Abstract
The implementation of antimicrobial stewardship programs (ASPs) is a promising strategy to help address the problem of antimicrobial resistance. We sought to determine the efficacy of ASPs and their effect on clinical and economic parameters. We searched PubMed, EMBASE, and Google Scholar looking for studies on the efficacy of ASPs in hospitals. Based on 26 studies (extracted from 24,917 citations) with pre- and postimplementation periods from 6 months to 3 years, the pooled percentage change of total antimicrobial consumption after the implementation of ASPs was −19.1% (95% confidence interval [CI] = −30.1 to −7.5), and the use of restricted antimicrobial agents decreased by −26.6% (95% CI = −52.3 to −0.8). Interestingly, in intensive care units, the decrease in antimicrobial consumption was −39.5% (95% CI = −72.5 to −6.4). The use of broad-spectrum antibiotics (−18.5% [95% CI = −32 to −5.0] for carbapenems and −14.7% [95% CI = −27.7 to −1.7] for glycopeptides), the overall antimicrobial cost (−33.9% [95% CI = −42.0 to −25.9]), and the hospital length of stay (−8.9% [95% CI = −12.8 to −5]) decreased. Among hospital pathogens, the implementation of ASPs was associated with a decrease in infections due to methicillin-resistant Staphylococcus aureus (risk difference [RD] = −0.017 [95% CI = −0.029 to −0.005]), imipenem-resistant Pseudomonas aeruginosa (RD = −0.079 [95% CI = −0.114 to −0.040]), and extended-spectrum beta-lactamase Klebsiella spp. (RD = −0.104 [95% CI = −0.153 to −0.055]). Notably, these improvements were not associated with adverse outcomes, since the all-cause, infection-related 30-day mortality and infection rates were not significantly different after implementation of an ASP (RD = −0.001 [95% CI = −0.009 to 0.006], RD = −0.005 [95% CI = −0.016 to 0.007], and RD = −0.045% [95% CI = −0.241 to 0.150], respectively). Hospital ASPs result in significant decreases in antimicrobial consumption and cost, and the benefit is higher in the critical care setting. Infections due to specific antimicrobial-resistant pathogens and the overall hospital length of stay are improved as well. Future studies should focus on the sustainability of these outcomes and evaluate potential beneficial long-term effects of ASPs in mortality and infection rates.
INTRODUCTION
About one-third of the hospitalized patients and more than two-thirds of critically ill patients are on antimicrobial therapy at any time (1, 2), and up to half of antibiotic prescriptions are inappropriate or not necessary (3). In 2013, the Centers for Disease Control and Prevention (CDC) reported that about 2 million patients are infected yearly with antimicrobial-resistant organisms in the United States, and about 23,000 deaths are directly attributed to these infections (3). This resulted in a call to action for acute care hospitals to implement antimicrobial stewardship programs (ASPs) (4, 5), a term that is used to describe the integrated strategy of improving antimicrobial use in order to enhance patient outcomes, reduce antimicrobial cost, and minimize the side effects associated with antimicrobial use, including drug resistance and nosocomial infections (4, 6, 7). Although there are studies that have already presented data on the efficacy of ASPs in the inpatient setting (8–10), limitations compromise their generalization (i.e., the studies were only conducted in the United States [8], age and study design limitations [9], a lack of clinical outcomes [10], etc.). The purpose of our systematic review and meta-analysis was to measure the efficacy of the implementation of an ASP expressed in daily defined doses (DDD) per 1,000 patient days in the hospital setting independently of the age and study design and to assess the subsequent clinical and economic outcomes.
MATERIALS AND METHODS
This systematic review and meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol (11).
Search strategy.
A systematic electronic search of PubMed, EMBASE, and Google Scholar databases was performed for pertinent studies up to 8 July 2015. All published studies reporting the efficacy of an ASP in a hospital were included in this analysis. Two independent investigators (S. Paudel and A. Kalbasi) reviewed the retrieved database results to determine potentially eligible articles which were read in full text. The precise search terms were “Hospitals AND (antimicrobial OR antibiotic OR stewardship).” Reference lists of the retrieved studies, systematic reviews, and meta-analyses pertaining to our study were also reviewed.
Study selection.
Studies were considered eligible for the analysis if they reported extractable data on the comparable efficacy of an ASP expressed in daily defined doses (DDD) per 1,000 patient days before and after the intervention among hospitalized patients. A restriction for English language was imposed, whereas abstracts from conference proceedings were excluded.
Data extraction and quality assessment.
Studies that were considered appropriate for inclusion in our study were independently evaluated by three reviewers (S. Karanika, S. Paudel, and A. Kalbasi), and discrepancies were discussed and resolved by consensus. The primary outcome of interest was the efficacy in terms of antimicrobial consumption before and after the implementation of an ASP in hospitals. Antimicrobial consumption was included if it was measured in DDD/1,000 patient days (12, 13). A restriction was applied to include only studies which mentioned the total antimicrobial consumption before and after the intervention, excluding those which reported only the restricted antimicrobial consumption. This exception was put in place in order to ensure that neither the effect of the intervention is overestimated nor we miss the phenomenon of “squeezing the balloon” (14) (discussed below). The efficacy was expressed in percentage change of antimicrobial consumption (15).
The secondary outcomes of interest were the effect of an ASP on a series of clinical outcomes, including measurement of antimicrobial consumption with high resistance potential (defined as the antimicrobials whose resistance occurs during drug development or clinical trials, or within 2 years of general use, such as carbapenems and glycopeptides [14, 16, 17]), overall and infection-related 30-day mortality, length of stay in hospital (LoS), and intensive care unit (ICU) stay, change in Clostridium difficile infection rate, change in rates of resistant strains throughout the hospitals, total infection rate, and consistency of antimicrobial treatment with ASP or national guidelines, as well as the change on the cost of antimicrobial treatment. In addition, for each study we extracted data on the midyear of the study, study design, location, ASP type and duration of pre- and postintervention periods, type of restricted antimicrobial agents (if applicable), patient age, and type of hospital setting.
The methodological quality of eligible studies was assessed by two reviewers (S. Paudel and S. Karanika) using the measurement tool Newcastle Ottawa scale (NOS). The three parameters used to evaluate the quality of individual studies were selection, comparability, and exposure/outcome assessments. The NOS assigns maximum four points for selection, two points for comparability, and three points for exposure/outcome. The study population was considered representative of the exposed cohort if data were available for inpatients on antimicrobial therapy and not among a specific subpopulation. Studies that received five stars were considered of adequate quality for extraction of relevant information, and nine stars were defined as the maximum score. Any discrepancies regarding quality assessment were resolved by joint reevaluation of the original article (see Table S1 in the supplemental material).
Data synthesis and analysis.
A random effects meta-analysis was carried out to calculate the combined percentage change and the 95% confidence intervals (95% CI), using the approach of DerSimonian and Laird (18). The variance of the raw proportions was stabilized using the Freeman-Tukey arcsine methodology (19), and studies with 0% or 100% proportions were not excluded from the meta-analysis (20, 21). The P value of each percentage change was extracted directly from the studies or was calculated using the Fisher exact test. The percent change and P value per study were used to calculate the 95% CI and standard error and vice versa according to the method of Altman et al. (22, 23). To check for publication bias, we used the Egger's test (24). The tau-squared statistic was calculated as a measure of heterogeneity (25), and a sensitivity analysis was performed to account for the following confounding factors: hospital setting (ICU versus wards), restricted versus total antimicrobial consumption, distribution per continent, and the inclusion of antifungal agents (26). The effect of an ASP on the secondary outcomes was expressed either as a percent change or unadjusted risk difference (RD), along with 95% CI and outliers, were removed upon their identification. We defined as an outlier a study which falls more than 1.5 times the interquartile range above the third quartile or below the first quartile (http://mathworld.wolfram.com/Outlier.html). Median values and their interquartile ranges or ranges extracted from included studies were transformed to means and standard deviations according to the method of Wan et al. (27). The year the study was conducted was used as the index year, and for studies whose study period extended for more than one calendar year the midyear was calculated. The Stata v13 software package (Stata Corporation, College Station, TX) and Excel Microsoft Office 2010 were used to perform the statistical analysis. The statistical significance threshold was set at 0.05.
RESULTS
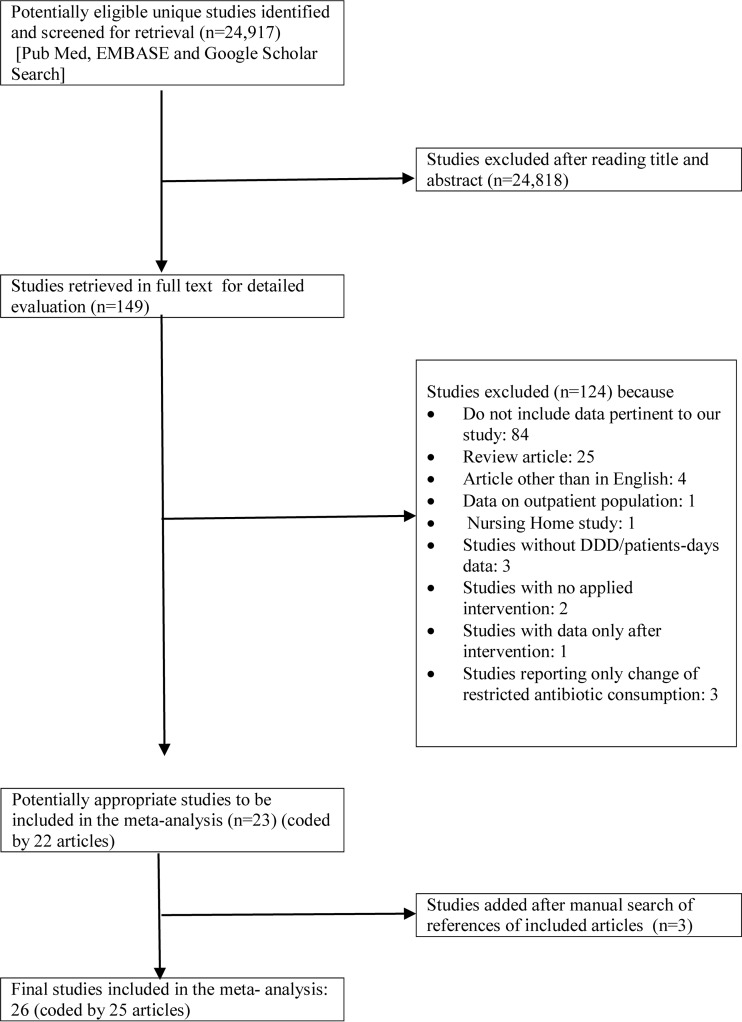
The initial database search retrieved 24,917 potentially relevant unique citations, out of which 149 studies were identified as potentially eligible for review and analysis through rigorous screening of titles and abstracts. After further investigation, 124 studies were excluded: 84 studies did not provide relevant data, 25 were review articles, 4 studies were in language other than English, 1 study focused on outpatients, and 1 study was conducted among nursing home patients. Also, three studies were excluded because they did not report antimicrobial consumption in DDD/1,000 patient days, two studies did not describe any applied intervention, one study described data only after intervention, and three studies reported only data on restricted antimicrobial consumption. The review of the reference lists of the full-texted articles yielded two additional studies. As a result, 26 studies coded from 25 articles (1 article presented data from two different hospitals [28]) were included in our meta-analysis. The information extracted from individual studies is exhibited in Table 1, and the detailed selection process is illustrated in a flow chart (Fig. 1). The implemented type of ASP strategy varied and included preapproval strategies, prospective audit and feedback, education, guidelines, and formulary restrictions, and most of the studies applied simultaneously multiple different types of the aforementioned ASP strategies (Table 1). Pre- and postintervention periods lasted from 6 months to 3 years, and the ASP was implemented to its full extent either outright or gradually over up to 3 months throughout the included studies (Table 1).
TABLE 1.
Individual studiesa
| Study designation (reference) | Pub yr | Mid-yr | Origin | Study design | ASP type | Duration | Antimicrobial restriction/control | % change | Age | Setting |
|---|---|---|---|---|---|---|---|---|---|---|
| Amer MR (47) | 2013 | 2010 | Saudi Arabia | Comparative historically controlled without intervention vs prospective arm under active ASP | Formulary restriction; preapproval strategies (antimicrobial order forms); prospective audit and feedback; education; guidelines; pharmacodynamic dose optimization; antimicrobial cycling | Pre-ASP: 6 mo (7–12/2009); ASP: started on 3/2011 | Piperacillin-tazobactam, imipenem, meropenem, vancomycin, tigecycline | −0.84 | Adults | Medical 20-bed ICU (of 894-bed tertiary hospital) |
| Apasarthanarak A (48) | 2006 | 2004 | Thailand | Prospective cohort; pre-ASP vs prospective cohort; post-ASP | Education; feedback: bedside discussion; use of IV antibiotic prescription forms; use of antibiogram; computerized system recording | Pre-ASP: 1 yr (7/2003–6/2004); post-ASP: 1 yr 7/2004–6/2005 | No | −0.13 | 65 ± 18 vs 66 ± 19 | 350-bed tertiary care university hospital |
| Bantar C (35) | 2003 | 2000 | Argentina | Comparative historically controlled without intervention vs prospective arm under active ASP | Introduction of an optional, and later obligatory, antibiotic order form; feedback: bedside discussion toward modification of prescription | Pre-ASP: 6 mo (1– 6/1999); ASP: 2 yrs (6/1999–6/2001) | No | −0.36 | Adults | 250-bed public teaching hospital & 10-bed ICU |
| Borde JP 1 (39) | 2015 | 2012 | Gernamy | Prospective cohort (pre- and post-ASP) | Daily rounds; written pocket-sized; formats of guidelines | Pre-ASP: >2 yrs (1/2011–3/2013); ASP: 4/2013; post-ASP: 1 yr (4/2013–3/2014) | Third-generation cephalosporins, fluoroquinolones | 0.02 | NA | 200-bed community hospital and 10-bed ICU |
| Borde JP 2 (37) | 2014 | 2010 | Germany | Prospective cohort medical service (applied ASP) vs surgical service (control) | Guideline; revision: written pocket-sized; formats and hospital intranet; information and education; regular ward rounds and intensified ID consultations; feedback; prospective audit | Pre-ASP: 3 yrs (1/2008–11/2011); ASP: 12/2011; post-ASP: >1 yr (1/2012–3/2013) | Cephalosporins, fluoroquinolones | −0.14 | NA | 300-bed medical service (of a 1,600 bed-academic teaching hospital) |
| Borde JP 3 (38) | 2015 | 2010 | Germany | Prospective cohort | Guideline revisions: written, pocket-sized formats and hospital intranet; information; education; intensified infectious diseases consultation and standardized treatment protocol | Pre-ASP: 3 yrs (1/2008–10/2011); ASP: 10/2011; post-ASP: >2 yrs (11/2011–10/2013) | Cephalosporin (especially third generation) fluoroquinolones | −0.07 | NA | Emergency department −39-bed capacity (of a 1,600-bed academic teaching hospital) |
| Boyles TH (40) | 2013 | 2012 | Cape Town, South Africa | Retrospective cohort- control arm; prospective cohort-intervention arm | Antibiotic prescription chart; antibiotic stewardship ward rounds; audit of antibiotic prescription chart use | Pre-ASP: 1 yr (1–12/2011); ASP: 11/22/2011; post-ASP: 1 yr (1–12/2012) | NA | −0.2 | 48 ± 18 vs 50 ± 18 | Two 32-bed medical wards |
| Bozkurt F (49) | 2014 | 2013 | Turkey | Cross-sectional study (before and after the intervention | Guidelines; education (monthly seminars); feedback; audit of antimicrobial prescription in terms of duration and appropriateness of the treatment | ASP: 5/16/2011–5/23/2015 | −0.33 | NA | 672-bed tertiary teaching and research hospital center with 6 ICUs, 10 medical and surgical units | |
| Cisneros JM (29) | 2014 | 2011 | Spain | Prospective recorded intervention | Antibiotic prescribers based on counseling interviews; guidelines | ASP: 1–3/2011; post-ASP: 9–12/2011 | No | −0.26 | NA | 1,251-bed tertiary care teaching hospital with 90 ICU beds and a transplant/BMT unit |
| Cook PP (30) | 2004 | 2001 | Louisville, KY | Retrospective cohort- control arm; prospective cohort-intervention arm | Enhanced feedback after two preauthorization approvals for restricted antibiotics; treatment days for controlled antibiotics | Pre-ASP: 2 yrs (1999–2000); ASP: 1/2001 introduced; post-ASP: 2 yrs (2002–2003) | Restricted: amikacin, caspofungin, traconazole, linezolid, quinupristin-dalfopristin, valganciclovir, oral vancomycin, amphotericin lipid formulation; controlled: ampicillin-sulbactam, azithromycin, aztreonam, cefepime, cefotaxime, ceftriaxone, ciprofloxacin, clindamycin, ertapenem, fluconazole, ganciclovir, imipenem-cilastatin, meropenem, moxifloxacin, piperacillin-tazobactam, tobramycin, vancomycin (IV) | −0.26 | Adults | 731-bed tertiary-care teaching hospital |
| Gould IM (41) | 2000 | 1994 | Scotland, United Kingdom | Prospective cohort for both arms | Drug restriction | Pre-ASP: >1 yr (1992–1993); ASP: 3/1993 introduced; post-ASP: >1 yr (1996–1997) | Didanosine, clarithromycin, zalcitabine, lipid; amphotericin, stavudine, meropenem, saquinavir, ceftriaxone, ritonavir, cefixime, indinavir, fosfomycin, famciclovir, ceftibuten, itraconazole, ofloxacin, terbinafine, valciclovir, azithromycin | 0.17 | NA | Multicenter: acute tertiary referral/teaching hospital, small district general hospital, long-stay hospital for the elderly, several small community hospitals, and two psychiatric hospitals |
| Hou D (31) | 2014 | 2011 | Taishan, China | Retrospective cohort- control arm; prospective cohort-intervention arm | Formulary restriction; preauthorization; education | Pre-ASP: 6 mo (10/2010–3/2011); ASP: 4/2011–8/2011; post-ASP: 6 mo (10/2011–3/2012) | Quinolones (perioperative use) | −0.27 | 53.10 ± 19.43 vs 54.59 ± 18.07 | 12-bed ICU (700-bed tertiary hospital) |
| Kim YC (50) | 2013 | 2008 | South Korea | Retrospective cohort- control arm; prospective cohort-intervention arm | Computerized prescription restriction; formulary restriction; report outcomes of the ASP | Pre-ASP: 1 yr (2006); ASP: 8/2008 started; post-ASP: 1 yr (2011) | Third-generation cephalosporin: surgery prophylaxis; aminoglycosides: surgery prophylaxis; inappropriate antibiotic combinations | −0.13 | NA | 2,000-bed tertiary hospital |
| Lin YS (32) | 2013 | 2010 | Taipei, Taiwan | Retrospective cohort- control arm; prospective cohort-intervention arm | Formulary, restriction; education concept; antibiotic stewardship, ward rounds: bedside evaluation, prospective audit, report outcomes of the program regularly to all staff | Pre-ASP: 6 mo (1–7/2009); ASP: 7/2009 introduced; post-ASP: 1 yr (7/2009–6/2012) | Imipenem, meropenem, vancomycin, tigecycline, colistin, linezolid | −0.21 | NA | 415-bed community public teaching hospital. |
| Mach R (42) | 2007 | 2002 | Czech Republic | Prospective computerized survey | New guidelines for antibiotic prophylaxis based on local microbial resistance patterns; prior authorization for the restricted antibiotics | Pre-ASP: 1 yr (2000–2001); ASP: 2002 introduced; post-ASP: 1 yr (2003–2004) | Aminopenicillins and β-lactamase inhibitors, piperacillin with β-lactamase inhibitors; meropenem, cefalothin, cefapirin, cefazolin, etc.; fluoroquinolones, colistin, vancomycin (prior authorization for the restricted ones) | −0.58 | NA | 500-bed general hospital |
| Meyer E (33) | 2007 | 2003 | Germany | Segmented regression analysis | Revised guidelines for pneumonia management; education | Pre-ASP: 1 yr (2002–2003); ASP: January 2004 introduced; post-ASP: 1 yr (2005) | No (revised guidelines: carbapenem removal for pneumonia) | −0.34 | Adults | Neurosurgical 12-bed ICU |
| Ng CK (51) | 2008 | 2004 | Hong Kong | Pretest/posttest analysis | Policy and guideline formulation; education; feedback; monthly antibiotic consumption; cost monitoring; antimicrobial susceptibility pattern reporting | Pre-ASP: 1 yr (7/2003–6/2004); ASP: 7/2004 introduced; post-ASP: 1 yr (7/2004–6/2005) | Antipseudomonal cephalosporins, carbapenems, IV vancomycin, IV fluoroquinolones, IV macrolides, fluconazole. | −0.06 | 71.4 ± 16.6 vs 72.9 ± 15.9 | 1,800-bed regional hospital providing acute care service |
| Nitsch-Osuch A 1 (43) | 2015 | 2013 | Poland | Retrospective analysis before and after of ASP implementation | Written guidelines for antibiotic prescription; preauthorization approval for broad-spectrum antibiotics (e.g., glycopeptides and carbapenems) | Pre-ASP: 1 yr (2012); ASP: 2013 introduced; post-ASP: 1 yr (2013) | Broad-spectrum antibiotics (e.g., glycopeptides and carbapenems) | 0.05 | 0-18 | General pediatric 21-bed ward (of an academic hospital) |
| Nitsch-Osuch A 2 (44) | 2015 | 2012 | Poland | Retrospective analysis before and after of ASP implementation | Preauthorization approval of broad-spectrum antibiotics | Pre-ASP: 1 yr (2011); ASP: 2012 introduced; post-ASP: 1 yr (2012) | Broad-spectrum antibiotics (e.g., glycopeptides and carbapenems) | −0.31 | neonates | 10-bed special neonatal care units (of an academic hospital) |
| Niwa T (52) | 2012 | 2010 | Japan | Retrospective cohort- control arm; prospective cohort-intervention arm | Review of antimicrobial orders-phone contact; IV antimicrobial administration limited to 2 weeks duration, otherwise preauthorization approval strategy; appropriateness of duration; education; feedback over mobile phone; printed information | Pre-ASP: 1 yr (8/2008–7/2009); ASP: 2 yrs (8/2009–7/2011) | No | −0.08 | 54 ± 22.5 vs 56 ± 22.6 | National 606-bed university hospital |
| Pate PG (36) | 2012 | 2010 | Dallas, TX | Retrospective cohort- control arm; prospective cohort-intervention arm | Prospective audit; ID consultation | Pre-ASP: <1 yr (1–11/2009); ASP: >1 yr (12/2009–2/2011) | No | −0.21 | 67 (54–77) vs 68 (56–77) | 60-bed LTACH & 6-bed high-acuity patients |
| Peto Z (45) | 2008 | 2003 | Hungary | Segmented regression analysis | ICU/ID specialist consultant in rounds and over telephone; preauthorization approval on every antibiotic apart from antibiotics for surgical prophylaxis | Pre-ASP: (2 yrs) 2000–2002; ASP: 11/2002; post-ASP: (2 yrs) 2003–2005 | All apart from antibiotics for surgical prophylaxis | −0.38 | 56.3 ± 17.2 vs 56.8 ± 17.6 | 6-bed surgical ICU (of a university tertiary referral hospital) |
| Ruttimann S (46) | 2004 | 1998 | Switzerland | Quasiexperimental study | Preauthorization approval for restricted drugs; educational program; written guidelines | Pre-ASP: 1 yr (1996); ASP: 1997 introduced; post-ASP: 1 yr (2001) | Ceftriaxone, ceftazidime, piperacillin-tazobactam, imipenem-cilastatin, vancomycin | 0.5 | Adults | 80-bed tertiary care center with 80 beds (including ICU) |
| Storey DF (34) | 2012 | 2010 | Dallas, TX | Retrospective cohort- control arm; prospective cohort-intervention arm | ASP team audited antimicrobial prescriptions provided nonbinding feedback | Pre-ASP: 8 mo (1/2009–8/2009); ASP: 9–12/2009; post-ASP: >1 yr (9/2009–12/2010) | No | −0.16 | 57.4 ± 18.6 vs 57.4 ± 18.7 | 43-bed medical-surgical services (24-bed medical-surgical wards, 11-bed; step-down unit and 8-bed ICU) |
| Yeo CL 1 (28) | 2012 | 2009 | Singapore | Prospective interrupted time-series study | Non-binding prospective audit of antibiotic prescription with direct feedback via a written form for discontinuation, change or de-escalation; drug restriction | Pre-ASP: 1.5 yrs (1/8/2008–6/30/2009); ASP: 7/2009; post-ASP: 1.5 yrs (8/1/2009–6/30/2010) | Carbapenems; third-generation and fourth-generation cephalosporins, piperacillin-tazobactam, vancomycin | 0.21 | Adults | National university cancer institute (including BMT) |
| Yeo CL 2 (28) | 2012 | 2009 | Singapore | Prospective interrupted time-series study | Nonbinding prospective audit of antibiotic prescription with direct feedback via a written form for discontinuation, change, de-escalation, change route; drug restriction | Pre-ASP: 1/8/2008–6/30/2009; ASP: 7/2009; post-ASP: 8/1/2009–6/30/2010 | Carbapenems; third-generation and fourth-generation cephalosporins, piperacillin-tazobactam, vancomycin | 0.29 | Adults | 990-bed tertiary public teaching hospital |
Characteristics of 26 studies: publication year, mid-year, origin, study design, duration of study, antimicrobials in restriction if applicable, the percent change of total antibiotic consumption, the mean age of the participants, and the type of setting. Note that study designations as set in column 1 correspond to the study designations used in the figures. BMT, bone marrow transplant unit.
FIG 1.
PRISMA flow diagram of meta-analysis.
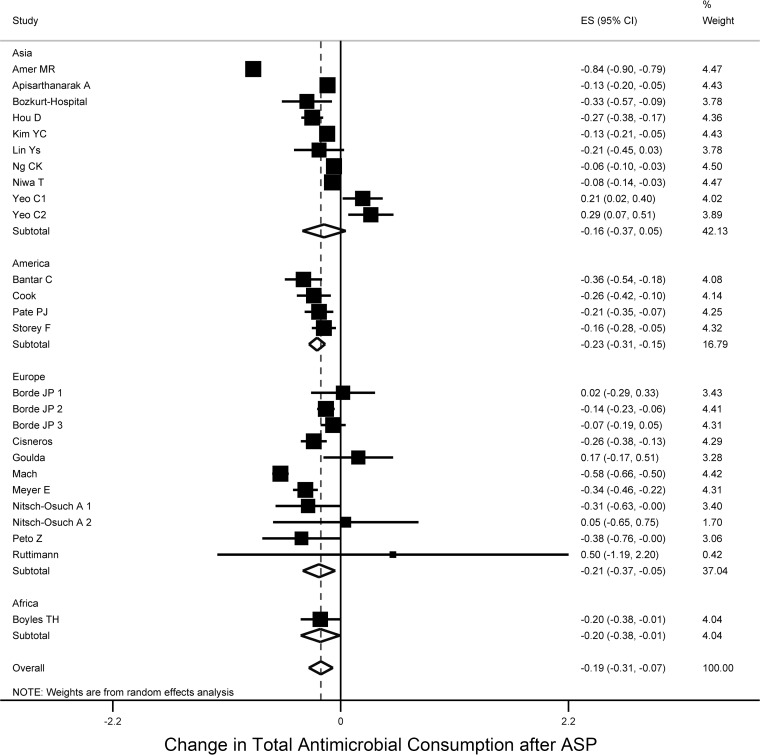
The pooled percentage change of antimicrobial consumption after ASP implementation was −19.1% (95% CI [−30.1 to −7.5], τ2 = 0.08), with no evidence of small-study effect across studies (Egger's bias = 0.33, P = 0.744) (Fig. 2). Interestingly, this decrease was not limited to antibacterial agents. More specifically, based on six studies (29–34), we found that the change in the consumption of antifungal agents after the implementation of an ASP decreased by −39.1% (95% CI [−62.3 to −16.0], τ2 = 0.05, Egger's bias = 1.89, P = 0.132) (see Fig. S1 in the supplemental material). Of note, only one of six studies applied antifungal restriction in their formulary (Table 1) (30).
FIG 2.
Forest plot of included studies stratified by continent. Individual and combined change of total antimicrobial consumption after ASP implementation among studies conducted in hospital settings.
Studies conducted in the United States (30, 34–36) and in Europe (29, 33, 37–46) reported the highest pooled decrease in antimicrobial consumption after the implementation of ASP (−19.9% [95% CI = −27.7 to −12.1, τ2 = 0.00] and −20.9% [95% CI = −30.5 to −15, τ2 = 0.05], respectively), whereas studies in Asia reported a reduction of −16% (95% CI = −36.5 to −5.3], τ2 = 0.11) (28, 31, 32, 47–52) (Fig. 2). Only one study was conducted in South America with a reduction of −35.9% (95% CI = −53.8 to −17.9) (35) and one study in South Africa with a reduction of −19.6% (95% CI = −38.5 to −0.8) (40).
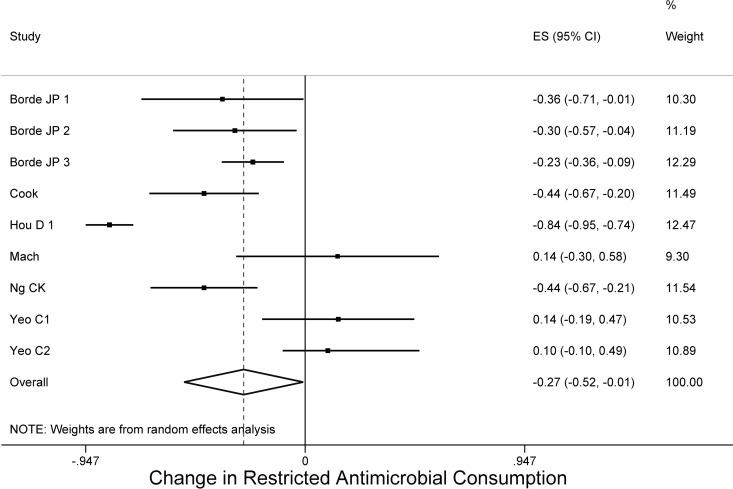
Regarding the changes in consumption of restricted antimicrobial agents, of the 17 studies that applied either audit of any kind of antimicrobial class or formulary restriction as a part of their ASP, 9 reported the change of the restricted antimicrobial consumption (28, 30, 31, 37–39, 42, 51), and the pooled decrease in consumption was 26.6% (95% CI = −52.3 to −0.8, τ2 = 0.14), without a publication bias (Egger's bias = 2.13, P = 0.071) (Fig. 3). Of note, all nine studies applied restriction mainly in last-resort antibiotics, including third-generation or fourth-generation cephalosporins, vancomycin, tigecycline, linezolid, imipenem, meropenem, and fluoroquinolones (Table 1). If we take into consideration the three studies that we excluded since they reported exclusively the change in consumption of restricted antibiotics (53–55). the pooled decrease in the consumption was 25% (95% CI = 34.2 to 15.8, τ2 = 0.02, Egger's bias = −1.84, P = 0.560). Notably, looking at specific categories of broad-spectrum antibacterial agents, the consumption of carbapenems (29, 33–35, 37–39, 48, 49, 52) (11 studies) and glycopeptides (33–39, 48–50) (10 studies) also decreased (−18.5% [95% CI = −32 to −5.0, τ2 = 0.02, Egger's bias = −2.61, P = 0.028] and −14.7% [95% CI = −27.7 to −1.7, τ2 = 0.02, Egger's bias = −2.51, P = 0.040], respectively) (see Fig. S2 and S3 in the supplemental material), but this decrease was significant only when they were not under restriction or preapproval authorization strategies prior the initiation of the ASP. Also, consistency of antimicrobial treatment with ASP or national guidelines increased after ASP implementation based on three studies (pooled RD = 0.078, 95% CI = 0.061 to 0.095, τ2 = 0.01, Egger's bias = 2.20, P = 0.271) (29, 47, 48).
FIG 3.
Forest plot of included studies. Individual and combined changes of consumption of restricted antimicrobials after ASP implementation.
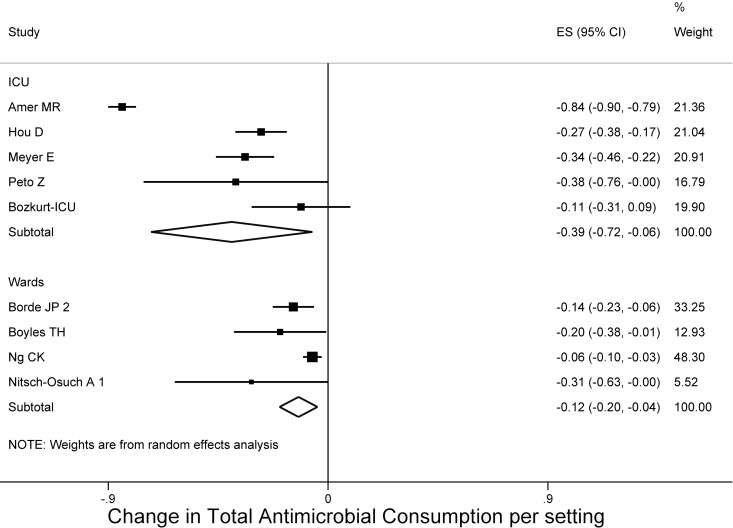
Stratifying the studies per hospital setting, we found that studies conducted in medical wards achieved an antimicrobial reduction of −12.1% (95% CI = −19.9 to −4.3%, τ2 = 0.00) (37, 40, 43, 51), whereas the studies conducted in an ICU reached a decrease of −39.5% (95% CI = −72.5 to −6.4, τ2 = 0.13) (31, 33, 45, 47), with no small-study effect (Egger's bias = −0.2, P = 0.823) based on four studies. This difference between medical wards and the critical care setting was −27% and was statistically significant (95% CI = −72.3 to −5.5) (Fig. 4).
FIG 4.
Forest plot of included studies per setting. Individual and combined changes of total antimicrobial consumption after ASP implementation in ICU and wards.
Regarding the change in mortality after ASP implementation, neither overall (30, 31, 33, 40-42, 45-47, 51) (10 studies), nor infection-related 30-day mortality (31, 33, 46, 51) (4 studies) were significantly different (pooled RD = −0.001 [95% CI = −0.009 to 0.006, τ2 = 0.00, Egger's bias = 0.19, P = 0.851] and pooled RD = −0.005 [95% CI = −0.016 to 0.007, τ2 = 0.00, Egger's bias = 0.11, P = 0.925], respectively) (see Fig. S4 and S5 in the supplemental material). The percent change in infection rate was also not significantly different before and after the implementation of the ASP based on seven studies (−4.5%, 95% CI = −24.1 to 15.0, τ2 = 0.00, Egger's bias = −0.37, P = 0.727) (31, 42–44, 46, 48, 49) (see Fig. S6 in the supplemental material). We also calculated the above parameters by region, but neither of these changed even after this kind of stratification (data not shown).
The mean hospital length of stay (LoS) was reduced by −8.9% based on four studies (95% CI = −12.8 to −5, Egger's bias = −0.31, P = 0.90) (Fig. 5) (34, 46, 51, 52). Of note, two studies were excluded from the calculation of the LoS, even though they provided relevant data, in order to avoid false estimation of the result. One was conducted in long-term acute care hospital (36), and the second was considered a significant outlier (35). Even including these studies, the decrease in the LoS remains significant (−15.7, 95% CI = −31.1 to −3). Notably, the LoS in the ICU did not change significantly after implementation of an ASP based on four studies (1.5%, 95% CI = −16.8 to 19.9, Egger's bias = 2.57, P = 0.080) (31, 33, 45, 47).
FIG 5.
Forest plot of included studies. Change in LoS after ASP.
In addition, we found that implementation of an ASP led to a decrease in antimicrobial cost of −33.9% based on 6 studies (95% CI = −42.0 to −25.9, τ2 = 0.05, Egger's bias = −0.77, P = 0.485) (32–34, 36, 42, 48) (Fig. 6). Evaluating the effect on the prevalence of resistant strains derived from infections, methicillin-resistant Staphylococcus aureus (MRSA) infections were significantly lower after the implementation of the ASP based on six studies with follow-up period of 1 year (33, 42, 48) or 2 years (35, 45, 52) (pooled RD = −0.017, 95% CI = −0.029 to −0.005, τ2 = 0.03, [Egger's bias = −1.25, P = 0.280) (33, 35, 42, 45, 48, 52), and the same was noted for imipenem-resistant Pseudomonas aeruginosa based on five studies with follow-up period of 1 year (33, 42) or 2 years (30, 35, 45, 52) (pooled RD = −0.079, 95% CI = −0.114 to −0.04, τ2 = 0.03, Egger's bias = −0.11, P = 0.918) (30, 33, 35, 45, 52) and infections associated with extended-spectrum beta-lactamase (ESBL)-Klebsiella spp. based on five studies with follow-up period of 1 year (33, 42, 48) or 2 years (35, 45) (pooled RD = −0.104, 95% CI = −0.153 to −0.055, τ2 = 0.02, Egger's bias = 1.53, P = 0.225) (33, 35, 42, 45, 48), whereas a significant decrease was not observed in ESBL-Escherichia coli infections based on five studies with follow-up period of 1 year (33, 42, 48) or 2 years (35, 45) (pooled RD = −0.009, 95% CI = −0.044 to 0.055], τ2 = 0.02, Egger's bias = −0.65, P = 0.560) (33, 35, 42, 45, 48) (see Fig. S7, S8, and S9 in the supplemental material). The C. difficile infection rate did not significantly change, but this finding was based on three studies (34, 36, 37), and the estimated publication bias was significant (71.9%, 95% CI = −119.5 to 26.32, τ2 = 1.64, Egger's bias = 32.96, P = 0.019). Notably, all three studies audited antimicrobial prescriptions and provided feedback to the prescribers (34, 36, 37), while two studies (34, 36) did not apply any formulary restriction, and a third study (37) restricted cephalosporins and fluoroquinolones, classes of antibiotics that are tightly linked with C. difficile infection (56).
FIG 6.
Forest plot of included studies. Change in antimicrobial cost after ASP implementation.
DISCUSSION
Evaluation of ASPs is based on their performance on antimicrobial consumption, as well as on clinical and microbiological outcomes and cost-effectiveness (45). However, because ASPs are highly variable, establishing specific targets and performance criteria requires the synthesis of data from different settings, making this topic ideal for a meta-analysis study. Using this approach, we found that the overall antimicrobial consumption among inpatients before and after the implementation of an ASP decreased by almost one-fifth, and the effect of ASPs was approximately double in the ICU setting. The consumption of carbapenems and glycopeptides was also reduced. ASPs also resulted in a decrease of the antimicrobial cost, length of hospital stay and infections from MRSA, imipenem-resistant P. aeruginosa, and ESBL-Klebsiella spp. decreased as well.
Given the decrease in new antimicrobial agents and the imminent emergence of resistance shortly after the introduction of new agents (57), the CDC, the World Health Organization, and the U.S. government have advocated the universal implementation of ASPs in hospitals as a promising strategy to preserve antimicrobial benefit (7, 58, 59). Our analysis showed that implementation of an ASP was associated with a decrease in total antimicrobial consumption by almost one-fifth, while the use of restricted or controlled antimicrobial agents was further reduced by over one-fourth. Interestingly, as noted above, in the ICU setting the antimicrobial consumption decreased by almost 40%, a finding that is reasonable if we consider that more than one-third of patients in ICUs are diagnosed with an infection (1, 60), and ICUs also represent the site of the hospital with the heaviest use of antimicrobial agents and high rates of multiresistant strains (47).
In addition, taking into account the potential for the “squeezing the balloon” phenomenon (a term that is used to describe the concern that restricting some antimicrobial agents might lead to an increase in the nonrestricted antimicrobials [61]), we estimated separately the restricted and nonrestricted antimicrobial agents, and we demonstrated that both were reduced. In addition, the finding that implementation of an ASP is associated with a decrease in the consumption of high potential resistance antimicrobial agents (14, 16), such as carbapenems and glycopeptides, indicates that not only the overall use of antimicrobial agents decreased, but the choices were probably more appropriate and ASPs seem to be effective not only because they result in a decrease in the quantity of antimicrobial consumption but also positively affect antimicrobial choices.
We also found that the implementation of ASPs was associated with a significant drop in antimicrobial cost by more than one-third. Notably, although this is an impressive decrease, it is only a partial estimation of savings (62). Indeed, in addition to the direct cost of antimicrobial agents, there are many indirect expenses which are expected to decrease proportionally, such as from drug side effects (63). One such potential indirect benefit is the decrease in the hospital LoS. Interestingly, we found that ASPs decreased hospital LoS. However, hospital LoS can be affected by several factors, such as admission diagnosis, institutional features and social status (64), and some hospital-acquired infections (65). Further studies are needed to quantify the impact of ASPs in hospital LoS and identify whether the decrease in the LoS is because of the impact of ASPs on infections due to certain resistant pathogens, earlier transition to oral therapy, the discontinuation of unnecessary antimicrobial agents, a decrease in drug side effects, or other reasons.
Regarding potential limitations of this study, the follow-up period in our analysis was fluctuated from 6 months to 3 years. Although most of the studies in the literature followed this period of time for a first assessment of outcomes, longer follow-up is needed to evaluate the longer-term effects of ASPs. For example, we did not find a change in all-cause and infection-related 30-day mortality after an ASP. This finding is reassuring since it supports previous reports that ASPs, at least, do not affect adversely the provided level of care depriving antibiotics from patients who really need them (66). However, in order to evaluate the hypothesis that ASPs can also improve these rates, a longer assessment period with adequate and stable implementation of an ASP is warranted. Although publication bias was sought through the Egger test and reported with each pooled result, estimations derived from fewer than 10 studies should be taken under consideration cautiously since the power of the test is attenuated in this case (http://handbook.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm). Also, a publication bias was found to be significant in our estimation for the change on the rate of C. difficile infection. This is an interesting finding indicating that negative results on the impact of ASPs are more likely to be published. Even though the effect of ASPs in C. difficile infection is generally accepted (67), additional reports are needed to confirm and quantify this finding. The implementation of ASPs is a relatively recent phenomenon and researchers should continue to publish their results, even in areas where the benefits of ASPs are considered “widely accepted.”
In conclusion, even though ASPs are highly variable, they are greatly effective in decreasing antimicrobial consumption, and they improve clinical and economic outcomes. This first aggregate statistical assessment of ASP implementation that includes multiple clinical and economic parameters, supports the implementation of ASPs and argues that ASP guidelines should be followed by clinicians and hospital administrators. Future studies should analyze each component of ASPs separately, while long-term evaluation of the effect of ASPs is also warranted to determine their lasting influence on mortality and infection rates.
Supplementary Material
ACKNOWLEDGMENTS
S.K. and E.M. accept full responsibility for the conduct of the study, have access to the data, and have control of the decision to publish. S.K. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. S.K. conceptualized and designed the study, participated in data collection, extraction, and interpretation, prepared tables and figures, performed the statistical analysis, wrote and drafted the initial manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. S.P. performed the literature search, participated in data collection extraction and interpretation, prepared tables and figures, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. C.G. participated in data interpretation, performed the statistical analysis, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. A.K. performed the literature search, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. E.M. conceptualized and designed the study, interpreted the data, reviewed and revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
We declare no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00825-16.
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Steward CD, Edwards JR, Pryor ER, McGowan JE Jr, Archibald LK, Gaynes RP, Tenover FC. 1999. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 29:245–252. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/. [Google Scholar]
- 4.Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 5.Fridkin SK, Baggs J, Fagan R, Magill S, Pollack LA, Malpiedi P, Slayton R, Khader K, Rubin MA, Jones M, Samore MH, Dumyati G, Dodds-Ashley E, Meek J, Yousey-Hindes K, Jernigan J, Shehab N, Herrera R, McDonald LC, Schneider A, Srinivasan A. 2014. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 6.MacDougall C, Polk RE. 2005. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The White House. 2014. National strategy for combating antibiotic-resistant bacteria. The White House, Washington, DC: https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf. [Google Scholar]
- 8.Wagner B, Filice GA, Drekonja D, Greer N, MacDonald R, Rutks I, Butler M, Wilt TJ. 2014. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 35:1209–1228. doi: 10.1086/678057. [DOI] [PubMed] [Google Scholar]
- 9.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. 2013. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 4:CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Schuts EC, Hulscher ME, Mouton JW, Verduin CM, Stuart JW, Overdiek HW, van der Linden PD, Natsch S, Hertogh CM, Wolfs TF, Schouten JA, Kullberg BJ, Prins JM. 2016. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis pii:S1473-3099(16)00065. doi: 10.1016/s1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson JM, Patrick DM, Marra F, Ng H, Bowie WR, Heule L, Muscat M, Monnet DL. 2004. Measurement of antibiotic consumption: a practical guide to the use of the Anatomical Therapeutic Chemical classification and defined daily dose system methodology in Canada. Can J Infect Dis 15:29–35. doi: 10.1155/2004/389092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2003. Drug utilization metrics and their applications. World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/en/d/Js4876e/7.html. [Google Scholar]
- 14.Cunha CB, Varughese CA, Mylonakis E. 2013. Antimicrobial stewardship programs (ASPs): the devil is in the details. Virulence 4:147–149. doi: 10.4161/viru.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Mathematical Society. 2013. MathJax: calculating percent increase and decrease. AMS/SIAM, Providence, RI: http://www.onemathematicalcat.org/algebra_book/online_problems/calc_percent_inc_dec.htm. [Google Scholar]
- 16.Cunha BA. 2002. Strategies to control antibiotic resistance. Semin Respir Infect 17:250–258. doi: 10.1053/srin.2002.34692. [DOI] [PubMed] [Google Scholar]
- 17.Cunha BA. 2001. Effective antibiotic-resistance control strategies. Lancet 357:1307–1308. doi: 10.1016/S0140-6736(00)04527-X. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Fazel S, Khosla V, Doll H, Geddes J. 2008. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med 5:e225. doi: 10.1371/journal.pmed.0050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyaga VN, Arbyn M, Aerts M. 2014. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsague X, Laporte L, Bosch FX, de Sanjose S, Trottier H. 2014. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 22.Altman DG, Bland JM. 2011. How to obtain the confidence interval from a P value. BMJ 343:d2090. doi: 10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Bland JM. 2011. How to obtain the P value from a confidence interval. BMJ 343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 24.Karanika S, Zervou FN, Zacharioudakis IM, Paudel S, Mylonakis E. 2015. Risk factors for methicillin-resistant Staphylococcus aureus colonization in dialysis patients: a meta-analysis. J Hosp Infect 91:257–263. doi: 10.1016/j.jhin.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Rucker G, Schwarzer G, Carpenter JR, Schumacher M. 2008. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol 8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. 2015. Colonization with toxinogenic Clostridium difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol 110:381–391. doi: 10.1038/ajg.2015.22. [DOI] [PubMed] [Google Scholar]
- 27.Wan X, Wang W, Liu J, Tong T. 2014. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo CL, Chan DS, Earnest A, Wu TS, Yeoh SF, Lim R, Jureen R, Fisher D, Hsu LY. 2012. Prospective audit and feedback on antibiotic prescription in an adult hematology-oncology unit in Singapore. Eur J Clin Microbiol Infect Dis 31:583–590. doi: 10.1007/s10096-011-1351-6. [DOI] [PubMed] [Google Scholar]
- 29.Cisneros JM, Neth O, Gil-Navarro MV, Lepe JA, Jimenez-Parrilla F, Cordero E, Rodriguez-Hernandez MJ, Amaya-Villar R, Cano J, Gutierrez-Pizarraya A, Garcia-Cabrera E, Molina J. 2014. Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect 20:82–88. doi: 10.1111/1469-0691.12191. [DOI] [PubMed] [Google Scholar]
- 30.Cook PP, Catrou PG, Christie JD, Young PD, Polk RE. 2004. Reduction in broad-spectrum antimicrobial use associated with no improvement in hospital antibiogram. J Antimicrob Chemother 53:853–859. doi: 10.1093/jac/dkh163. [DOI] [PubMed] [Google Scholar]
- 31.Hou D, Wang Q, Jiang C, Tian C, Li H, Ji B. 2014. Evaluation of the short-term effects of antimicrobial stewardship in the intensive care unit at a tertiary hospital in China. PLoS One 9:e101447. doi: 10.1371/journal.pone.0101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YS, Lin IF, Yen YF, Lin PC, Shiu YC, Hu HY, Yang YP. 2013. Impact of an antimicrobial stewardship program with multidisciplinary cooperation in a community public teaching hospital in Taiwan. Am J Infect Control 41:1069–1072. doi: 10.1016/j.ajic.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Meyer E, Buttler J, Schneider C, Strehl E, Schroeren-Boersch B, Gastmeier P, Ruden H, Zentner J, Daschner FD, Schwab F. 2007. Modified guidelines impact on antibiotic use and costs: duration of treatment for pneumonia in a neurosurgical ICU is reduced. J Antimicrob Chemother 59:1148–1154. doi: 10.1093/jac/dkm088. [DOI] [PubMed] [Google Scholar]
- 34.Storey DF, Pate PG, Nguyen AT, Chang F. 2012. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control 1:32. doi: 10.1186/2047-2994-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bantar C, Sartori B, Vesco E, Heft C, Saul M, Salamone F, Oliva ME. 2003. A hospital-wide intervention program to optimize the quality of antibiotic use: impact on prescribing practice, antibiotic consumption, cost savings, and bacterial resistance. Clin Infect Dis 37:180–186. doi: 10.1086/375818. [DOI] [PubMed] [Google Scholar]
- 36.Pate PG, Storey DF, Baum DL. 2012. Implementation of an antimicrobial stewardship program at a 60-bed long-term acute care hospital. Infect Control Hosp Epidemiol 33:405–408. doi: 10.1086/664760. [DOI] [PubMed] [Google Scholar]
- 37.Borde JP, Kaier K, Steib-Bauert M, Vach W, Geibel-Zehender A, Busch H, Bertz H, Hug M, de With K, Kern WV. 2014. Feasibility and impact of an intensified antibiotic stewardship programme targeting cephalosporin and fluoroquinolone use in a tertiary care university medical center. BMC Infect Dis 14:201. doi: 10.1186/1471-2334-14-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borde JP, Kern WV, Hug M, Steib-Bauert M, de With K, Busch HJ, Kaier K. 2015. Implementation of an intensified antibiotic stewardship programme targeting third-generation cephalosporin and fluoroquinolone use in an emergency medicine department. Emerg Med J 32:509–515. doi: 10.1136/emermed-2014-204067. [DOI] [PubMed] [Google Scholar]
- 39.Borde JP, Litterst S, Ruhnke M, Feik R, Hubner J, de With K, Kaier K, Kern WV. 2015. Implementing an intensified antibiotic stewardship programme targeting cephalosporin and fluoroquinolone use in a 200-bed community hospital in Germany. Infection 43:45–50. doi: 10.1007/s15010-014-0693-2. [DOI] [PubMed] [Google Scholar]
- 40.Boyles TH, Whitelaw A, Bamford C, Moodley M, Bonorchis K, Morris V, Rawoot N, Naicker V, Lusakiewicz I, Black J, Stead D, Lesosky M, Raubenheimer P, Dlamini S, Mendelson M. 2013. Antibiotic stewardship ward rounds and a dedicated prescription chart reduce antibiotic consumption and pharmacy costs without affecting inpatient mortality or re-admission rates. PLoS One 8:e79747. doi: 10.1371/journal.pone.0079747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould IM, Jappy B. 2000. Trends in hospital antimicrobial prescribing after 9 years of stewardship. J Antimicrob Chemother 45:913–917. doi: 10.1093/jac/45.6.913-a. [DOI] [PubMed] [Google Scholar]
- 42.Mach R, Vlcek J, Prusova M, Batka P, Rysavy V, Kubena A. 2007. Impact of a multidisciplinary approach on antibiotic consumption, cost and microbial resistance in a Czech hospital. Pharm World Sci 29:565–572. doi: 10.1007/s11096-006-9059-x. [DOI] [PubMed] [Google Scholar]
- 43.Nitsch-Osuch A, Kuchar E, Zycinska K, Gyrczuk E, Miskiewicz K, Korzeniewski K. 2015. Implementation of hospital's antibiotic policy decreases antimicrobial use in the general pediatric ward. Adv Exp Med Biol 857:67–74. doi: 10.1007/5584_2015_124. [DOI] [PubMed] [Google Scholar]
- 44.Nitsch-Osuch A, Kurpas D, Kuchar E, Zycinska K, Zielonka T, Wardyn K. 2015. Antibiotic consumption pattern in the neonatal special care unit before and after implementation of the hospital's antibiotic policy. Adv Exp Med Biol 835:45–51. doi: 10.1007/5584_2014_32. [DOI] [PubMed] [Google Scholar]
- 45.Peto Z, Benko R, Matuz M, Csullog E, Molnar A, Hajdu E. 2008. Results of a local antibiotic management program on antibiotic use in a tertiary intensive care unit in Hungary. Infection 36:560–564. doi: 10.1007/s15010-008-7377-8. [DOI] [PubMed] [Google Scholar]
- 46.Ruttimann S, Keck B, Hartmeier C, Maetzel A, Bucher HC. 2004. Long-term antibiotic cost savings from a comprehensive intervention program in a medical department of a university-affiliated teaching hospital. Clin Infect Dis 38:348–356. doi: 10.1086/380964. [DOI] [PubMed] [Google Scholar]
- 47.Amer MR, Akhras NS, Mahmood WA, Al-Jazairi AS. 2013. Antimicrobial stewardship program implementation in a medical intensive care unit at a tertiary care hospital in Saudi Arabia. Ann Saudi Med 33:547–554. doi: 10.5144/0256-4947.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apisarnthanarak A, Danchaivijitr S, Khawcharoenporn T, Limsrivilai J, Warachan B, Bailey TC, Fraser VJ. 2006. Effectiveness of education and an antibiotic-control program in a tertiary care hospital in Thailand. Clin Infect Dis 42:768–775. doi: 10.1086/500325. [DOI] [PubMed] [Google Scholar]
- 49.Bozkurt F, Kaya S, Tekin R, Gulsun S, Deveci O, Dayan S, Hosoglu S. 2014. Analysis of antimicrobial consumption and cost in a teaching hospital. J Infect Public Health 7:161–169. doi: 10.1016/j.jiph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Kim YC, Kim MH, Song JE, Ahn JY, Oh DH, Kweon OM, Lee D, Kim SB, Kim HW, Jeong SJ, Ku NS, Han SH, Park ES, Yong D, Song YG, Lee K, Kim JM, Choi JY. 2013. Trend of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in an institution with a high rate of MRSA after the reinforcement of antibiotic stewardship and hand hygiene. Am J Infect Control 41:e39–e43. doi: 10.1016/j.ajic.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Ng CK, Wu TC, Chan WM, Leung YS, Li CK, Tsang DN, Leung GM. 2008. Clinical and economic impact of an antibiotics stewardship programme in a regional hospital in Hong Kong. Qual Saf Health Care 17:387–392. doi: 10.1136/qshc.2007.023267. [DOI] [PubMed] [Google Scholar]
- 52.Niwa T, Shinoda Y, Suzuki A, Ohmori T, Yasuda M, Ohta H, Fukao A, Kitaichi K, Matsuura K, Sugiyama T, Murakami N, Itoh Y. 2012. Outcome measurement of extensive implementation of antimicrobial stewardship in patients receiving intravenous antibiotics in a Japanese university hospital. Int J Clin Pract 66:999–1008. doi: 10.1111/j.1742-1241.2012.02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. 2013. Clinical and economic outcomes from a community hospital's antimicrobial stewardship program. Am J Infect Control 41:145–148. doi: 10.1016/j.ajic.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Agwu AL, Lee CK, Jain SK, Murray KL, Topolski J, Miller RE, Townsend T, Lehmann CU. 2008. A world wide web-based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis 47:747–753. doi: 10.1086/591133. [DOI] [PubMed] [Google Scholar]
- 55.Cairns KA, Jenney AW, Abbott IJ, Skinner MJ, Doyle JS, Dooley M, Cheng AC. 2013. Prescribing trends before and after implementation of an antimicrobial stewardship program. Med J Aust 198:262–266. doi: 10.5694/mja12.11683. [DOI] [PubMed] [Google Scholar]
- 56.Gerding DN. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin Infect Dis 38:646–648. doi: 10.1086/382084. [DOI] [PubMed] [Google Scholar]
- 57.Morel CM, Mossialos E. 2010. Stoking the antibiotic pipeline. BMJ 340:c2115. doi: 10.1136/bmj.c2115. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. 2010. Get smart: know when antibiotics work. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/getsmart/healthcare/pdfs/getsmart-healthcare.pdf. [Google Scholar]
- 59.World Health Organization. 2011. Antimicrobial resistance: no action today, no cure tomorrow. World Health Organization, Geneva, Switzerland: http://www.who.int/world-health-day/2011/en/. [Google Scholar]
- 60.Brusselaers N, Vogelaers D, Blot S. 2011. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care 1:47. doi: 10.1186/2110-5820-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burke JP. 1998. Antibiotic resistance: squeezing the balloon? JAMA 280:1270–1271. doi: 10.1001/jama.280.14.1270. [DOI] [PubMed] [Google Scholar]
- 62.Paladino JA. 2004. Economics of antibiotic use policies. Pharmacotherapy 24:232s–238s. doi: 10.1592/phco.24.18.232S.52234. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. 2013. Measuring and comparing drug costs. World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/en/d/Js4882e/6.5.html. [Google Scholar]
- 64.Wright SP, Verouhis D, Gamble G, Swedberg K, Sharpe N, Doughty RN. 2003. Factors influencing the length of hospital stay of patients with heart failure. Eur J Heart Fail 5:201–209. doi: 10.1016/S1388-9842(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 65.Glance LG, Stone PW, Mukamel DB, Dick AW. 2011. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg 146:794–801. doi: 10.1001/archsurg.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention. 2015. Impact of antibiotic stewardship programs on Clostridium difficile infections. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/getsmart/healthcare/evidence/asp-int-cdiff.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.