) such as sodium stibogluconate (Pentostam) and N-methyl-glucamine (Glucantime) made from sodium or potassium hexahydroxoantimonate salts have been used as first-line therapies against all forms of leishmaniasis. They are prodrugs and must be reduced in vivo to the trivalent active form (SbIII) (10).
The major entry route of SbIII in Leishmania parasites is through aquaglyceroporin 1 (AQP1), a six-helix plasma transmembrane pore-forming protein that also allows the transport of water, glycerol, urea, and other small uncharged solutes and that thus plays an important role in osmoregulation (11). At physiological pH, the affinity of trivalent antimony through AQP1 is explained by its uncharged Sb(OH)3 form, which is structurally similar to glycerol (12). AQP1-mediated antimony sensitivity is species specific, with cutaneous leishmaniasis-associated species being more sensitive to the drug. This phenotype is a result of posttranscriptionally regulated AQP1 expression (13). Although the molecular basis of the mechanisms of resistance are not fully understood, the downregulation, mutation, and deletion of the AQP1-encoding gene have been clearly associated with Sb resistance in both laboratory-selected and field isolates of Leishmania (14–16). In Leishmania, AQP1 has also been implicated in the transport of the Sb-related metal arsenite (AsIII) (17). Indeed, disruption of the AQP1 homolog in the Leishmania-related parasite Trypanosoma brucei (the etiological agent of human African trypanosomiasis), TbAQP2, generates melarsoprol (As-based drug)-resistant parasites, and laboratory-selected melarsoprol-resistant trypanosomes also showed a disrupted TbAQP2 (18). In fact, T. brucei gambiense field isolates with reduced susceptibility to melarsoprol harbor TbAQP2 mutations and gene loss (19). A similar functional correlation was demonstrated in L. mexicana, in which the heterologous expression of TbAQP2 increased the sensitivity of arsenicals >1,000-fold (20).
We have recently proposed theoretical transport models for the characterization of Sb resistance by considering different pathways where decreased drug influx plays a critical role (21). Despite biophysical characterization, pharmacological approaches are useful for the study of drug resistance mechanisms in Leishmania. In this regard, specific AQP1 modulators would support functional in vitro drug transport and competition studies. The silver-containing compounds silver nitrate (AgNO3) and silver sulfadiazine (SAg) are known to inhibit plant aquaporins and human aquaporin 1 (hAQP1) more effectively than gold- or mercury-containing compounds (22). Here, we performed a functional analysis of the AQP1 transporter gene in Leishmania (Viannia) species and investigated the role of silver and nitrate on SbIII susceptibility in wild-type (WT) and AQP1-overexpressing parasites.
MATERIALS AND METHODS
Parasite cultures.
Promastigote forms of L. (Viannia) guyanensis (IUMB/BR/85/M9945) and L. (Viannia) braziliensis (MHOM/BR/75/M2904) were cultured in vitro at 26°C in medium M199 supplemented as described by Liarte and Murta (23).
Transfection of L. guyanensis and L. braziliensis with LbAQP1.
pIR1-BSD-LbAQP1 was constructed as described previously using blasticidin S deaminase (BSD) as a selectable marker (24). An 858-bp segment corresponding to the open reading frame of L. braziliensis AQP1 (LbAQP1; L. braziliensis M.31.0020; genome version 2013-01-16, available at tritrypdb.org) was amplified using forward primer 5′-tAGATCTccaccATGGCGATTGAAAACCACAT-3′ and reverse primer 5′-ttAGATCTCTACGCACCGCTCGGTATTA-3′, in which the underlined sequences correspond to the BgIII restriction site and the lowercase nucleotides correspond to the Kozak sequence. The PCR product obtained was cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), sequenced for confirmation of the correct fragment, and subcloned into the pIR1-BSD expression vector. The constructs pIR1-BSD (empty vector) and pIR1-BSD-LbAQP1 were linearized by SwaI digestion and electroporated into L. braziliensis and L. guyanensis lines using a GenePulser Xcell electroporation system (Bio-Rad, Hercules, CA, USA). This stable transfection allowed integration of the vector into the 18S ribosomal DNA small-subunit locus of the parasite (25).
Production of polyclonal antiserum and Western blotting.
Polyclonal antibodies against LbAQP1 were obtained in order to monitor protein overexpression. Representative peptides of aquaglyceroporin 1 were analyzed by the use of two predictors for B cells: the BEPIPRED and BCPRED12 programs (26). In addition, the sequences were subjected to analysis using SIGCLEAVE software for the identification of the signal peptide and TMHMM software to check the presence of transmembrane domains. The peptides QHFDDAGVMLLPNETMASKFSGVFVT and NPTRDLGPRIFTAMLWGKEPFTLHGY are considered highly immunogenic. Both peptides were synthetized using the 9-fluorenylmethoxy carbonyl synthesis technique developed by Merrifield (27). Polyclonal antiserum was produced in New Zealand White rabbits, whose use was approved by the appropriate ethical committee for animal research (CEUA-375/2012, Universidade Federal de Minas Gerais). The rabbits were inoculated with Freund's adjuvant (Sigma, St. Louis, MO, USA) containing 1,500 μg of both synthetic peptides on days 0, 15, and 30. The rabbits received three subcutaneous inoculations, the first with complete Freund's adjuvant and the other two with incomplete Freund's adjuvant. Serum was obtained 10 and 20 days after the last inoculation.
Western blotting was carried out to investigate the AQP1 protein levels in transfected Leishmania clonal lines. Protein extracts from L. braziliensis and L. guyanensis clonal lines were obtained as previously described (28). The protein concentration was determined by the Bradford method. Proteins (100 μg) were separated by electrophoresis on a 12% SDS-polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. The blots were incubated overnight in the presence of the anti-AQP1 antiserum at a dilution of 1:5. Membranes were then incubated for 1 h at 25°C with alkaline phosphatase-conjugated affinity-purified anti-rabbit IgG (Sigma, St. Louis, MO, USA) at a 1:6,000 dilution. The bands on the nitrocellulose membrane were then visualized by staining with 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT) following the manufacturer's instructions (Bio-Rad, Hercules, CA, USA).
SbIII and AgNO3 susceptibility assays.
The effective concentration required to decrease growth by 50% (EC50) for SbIII was determined using a Z1 Coulter Counter (Beckman Coulter, Fullerton, CA, USA), as described by Andrade and Murta (24). Promastigotes of wild-type L. guyanensis and L. braziliensis clonal lines transfected with the construct pIR1-BSD (empty vector) or pIR1-BSD-LbAQP1 or untransfected clonal lines were submitted to the SbIII susceptibility test. Parasites were seeded in medium M199 at 2 × 106 cells ml−1 in 24-well plates in the presence of several concentrations of SbIII (potassium antimonyl tartrate with Sb at concentrations of up to 3 mM) and AgNO3 (0.125 to 10 μM) for 48 h. EC50s were determined from three independent measurements performed in triplicate, using the linear interpolation method (29).
Competition assays.
Since AgNO3 has been described to be a potential inhibitor of AQP1 (22), we performed competition assays using different sources of silver or nitrate salts (AgNO3, SAg, AgC2H3O2, NaNO3, KNO3) concomitantly or not with SbIII (potassium antimonyl tartrate) to better investigate the role of silver and/or nitrate on the modulation of AQP1 activity. All reagents were purchased from Sigma and were of the purest grade available. First, we determined the EC50 of each compound. For competition assays, 2 × 106 parasites were seeded into 24-well cell culture plates containing medium M199. Subsequently, the EC50 of each compound (AgNO3, AgS, AgC2H3O2, NaNO3, or KNO3) was added to the parasite culture 5 min prior to SbIII treatment (with the corresponding SbIII EC50s; see Table 1), followed by incubation for 48 h. Growth as a percentage of that of the parental WT was determined by automated cell counting using a Z1 Coulter Counter.
TABLE 1.
EC50s of SbIII and AgNO3 and corresponding SIs for wild-type or LbAQP1-overexpressing Leishmania (Viannia) speciesa
| Leishmania line | SbIII |
AgNO3 |
||
|---|---|---|---|---|
| EC50 (95% CI) (μM) | SI | EC50 (95% CI) (μM) | SI | |
| L. guyanensis WT | 48.72 (43.09–55.08) | 1.37 (1.25–1.49) | ||
| L. guyanensis/pIR1-BSD | 48.96 (42.79–56.02) | 1.4 (1.28–1.51) | ||
| L. guyanensis/pIR1-BSD-AQP1 cl 16 | 30.09 (27.21–33.26)** | 0.61 | 1.62 (1.47–1.8)* | 1.18 |
| L. guyanensis/pIR1-BSD-AQP1 cl 18 | 29.50 (26.12–33.31)** | 0.6 | 1.61 (1.48–1.74)* | 1.17 |
| L. braziliensis WT | 108 (103.3–112.9) | 0.95 (0.88–1.03) | ||
| L. braziliensis/pIR1-BSD | 94.21 (88.06–100.8) | 0.94 (0.86–1.02) | ||
| L. braziliensis/pIR1-BSD-AQP1 cl 12 | 40.94. (37.94–44.17)*** | 0.38 | 1.5 (1.4–1.62)*** | 1.56 |
| L. braziliensis/pIR1-BSD-AQP1 cl 15 | 46 (43.05–49.13)*** | 0.42 | 1.57 (1.47–1.67)*** | 1.65 |
Abbreviations and symbols: CI, confidence interval; cl, clone; SI, susceptibility index, which is equal to the EC50 for the LgAQP1-overexpressing isolate/EC50 for the WT isolate; ***, P < 0.0001; **, P = 0.0012; *, P = 0.0121 (analysis of variance). EC50s are averages from at least two independent experiments performed in triplicate and were obtained after 48 h of exposure to potassium antimonyl tartrate, which was used as the SbIII source (Sigma-Aldrich, St. Louis, MO, USA), or AgNO3 (silver nitrate).
Antimony uptake.
The intracellular accumulation of antimony was measured as previously reported (30). Briefly, log-phase Leishmania promastigotes were washed twice with HEPES-glucose (HG) buffer (20 mM HEPES, 0.15 M NaCl, 10 mM glucose, pH 7.2) and resuspended in this buffer at a density of 1.0 × 108 cells/ml. This parasite suspension was aliquoted (1 ml) and placed in quadruplicate into tubes containing (i) parasites only (blank), (ii) parasites and 540 μM SbIII (potassium antimonyl tartrate, positive control), (iii) parasites and 540 μM SbIII plus 30 μM AgC2H3O2, (iv) parasites and 540 μM SbIII plus 160 μM NaNO3, and (v) parasites and 540 μM SbIII plus 120 μM KNO3. Parasites treated only with SbIII and silver or nitrate salt were used as controls. Cells were then incubated for 1 h, centrifuged, and washed three times with HG buffer. The pellets were then resuspended in 100 μl of HG buffer. One aliquot of 10 μl from each tube was used for normalization (parasite quantification); after the last centrifugation, the pellet was resuspended in 100 μl of nitric acid (65%) for cell digestion. The intracellular antimony content was quantified by graphite furnace atomic absorption spectrometry (AAnalyst 600; PerkinElmer). Each uptake assay was performed three times, and the Sb dosage from blanks was used for background subtraction. The total Sb content was normalized by 1.0 × 108 promastigote forms.
RESULTS
Overexpression of AQP1 in Leishmania spp.
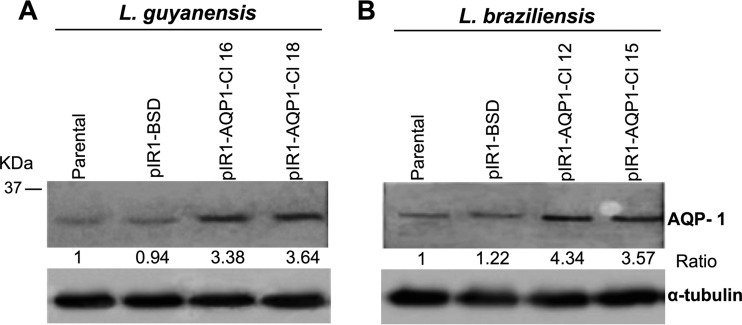
In order to evaluate whether the overexpression of AQP1 interferes with the SbIII resistance phenotype, L. guyanensis and L. braziliensis lines were transfected with the LbAQP1 gene. To confirm the transfection, genomic DNA from the transfected clones was subjected to PCR using primers specific for the BSD gene, which confers resistance to blasticidin (data not shown) (31). Leishmania clones PCR positive for the BSD gene were subjected to a Western blot assay to assess the AQP1 expression levels in the transfected parasites. The AQP1 expression level in LbAQP1-overexpressing L. braziliensis and L. guyanensis (wild-type background) was 3.3- to 4.3-fold higher than that in the nontransfected parental counterpart. AQP1 expression levels were normalized by the level of expression of α-tubulin (Fig. 1). These transfected clones were subjected to an SbIII susceptibility test and competition assays using silver and nitrate salts.
FIG 1.
LbAQP1 expression levels in Leishmania (Viannia) species. (A) L. guyanensis and (B) L. braziliensis. Proteins (100 μg) were separated by electrophoresis on a 12% SDS-polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. The blots were probed with an anti-AQP1 antiserum at a dilution of 1:5 and with alkaline phosphatase-conjugated affinity-purified anti-rabbit IgG at a 1:6,000 dilution and developed with NBT-BCIP. First lane in each panel, untransfected parental wild-type strains; second lane in each panel, parental wild type transfected with pIR1-BSD (empty expression vector); third and fourth lanes in each panel, clonal lines (Cl) constitutively expressing LbAQP1 (transfected with the expression vector pIR1-BSD containing LbAQP1 18S ribosomal small-subunit DNA integration cassette). Densitometric analysis of the bands on the Western blot was performed by normalization to the α-tubulin antibody signal and performed using ImageJ software (version 1.48).
Susceptibility of Leishmania spp. to potassium antimonyl tartrate (SbIII) and silver nitrate.
Antileishmanial drug susceptibility assays were carried out in order to evaluate the SbIII and AgNO3 sensitivity phenotypes of L. guyanensis and L. braziliensis. Table 1 depicts the half-maximal effective concentration (EC50) values for the different Leishmania lines overexpressing or not expressing AQP1. Although they are members of the same subgenus, L. guyanensis was two times more sensitive to SbIII than L. braziliensis, presenting EC50s of 48.72 and 108 μM, respectively. The overexpression of AQP1 in both L. guyanensis and L. braziliensis resulted in 1.6-fold and 2.3- to 2.6-fold greater susceptibility to SbIII, respectively, with the corresponding susceptibility indexes (SIs) being approximately 0.6 and 0.4 (Table 1). Upon AgNO3 treatment, AQP1-overexpressing mutants presented a slightly increased resistance phenotype, with the L. guyanensis and L. braziliensis mutants showing SIs of approximately 1.2 and 1.6, respectively (Table 1). This observation suggests that AQP1 would also behave as a target for AgNO3 in Leishmania. Additionally, AgNO3 was highly toxic against Leishmania parasites, showing EC50s of approximately 1 μM (Table 1). The drug susceptibilities of parasites transfected with the empty vector were not different from those of their wild-type counterparts.
Combined SbIII-silver or SbIII-nitrate decreases or enhances antimony sensitivity in Leishmania, respectively.
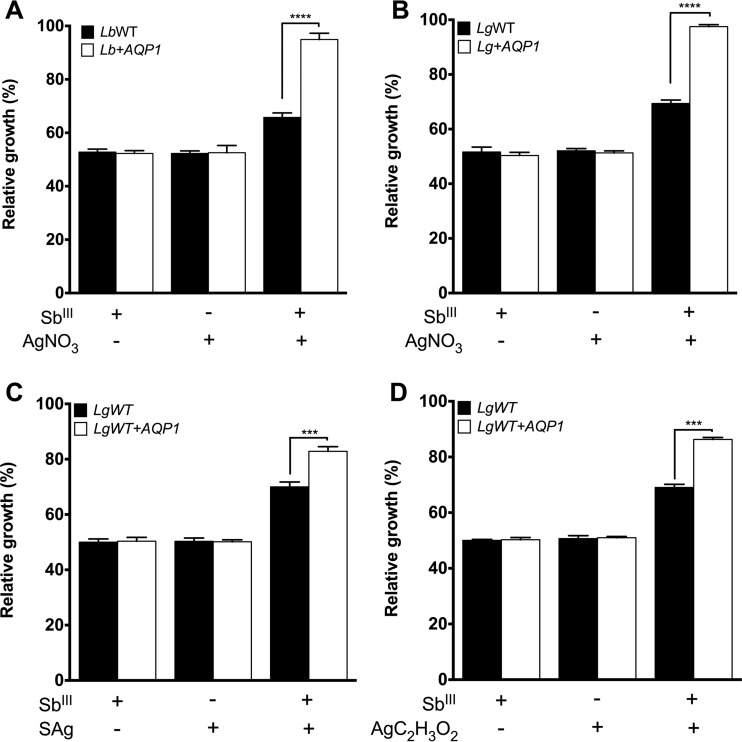
Since AgNO3 has been described to be an aquaporin inhibitor, we performed a competition assay using AgNO3 concomitantly with or not concomitantly with SbIII treatment. Similar growth profiles were observed when the L. guyanensis and L. braziliensis WT strains were exposed to the drug combinations, leading to reduced sensitivity (Fig. 2A and B). This behavior could be explained by a reduced SbIII activity influenced by AgNO3. In order to investigate the role of AQP1, AQP1-overexpressing L. guyanensis and L. braziliensis mutants were submitted to a competition assay, as described above. Both L. guyanensis and L. braziliensis AQP1-overexpressing mutants became resistant at 48 h under AgNO3-SbIII exposure. Indeed, all LbAQP1-overexpressing mutants presented results statistically significantly different from those for the WT strains when they were exposed to the drug combination (Fig. 2).
FIG 2.
Effect of silver nitrate (AgNO3) (A and B), silver sulfadiazine (SAg) (C), and silver acetate (AgC2H3O2) (D) on the growth of Leishmania (Viannia) species upon SbIII exposure. (A) L. braziliensis; (B to D) L. guyanensis. Parasites (2 × 106) were seeded into 24-well cell culture plates containing medium M199. Cells were exposed to the EC50s of SbIII and AgNO3 (Table 1), SAg (3.0 and 3.5 μM for WT L. guyanensis and WT L. guyanensis overexpressing AQP1, respectively), and AgC2H3O2 (3.1 and 3.6 μM for WT L. guyanensis and WT L. guyanensis overexpressing AQP1, respectively) independently or were treated with the silver salt 5 min prior to SbIII exposure, followed by incubation for 48 h. Parasite numbers were quantified by cell counting using a Coulter Counter. The data represent the means ± standard errors from three independent experiments performed in triplicate. Statistical analysis was carried out using Student's t test and one-way analysis of variance followed by Bonferroni's multiple-comparison test. ***, P < 0.001; ****, P < 0.0001. LbWT, L. braziliensis WT; Lb+AQP1, L. braziliensis/pIR1-BSD-LbAQP1 clone 12; LgWT, L. guyanensis WT; Lg+AQP1, L. guyanensis/pIR1-BSD-LbAQP1 clone 18. The growth of Leishmania mutants transfected with pIR1-BSD only (empty vector) was similar to that of the parental WT (data not shown).
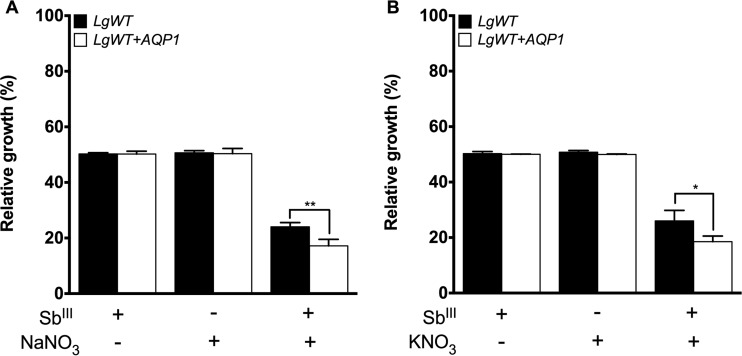
These results show evidence of drug susceptibility modulation by the AgNO3 and SbIII combination mediated by AQP1. To investigate the role of silver on the modulation of susceptibility mediated by AQP1, we performed functional competition assays with WT and AQP1-overexpressing L. guyanensis lines using different silver and nitrate sources. The silver sulfadiazine (SAg) EC50s were 3 and 3.5 μM for the wild-type and AQP1-overexpressing L. guyanensis lines, respectively. These values were very similar to the silver acetate EC50s for both lines, which were 3.1 μM and 3.6 μM, respectively. On the other hand, nitrate salts were less toxic to the Leishmania lines than the silver salts. The potassium nitrate EC50s were 120 and 160 μM for the wild-type and AQP1-overexpressing L. guyanensis lines, respectively, whereas the sodium nitrate EC50 was 160 μM for both Leishmania lines. When both parasites were exposed to the SbIII-silver sulfadiazine or SbIII-silver acetate drug combination, they became more resistant to SbIII (Fig. 2C and D), as was observed for SbIII-silver nitrate. Moreover, this increased SbIII resistance was higher in AQP1-overexpressing L. guyanensis parasites than in WT L. guyanensis (Fig. 2C and D, white bars). Surprisingly, a pronounced antileishmanial activity was observed when both WT and AQP1-overexpressing L. guyanensis parasites were exposed to the SbIII-sodium nitrate and SbIII-potassium nitrate combinations (Fig. 3A and B). Additionally, this increased SbIII susceptibility was higher in AQP1-overexpressing L. guyanensis parasites than in the WT L. guyanensis parasites (Fig. 3C and D), suggesting the involvement of AQP1 in this activity. No change in the relative growth of parasites incubated only with the EC50 of each nitrate salt was observed (Fig. 3).
FIG 3.
Effect of sodium nitrate (NaNO3) and potassium nitrate (KNO3) on the growth of L. (Viannia) guyanensis lines upon SbIII exposure. Parasites (2 × 106) were seeded into 24-well cell culture plates containing medium M199. Cells were exposed to the EC50 of SbIII (48.72 and 29.50 μM for the L. guyanensis WT and the L. guyanensis WT overexpressing AQP1, respectively) and the EC50 of each compound (160 μM NaNO3 for both lines; 120 and 160 μM KNO3 for the L. guyanensis WT and the L. guyanensis WT overexpressing AQP1, respectively) independently or were treated with each compound for 5 min prior to SbIII exposure, followed by incubation for 48 h. Parasite numbers were quantified by cell counting using a Coulter Counter. The data represent the means ± standard errors from three independent experiments performed in triplicate. Statistical analysis was carried out using Student's t test and one-way analysis of variance followed by Bonferroni's multiple-comparison test. *, P < 0.04; **, P < 0.001. LgWT, L. guyanensis WT; Lg+AQP1, L. guyanensis/pIR1-BSD-LbAQP1 clone 18.
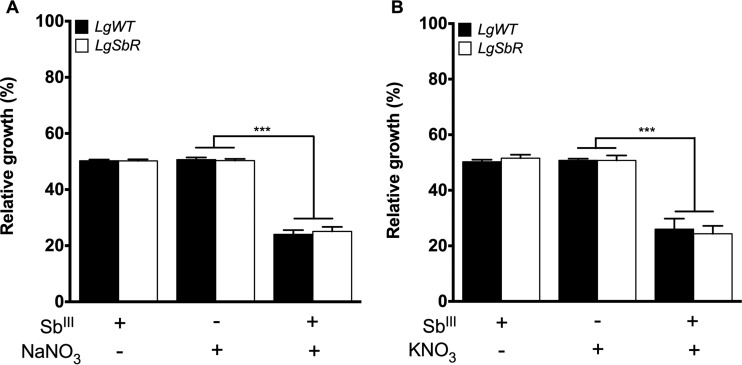
In order to further investigate this antileishmanial activity, an SbIII-resistant L. guyanensis line was used in this competition assay. This resistant line was previously selected in vitro by stepwise increased SbIII pressure and is 19-fold more resistant than its WT susceptible counterpart (23). One of the promising findings here is that SbIII-resistant parasites were also susceptible to treatment with the SbIII-sodium nitrate and SbIII-potassium nitrate combinations, similar to the findings for WT L. guyanensis (Fig. 4A and B). This result indicates that the combination of nitrate salts and SbIII is toxic for Leishmania, even SbIII-resistant parasites, reverting this phenotype and leading to antimony resensitization.
FIG 4.
Effect of sodium nitrate (NaNO3) and potassium nitrate (KNO3) on the growth of L. (Viannia) guyanensis wild-type (LgWT) and SbIII-resistant (LgSbR) lines upon SbIII exposure. Parasites (2 × 106) were seeded into 24-well cell culture plates containing medium M199. Cells were exposed to the EC50 of SbIII (48.72 and 912 μM for the L. guyanensis WT and the SbIII-resistant L. guyanensis, respectively) and the EC50 of each compound (160 and 200 μM NaNO3 for the L. guyanensis WT and SbIII-resistant L. guyanensis, respectively; 120 and 180 μM KNO3 for the L. guyanensis WT and SbIII-resistant L. guyanensis, respectively) independently or were treated with each compound for 5 min prior to SbIII exposure, followed by incubation for 48 h. Parasite numbers were quantified by cell counting using a Coulter Counter. The data represent the means ± standard errors from three independent experiments performed in triplicate. Statistical analysis was carried out using Student's t test and one-way analysis of variance followed by Bonferroni's multiple-comparison test. ***, P < 0.0001.
The SbIII-nitrate combination favors intracellular antimony accumulation in Leishmania guyanensis.
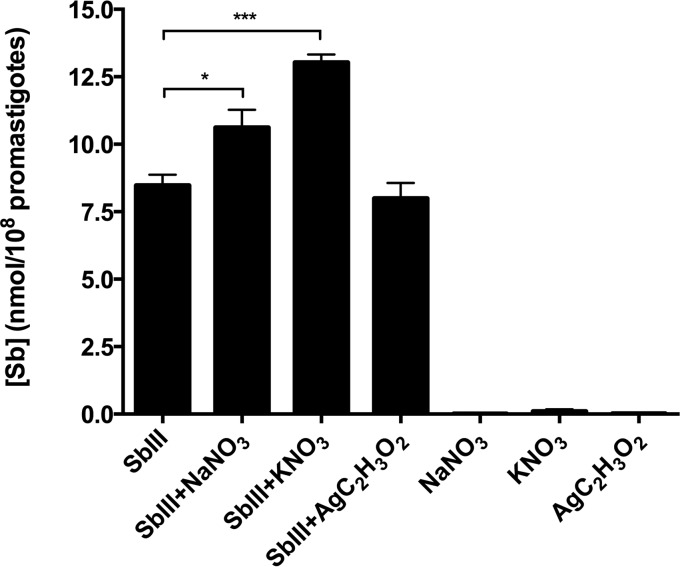
We evaluated the level of antimony uptake in wild-type L. guyanensis parasites incubated in the presence of SbIII and silver or nitrate salts. Interestingly, parasites treated with SbIII-sodium nitrate and SbIII-potassium nitrate exhibited higher intracellular antimony concentrations than parasites exposed only to SbIII (control) and SbIII-silver acetate (Fig. 5). This result shows that the combination of SbIII and nitrate favors SbIII accumulation in the parasite, increasing drug toxicity and leading to parasite death. Further investigations are needed to mechanistically explore the transport pathways involved in this accumulation of increased amounts of antimony.
FIG 5.
Levels of antimony in promastigote forms of L. (Viannia) guyanensis wild-type (LgWT) incubated in the presence of SbIII, SbIII-sodium nitrate (NaNO3), SbIII-potassium nitrate (KNO3), or SbIII-silver acetate (AgC2H3O2). As a control, the parasites were incubated only with sodium nitrate, potassium nitrate, or silver acetate. The intracellular level of antimony was quantified by graphite furnace atomic absorption spectrometry. The data represent the means ± standard errors from three independent experiments performed in quadruplicate. Statistical analysis was carried out using Student's t test and one-way analysis of variance followed by Bonferroni's multiple-comparison test. *, P < 0.001; ***, P < 0.0004.
In order to investigate the formation of novel chemical entities in culture medium upon exposure to antimonyl tartrate and nitrate salts combined, mass spectrometry analysis was conducted; however, no complexes were identified (data not shown).
DISCUSSION
Drug resistance is one of the major barriers to successful leishmaniasis chemotherapy. Molecular mechanisms of antimony resistance in Leishmania are complex and multifactorial and take place by several pathways, such as entry, metabolism, efflux, sequestration, and cell death (32). Since the plasma membrane pore-forming protein aquaglyceroporin 1 (AQP1) is the major route of Sb uptake in Leishmania, we performed functional studies to characterize drug transport pathways in the parasite. In order to investigate AQP1 involvement in this process, AQP1-overexpressing L. guyanensis and L. braziliensis mutants were obtained and treated with SbIII in the presence of silver or nitrate salts to investigate the role of these ions in the modulation of AQP1 activity.
Protein levels of LbAQP1-overexpressing parasites were determined by Western blot analysis. Both L. guyanensis and L. braziliensis presented at least 3.3 times increased AQP1 levels than their parental counterparts. Unlike previously described anti-AQP1 antibodies (33), the polyclonal antibodies presented here were able to recognize AQP1 in both untransfected and AQP1-overexpressing parasites, an important tool to monitor Leishmania AQP1 expression at the relevant protein level in resistant mutant isolates either selected in the laboratory or collected in the field.
Functional analysis showed that L. guyanensis and L. braziliensis clones overexpressing AQP1 were 1.6- and 2.6-fold more susceptible to SbIII, respectively, than the nontransfected parental lines. Gourbal et al. also observed that AQP1-overexpressing L. tarentolae, L. major, and L. infantum parasites presented hypersensitivity to both AsIII and SbIII compounds (34). These parasites exhibited higher rates of uptake of both metalloids than those transfected with the empty vector. These authors also showed that transfection of the AQP1 gene into a sodium stibogluconate-resistant clinical isolate sensitized amastigote forms of the parasite to SbIII. On the other hand, the deletion of one allele of the L. mexicana AQP1 (LmAQP1) gene resulted in a mutant parasite 10-fold more resistant to SbIII (34). A dominant negative functional cloning strategy led to the isolation of a cosmid containing the AQP1 gene, showing that AQP1 is an important route of entry of SbIII in Leishmania cells (35). These authors further showed that transfection of AQP1 increases the level of SbIII accumulation in cells. The findings presented here are in accordance with all these data obtained in studies using different Leishmania species, showing that transfection of AQP1 leads to SbIII sensitization.
Since LbAQP1 is harbored on polyploid chromosome 31 (36), it was thought to be essential for Leishmania spp. (37). However, we have recently identified an Sb-resistant L. guyanensis strain from which a locus containing LgAQP1 was deleted during an in vitro stepwise drug selection process (16). Additionally, LmAQP1 was successfully disrupted in L. major null mutants (38). These observations discourage a focus on AQP1 as a drug target; however, further studies are needed to address the development of rational strategies.
Silver is a metal that presents low toxicity for mammalian cells, and silver salts, such as silver nitrate and silver sulfadiazine, present antimicrobial activity (39, 40). When Staphylococcus aureus and Escherichia coli are exposed to silver nitrate, the Ag+ ions activate the stress response and DNA damage, preventing cell replication and causing disruption of the cell wall (39). Our results revealed that silver salts (silver nitrate, silver sulfadiazine, and silver acetate) are highly toxic against Leishmania parasites, showing EC50s of 1 to 3 μM. Under cell culture medium conditions, AgNO3 dissociation is favored by the surrounding NaCl and proteins, where Ag+ forms colloidal dispersed AgCl nanocomplexes related to cell toxicity (41).
Silver compounds were previously described to be potential inhibitors of aquaporins from plants and humans (22). In order to check the inhibitory potential of silver and nitrate ions, we performed competition assays on L. braziliensis and L. guyanensis lines overexpressing AQP1. Interestingly, our results showed a reduced sensitivity to SbIII upon cotreatment with SbIII and silver nitrate, SbIII and silver sulfadiazine, or SbIII and silver acetate. Metal ions, such as gold and silver ions, were described to be potent inhibitors of water transport through AQPs. Silver reacts with the sulfhydryl group from a motif conserved near the NPA (asparagine-proline-alanine) cysteine of the AQPs and then effectively blocks the region of channel constriction (22). The reduced SbIII sensitivity phenotype that emerged upon silver treatment is compatible with the suggestion that the metal inhibits AQP1, since lines that overexpressed AQP1 presented pronounced differences in sensitivity from that of lines that did not. This is the first report showing evidence of the modulation of the drug susceptibility mediated by AQP1 in Leishmania by silver and SbIII in combination. Thus, modulation of the drug susceptibility phenotype by silver can be a pharmacological tool to study AQP1-mediated drug resistance in Leishmania parasites.
Competition assays revealed that cotreatment with sodium or potassium nitrate and SbIII enhanced the susceptibility of Leishmania parasites to SbIII, especially AQP1-overexpressing mutants. Our results also showed that nitrate compounds presented very low toxicity against Leishmania and had high EC50s (120 to 160 μM). To further investigate this antileishmanial activity, we measured the amount of antimony accumulated in the L. guyanensis wild-type parasites incubated in the presence of SbIII and silver or nitrate salts. The data showed high intracellular antimony concentrations in parasites incubated with SbIII and sodium or potassium nitrate. Interestingly, the results showed that antimony-mediated antileishmanial activity is enhanced when antimony is combined with a nitrate source. Indeed Sb-nitrate cotreatment favors intracellular antimony accumulation, increasing drug toxicity and leading to parasite death. We checked by electrospray ionization-mass spectrometry that potassium antimonyl tartrate, used as a source of SbIII, did not form any new chemical species when mixed with potassium or sodium nitrate (data not shown). Thus, our data taken altogether indicate an additional effect of nitrate on SbIII activity, explaining the expression of antileishmanial activity, and suggest the possible modulation of AQP1 activity by nitrate.
Studies indicated that AQP6 may function as a nitrate channel in mammalian cells (42). However, unlike other aquaporins, AQP6 is permeated by ions. AQP1 from Leishmania, the closest homologue of human AQP9, is permeated by water or small uncharged solutes, and nitrate ions probably do not pass through AQP1. In aqueous solution at physiological pH, trivalent antimoniate (SbIII) exists mainly in the trihydroxylated uncharged form, Sb(OH)3, which structurally resembles glycerol (12). Thus, the major entry route of SbIII in Leishmania parasites is through AQP1, which water and glycerol are able to permeate. How nitrate may modulate the activity of AQP1 requires further evaluation. It has been suggested that SbIII can also enter cells via hexose transporters (43), and nitrate could perhaps facilitate SbIII entry into the cell using this route.
The findings presented here show that SbIII-resistant L. guyanensis parasites are also susceptible to treatment with the SbIII-sodium nitrate and SbIII-potassium nitrate combinations. This result indicates that this cotreatment also affects the mechanisms of SbIII resistance, as the SbIII-resistant parasite is resensitized. Previous results demonstrated that this SbIII-resistant L. guyanensis line presents a decrease in AQP1 expression and reduced antimony accumulation (30). Interestingly, both of these SbIII and nitrate combinations are very toxic for these resistant parasites. These results suggesting the potential of combined treatment with nitrate salts and SbIII to have antileishmanial activity are very promising and should be further explored.
Our findings showed that overexpression of the AQP1 gene increased the sensitivity of L. braziliensis and L. guyanensis to SbIII. In addition, the present study suggests for the first time the role of silver as a putative AQP1 inhibitor in Leishmania, reducing SbIII entry into the cell and thereby making it more resistant to SbIII. Interestingly, treatment with nitrate salts and SbIII favors the accumulation of antimony into the parasite and increases the toxicity of the drug, culminating with parasite death. These data support the rational drug development approaches urgently needed to overcome resistance.
ACKNOWLEDGMENTS
We thank Stephen Beverley for kindly providing the pIR1-BSD expression vector and the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for the use of its facilities.
Funding Statement
This study received financial support from the following agencies: CNPq/Universal (475782/2012-7), CNPq/BJT (401936/2013-9), CNPq/Pq (304483/2015-0), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-CBB-PPM00196/13 and 00610/15), UNICEF/UNDP/World Bank/WHO (TDR), and the P3D-Programa de Descoberta e Desenvolvimento de Drogas (PROEP/CNPq/FIOCRUZ 401988/2012-0). R.L.M.-N. and S.M.F.M. are researchers supported by CNPq-Brasil (National Council for the Scientific and Technological Development). J.M.A. is supported by a doctoral fellowship from CAPES (Coordination for the Improvement of Higher Education Personnel). R.L.M.-N. holds a young talent fellowship (CNPq/BJT-A 314024/2013-1).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Guerra JA, Prestes SR, Silveira H, Coelho LI, Gama P, Moura A, Amato V, Barbosa M, Ferreira LC. 2011. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis 5:e980. doi: 10.1371/journal.pntd.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verastegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis 195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 5.Banjara MR, Hirve S, Siddiqui NA, Kumar N, Kansal S, Huda MM, Das P, Rijal S, Gurung CK, Malaviya P, Arana B, Kroeger A, Mondal D. 2012. Visceral leishmaniasis clinical management in endemic districts of India, Nepal, and Bangladesh. J Trop Med 2012:126093. doi: 10.1155/2012/126093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman J. 2015. Amphotericin B formulations and other drugs for visceral leishmaniasis. Am J Trop Med Hyg 92:471–473. doi: 10.4269/ajtmh.14-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorlo TP, Rijal S, Ostyn B, de Vries PJ, Singh R, Bhattarai N, Uranw S, Dujardin JC, Boelaert M, Beijnen JH, Huitema AD. 2014. Failure of miltefosine in visceral leishmaniasis is associated with low drug exposure. J Infect Dis 210:146–153. doi: 10.1093/infdis/jiu039. [DOI] [PubMed] [Google Scholar]
- 8.Morizot G, Jouffroy R, Faye A, Chabert P, Belhouari K, Calin R, Charlier C, Miailhes P, Siriez JY, Mouri O, Yera H, Gilquin J, Tubiana R, Lanternier F, Mamzer MF, Legendre C, Peyramond D, Caumes E, Lortholary O, Buffet P. 2016. Antimony to cure visceral leishmaniasis unresponsive to liposomal amphotericin B. PLoS Negl Trop Dis 10:e0004304. doi: 10.1371/journal.pntd.0004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 56:1031–1041. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaked-Mishan P, Ulrich N, Ephros M, Zilberstein D. 2001. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem 276:3971–3976. doi: 10.1074/jbc.M005423200. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharjee H, Rosen BP, Mukhopadhyay R. 2009. Aquaglyceroporins and metalloid transport: implications in human diseases. Handb Exp Pharmacol 16:309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porquet A, Filella M. 2007. Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: implications for their uptake. Chem Res Toxicol 20:1269–1276. doi: 10.1021/tx700110m. [DOI] [PubMed] [Google Scholar]
- 13.Mandal G, Mandal S, Sharma M, Charret KS, Papadopoulou B, Bhattacharjee H, Mukhopadhyay R. 2015. Species-specific antimonial sensitivity in Leishmania is driven by post-transcriptional regulation of AQP1. PLoS Negl Trop Dis 9:e0003500. doi: 10.1371/journal.pntd.0003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal S, Maharjan M, Singh S, Chatterjee M, Madhubala R. 2010. Assessing aquaglyceroporin gene status and expression profile in antimony-susceptible and -resistant clinical isolates of Leishmania donovani from India. J Antimicrob Chemother 65:496–507. doi: 10.1093/jac/dkp468. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee A, Boisvert S, Monte-Neto RL, Coelho AC, Raymond F, Mukhopadhyay R, Corbeil J, Ouellette M. 2013. Telomeric gene deletion and intrachromosomal amplification in antimony-resistant Leishmania. Mol Microbiol 88:189–202. doi: 10.1111/mmi.12178. [DOI] [PubMed] [Google Scholar]
- 16.Monte-Neto R, Laffitte MC, Leprohon P, Reis P, Frezard F, Ouellette M. 2015. Intrachromosomal amplification, locus deletion and point mutation in the aquaglyceroporin AQP1 gene in antimony resistant Leishmania (Viannia) guyanensis. PLoS Negl Trop Dis 9:e0003476. doi: 10.1371/journal.pntd.0003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brochu C, Wang J, Roy G, Messier N, Wang XY, Saravia NG, Ouellette M. 2003. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob Agents Chemother 47:3073–3079. doi: 10.1128/AAC.47.10.3073-3079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker N, Glover L, Munday JC, Aguinaga Andres D, Barrett MP, de Koning HP, Horn D. 2012. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A 109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf FE, Ludin P, Wenzler T, Kaiser M, Brun R, Pyana PP, Buscher P, de Koning HP, Horn D, Maser P. 2013. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl Trop Dis 7:e2475. doi: 10.1371/journal.pntd.0002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munday JC, Eze AA, Baker N, Glover L, Clucas C, Aguinaga Andres D, Natto MJ, Teka IA, McDonald J, Lee RS, Graf FE, Ludin P, Burchmore RJ, Turner CM, Tait A, MacLeod A, Maser P, Barrett MP, Horn D, De Koning HP. 2014. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J Antimicrob Chemother 69:651–663. doi: 10.1093/jac/dkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frézard F, Monte-Neto RL, Reis PG. 2014. Antimony transport mechanisms in resistant Leishmania. Biophys Rev 6:119–132. doi: 10.1007/s12551-013-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemietz CM, Tyerman SD. 2002. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett 531:443–447. doi: 10.1016/S0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- 23.Liarte DB, Murta SM. 2010. Selection and phenotype characterization of potassium antimony tartrate-resistant populations of four New World Leishmania species. Parasitol Res 107:205–212. doi: 10.1007/s00436-010-1852-8. [DOI] [PubMed] [Google Scholar]
- 24.Andrade JM, Murta SM. 2014. Functional analysis of cytosolic tryparedoxin peroxidase in antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum lines. Parasit Vectors 7:406. doi: 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson KA, Beverley SM. 2003. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol 128:217–228. doi: 10.1016/S0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 26.Larsen JE, Lund O, Nielsen M. 2006. Improved method for predicting linear B-cell epitopes. Immunome Res 2:2. doi: 10.1186/1745-7580-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrifield RB. 1969. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol 32:221–296. [DOI] [PubMed] [Google Scholar]
- 28.Matrangolo FS, Liarte DB, Andrade LC, de Melo MF, Andrade JM, Ferreira RF, Santiago AS, Pirovani CP, Silva-Pereira RA, Murta SM. 2013. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol Biochem Parasitol 190:63–75. doi: 10.1016/j.molbiopara.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Huber W, Koella JC. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55:257–261. doi: 10.1016/0001-706X(93)90083-N. [DOI] [PubMed] [Google Scholar]
- 30.Moreira DS, Monte Neto RL, Andrade JM, Santi AM, Reis PG, Frezard F, Murta SM. 2013. Molecular characterization of the MRPA transporter and antimony uptake in four New World Leishmania spp. susceptible and resistant to antimony. Int J Parasitol Drugs Drug Resist 3:143–153. doi: 10.1016/j.ijpddr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyard S, Beverley SM. 2000. Blasticidin resistance: a new independent marker for stable transfection of Leishmania. Mol Biochem Parasitol 108:249–252. doi: 10.1016/S0166-6851(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 32.Jeddi F, Piarroux R, Mary C. 2011. Antimony resistance in leishmania, focusing on experimental research. J Trop Med 2011:695382. doi: 10.1155/2011/695382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. 2007. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol 65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 34.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem 279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 35.Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. 2005. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol Microbiol 57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- 36.Rogers MB, Hilley JD, Dickens NJ, Wilkes J, Bates PA, Depledge DP, Harris D, Her Y, Herzyk P, Imamura H, Otto TD, Sanders M, Seeger K, Dujardin JC, Berriman M, Smith DF, Hertz-Fowler C, Mottram JC. 2011. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res 21:2129–2142. doi: 10.1101/gr.122945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal G, Sharma M, Kruse M, Sander-Juelch C, Munro LA, Wang Y, Vilg JV, Tamas MJ, Bhattacharjee H, Wiese M, Mukhopadhyay R. 2012. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol 85:1204–1218. doi: 10.1111/j.1365-2958.2012.08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plourde M, Ubeda JM, Mandal G, Monte-Neto RL, Mukhopadhyay R, Ouellette M. 2015. Generation of an aquaglyceroporin AQP1 null mutant in Leishmania major. Mol Biochem Parasitol 201:108–111. doi: 10.1016/j.molbiopara.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. 2000. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668. doi:. [DOI] [PubMed] [Google Scholar]
- 40.Kong H, Jang J. 2008. Antibacterial properties of novel poly(methyl methacrylate) nanofiber containing silver nanoparticles. Langmuir 24:2051–2056. doi: 10.1021/la703085e. [DOI] [PubMed] [Google Scholar]
- 41.Loza K, Sengstock C, Chernousova S, Koller M, Epple M. 2014. The predominant species of ionic silver in biological media is colloidally dispersed nanoparticulate silver chloride. RSC Advances 4:35290–35297. doi: 10.1039/C4RA04764H. [DOI] [Google Scholar]
- 42.Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. 2002. Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem 277:39873–39879. [DOI] [PubMed] [Google Scholar]
- 43.Maciaszczyk-Dziubinska E, Wawrzycka D, Wysocki R. 2012. Arsenic and antimony transporters in eukaryotes. Int J Mol Sci 13:3527–3548. doi: 10.3390/ijms13033527. [DOI] [PMC free article] [PubMed] [Google Scholar]