Abstract
Candida species are the cause of many bloodstream infections through contamination of indwelling medical devices. These infections account for a 40% mortality rate, posing a significant risk to immunocompromised patients. Traditional treatments against Candida infections include amphotericin B and various azole treatments. Unfortunately, these treatments are associated with high toxicity, and resistant strains have become more prevalent. As a new frontier, light-activated phenylene ethynylenes have shown promising biocidal activity against Gram-positive and -negative bacterial pathogens, as well as the environmental yeast Saccharomyces cerevisiae. In this study, we monitored the viability of Candida species after treatment with a cationic conjugated polymer [poly(p-phenylene ethynylene); PPE] or oligomer [“end-only” oligo(p-phenylene ethynylene); EO-OPE] by flow cytometry in order to explore the antifungal properties of these compounds. The oligomer was found to disrupt Candida albicans yeast membrane integrity independent of light activation, while PPE is able to do so only in the presence of light, allowing for some control as to the manner in which cytotoxic effects are induced. The contrast in killing efficacy between the two compounds is likely related to their size difference and their intrinsic abilities to penetrate the fungal cell wall. Unlike EO-OPE-DABCO (where DABCO is quaternized diazabicyclo[2,2,2]octane), PPE-DABCO displayed a strong propensity to associate with soluble β-glucan, which is expected to inhibit its ability to access and perturb the inner cell membrane of Candida yeast. Furthermore, treatment with PPE-DABCO unmasked Candida albicans β-glucan and increased phagocytosis by Dectin-1-expressing HEK-293 cells. In summary, cationic phenylene ethynylenes show promising biocidal activity against pathogenic Candida yeast cells while also exhibiting immunostimulatory effects.
INTRODUCTION
Bloodstream infections affect a huge patient population in the United States, with more than 250,000 cases reported each year (1). Patients with indwelling medical devices, such as central venous catheters (CVCs), are most at risk for these infections (2). Frequently, various microorganisms from the skin of the patient, or the respective health care professional, can gain access through the catheter wound as a result of nonsterile conditions (3–6). Of these resulting bloodstream infections, Candida species account for 9% of all instances and are associated with an ∼40% mortality rate (2, 7). The most commonly isolated fungal pathogen from bloodstream infections is Candida albicans, but the prevalence of other species, such as Candida parapsilosis, Candida glabrata, and Candida tropicalis, is increasing (8, 9).
An important determinant of pathogenicity among Candida spp. is the outer cell wall. The cell wall is composed primarily of carbohydrates and, structurally, is separated into two layers. The outer layer is composed mostly of cell wall glycoproteins with N- and O-linked mannans, and the inner layer is composed of β-glucan and chitin. The complexity of the cell wall contributes to various pathogenic factors, including adherence of the fungus and establishment of cross talk with the host, which is known as “glycan code” (10–12). Cell wall components, such as β-glucan and other polysaccharides, are also found in the extracellular matrix secreted by Candida species biofilms, which can contaminate the synthetic material surfaces of indwelling medical devices. β-Glucan in the extracellular matrix of Candida biofilms has been shown to sequester antifungal drugs, which contributes to the decreased drug susceptibility of biofilms (13–15).
Various antimicrobial impregnation approaches have been devised to prevent catheter infections. Catheter materials coated with chlorhexidine-silver sulfadiazine and minocycline/rifampin have shown trends in reduced infection rates, but their clinical effectiveness remains questionable (16, 17). Other treatments, including the use of silver-impregnated subcutaneous collagen cuffs, have also shown to be ineffective in recent trials (18, 19). CVC contamination generally requires device removal and replacement, in addition to a prolonged course of antifungal drug therapy, which raises concerns regarding drug toxicity and the development of antifungal resistance. Antifungal chemotherapy is also problematic, as many antifungal drugs are either toxic to the host (amphotericin B) or result in drug-resistant strains (triazoles) (20). Due to the high morbidity and mortality rates of catheter-related Candida species bloodstream infections, strategies for preventing medical device contamination by fungal pathogens remain a top priority for infection control.
In this study, we sought to elucidate the antimicrobial effectiveness of two p-phenylene ethynylene (PE) compounds against Candida species. A subset of conjugated polyelectrolytes, phenylene ethynylenes, have shown promising biocidal activity against Gram-positive and -negative bacterial pathogens, as well as the environmental yeast Saccharomyces cerevisiae (21–23). The chemical structure of these compounds renders them capable of inducing broad-spectrum cell damage. The phenylene ethynylenes studied consist of alternating phenyl and acetylenic groups with appended cationic groups (Fig. 1). The interaction of the cationic quaternary ammonium groups with net-anionic membranes and cell walls facilitates interactions with microbes, leading to membrane perturbation, pore formation, and the leakage of cell contents (24). In addition, when PEs are irradiated by the appropriate wavelength, the backbone produces reactive oxygen species (ROS) that induce rapid cell death (25, 26).
FIG 1.
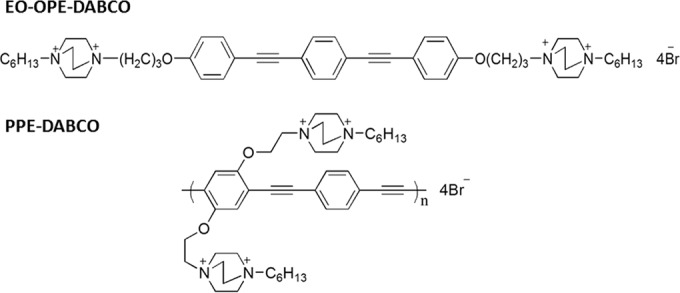
Molecular structures of oligomeric EO-OPE-DABCO and polymeric PPE-DABCO.
In previous studies, the oligomeric and polymeric molecular sizes of PE compounds have played an important role in their mechanisms of killing. The antimicrobial activity of these compounds is dependent on various factors, including molecular conformation, size, functional groups, and, of course, membrane composition of the target pathogen (22). The studies described here serve as a preliminary investigation into the utility of a cationic conjugated polymer, poly(p-phenylene ethynylene) (PPE), and an oligomer, “end-only” oligo(p-phenylene ethynylene) (EO-OPE), as antifungals. After treatment with the compounds—here referred to, respectively, as PPE-DABCO and EO-OPE-DABCO (where DABCO is quaternized diazabicyclo[2,2,2]octane)—the viability of Candida spp. was monitored using flow cytometry. This novel class of compounds may provide an innovative approach to the prevention of catheter-related bloodstream infections caused by C. albicans and other Candida species.
MATERIALS AND METHODS
Fungal culture.
Candida albicans (ATCC MYA-2876), C. parapsilosis (ATCC 22019), and C. glabrata (ATCC 2001) were grown from glycerol stocks and stored at −80°C. This stock was transferred to 5 ml filter-sterilized yeast extract-peptone-dextrose (YPD) medium (Becton Dickinson) and grown for 16 h at 30°C, with a shaking speed of 300 rpm. Such growth conditions yielded yeast cells at the late log phase (27).
Following a 10-min centrifugation at 2,900 relative centrifugal force (RCF), the supernatant was replaced with sterile phosphate-buffered saline (PBS) and subsequently vortexed. This washing step was repeated a second time to mitigate cell debris. The cell concentration was then determined using a disposable hemocytometer (C-Chip; Bulldog Bio catalog no. DHC-N01).
Derivation of clinical isolate strains of C. albicans.
Patient specimens (peripheral blood or catheter tips) were processed by Tricore Reference Laboratories (Albuquerque, NM) and identified as C. albicans by use of a Bruker Biotyper MALDI-TOF (matrix-assisted laser desorption ionization–time of flight mass spectrometry) system (MS ID score > 2.0). Clonal isolates so identified were subcultured on Sabouraud agar slants and provided to the investigators as unique strains. Isolate strains were provided in completely deidentified form according to procedures approved by the University of New Mexico School of Medicine Human Research Protections Office. For biocidal assays, clinical isolates were grown in YPD broth as described above.
Biocidal testing.
All biocidal experiments were carried out in either translucent or opaque 1.5-ml microcentrifuge tubes, at cell concentrations of 5 × 106/ml in PBS. Two cationic compounds were tested, both based on the p-phenylene ethynylene backbone, with quaternized diazabicyclo[2,2,2]octane (DABCO) functionalities. EO-OPE-DABCO and PPE-DABCO stocks were prepared in sterile deionized water (18.2 MΩ · cm at 25°C) and contained 0.47% dimethyl sulfoxide (by volume) to improve solubility and minimize aggregate formation. Negative controls contained equal amounts of dimethyl sulfoxide.
Samples were exposed to controlled amounts of light by use of a 14-lamp photoreactor (LZC-4V; Luzchem Research, Ottawa, Ontario, Canada). A rotating carousel ensured that all samples receive equivalent levels of light exposure; ventilation kept the photoreactor below 30°C. EO-OPE-DABCO absorbs in the UV region (28), warranting the use of UVA lamps (350-nm emission peak; 4.46 ± 2.41 mW/cm2) to optimize singlet oxygen yields. Conversely, 420-nm blue-light lamps (6.62 ± 2.93 mW/cm2) were used in PPE-DABCO tests; unlike its oligomeric counterpart, polymeric PPE-DABCO absorbs in the near-visible range (29). Power density output was measured at the peak excitation wavelength for both lighting configurations. Data shown are an aggregation of the results of two independent replicate experiments.
Samples were then stained with 5 μM membrane-permeable SYTO 9 (Invitrogen catalog no. S34854) and 1 μM membrane-impermeable TO-PRO-3 (Invitrogen catalog no. T3605), both of which are nucleic acid stains. After 30 min, samples were evaluated by flow cytometry (FACSCalibur; Becton Dickinson). At least 10,000 events were evaluated in each trial. A heat-killed sample (70°C for 30 min) was used to identify the fluorescence characteristics of dead cells.
Fifteen-minute assays of activity in the dark were carried out in a somewhat different manner. Samples were prepared and stained with SYTO 9 and TO-PRO-3, albeit in the absence of any biocide. After a 30-min staining duration, EO-OPE-DABCO was added (10 μg/ml final concentration); the sample was then vortexed and analyzed by flow cytometry. Every minute, viability data were collected (again, 10,000 events/sample), for a total of 15 min. It is important to note that EO-OPE-DABCO was added one sample at a time, so that, in each case, flow cytometry readings could begin within 1 min of the introduction of the biocide.
Spectroscopy of β-glucan interactions.
Stocks of S. cerevisiae β-(1, 3)-glucan (high, medium, or low molecular weight [MW]; gift of Biothera, Eagan, MN), PPE-DABCO, and EO-OPE-DABCO were mixed with 10 mM phosphate buffer at pH 7.4 to a final concentration of 2 μg/ml for PPE-DABCO or EO-OPE-DABCO and 100 μg/ml for β-glucan. Solutions of 200 μl were transferred to a 160-μl-nominal-volume fused quartz fluorimetry cuvette and read on a PTI QuantaMax 40 steady-state fluorescence spectrophotometer (HORIBA Scientific, Edison, NJ) with photomultiplier tube detection. Emission spectra were obtained using excitation wavelengths of 350 nm for EO-OPE-DABCO and 420 nm for PPE-DABCO, and excitation spectra were obtained with the corresponding maximum emission wavelength.
Interactions of PPE-DABCO with glucan microparticles.
Glucan microparticles were prepared from C. albicans SC5314 yeast cells using the extraction techniques described by Lowman et al. (30). Glucan microparticles were then suspended in PBS at a concentration of 1 × 105 ml−1. Microparticles were sonicated for 10 min and subsequently vortexed for another 5 min. PPE-DABCO was then added at a concentration of 2 μg/ml and incubated at room temperature for 1 h. No PPE-DABCO was added to the negative control. Five hundred microliters of each sample was transferred to petri dishes for examination by confocal microscopy. Excitation at 405 nm was used to induce fluorescence of PPE-DABCO.
Surface exposure of β-glucan.
C. albicans yeast cells were treated in a manner similar to that of the previously described biocidal experiments. In an effort to maintain a consistent degree of cell death across samples, OPE-DABCO exposure to UVA light was limited to just 10 min. A thermal positive control was also implemented, which entailed heating samples to 100°C for 30 min. Following the appropriate treatment and removal from the photoreactor, samples were blocked with 1% (wt/vol) bovine serum albumin (BSA) for 30 min at room temperature. The samples were then treated with a primary antibody, anti-β-glucan IgG (catalog no. 400-2; Biosupplies, Victoria, Australia), at a final concentration of 10 μg/ml for an additional 30 min. Negative controls contained 10 μg/ml isotype-matched murine IgG in place of anti-β-glucan IgG. An Alexa Fluor 647-conjugated secondary antibody (Invitrogen catalog no. A21235) (1 µg/ml) and SYTO 9 (5 µM) in PBS plus 1% BSA were simultaneously added and allowed to stain cells for 30 min at 25°C prior to analysis by flow cytometry. Data shown are an aggregation of the results of two independent replicate experiments.
Tissue culture and transfection.
HEK-293 cells (ATCC CRL-1573) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum (CS), 1% penicillin-streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate at 37°C, 5% CO2. The cells were then plated in 6-well plates at 1 × 105 cells per well. mApple-human Dectin-1A-C-10 (gift of Michael Davidson; Addgene plasmid no. 54883) was transfected into cells by following standard protocols using Fugene 6 (Promega product no. E2691). Cell cultures were used for further experimentation at 24 h posttransfection with growth in normal medium, as described above.
Phagocytosis assay.
C. albicans yeast cells were subjected to the same treatment conditions described above for the β-glucan exposure study, before being spun down and washed in PBS. Following the last washing step, the C. albicans yeast cells were stained with 7.5 μM SYTO 9 and 75 μM CypHer5E NHS ester (GE Healthcare; PA 15401) for 1 h at 25°C. After the C. albicans cells were stained, they were added to live, Dectin-1A-C-10-transfected HEK-293 cells for 1 h. Next, ice-cold PBS was used to lift the HEK-293 cells off the plate. The controls with C. albicans or HEK-293 cells alone or the above samples with a mixture of C. albicans and HEK-293 cells were analyzed using an LSR Fortessa flow cytometer (Becton Dickinson) and FlowJo software (FlowJo, Ashland, OR). At least 10,000 side scatter (SSC)-positive events are evaluated in each trial. CypHer5E, SYTO 9, and mApple fluorescence was excited using the following laser lines: CypHer5E, 640 nm; SYTO 9, 488 nm; mApple, 561 nm. Emission from the same dyes was observed using the following optics: CypHer5E, band-pass 670/14 nm filter (center/full-width half maximum); SYTO 9, long pass 505 nm dichroic mirror and band-pass 530/30 nm filter; mApple, band-pass 582/15 nm filter. Temporal separation of excitation from independent laser lines and satisfactory spectral separation of emissions ensured negligible cross-talk between these three fluorescent probes. Data shown are an aggregation of the results two independent replicate experiments.
RESULTS
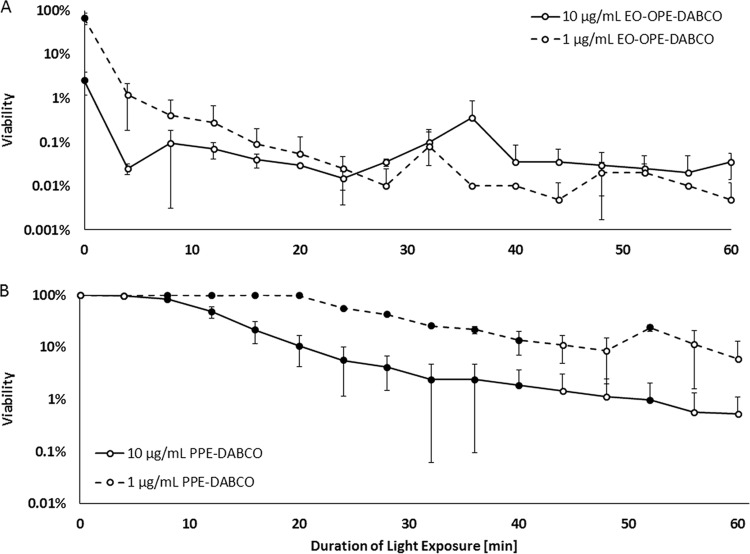
A series of biocidal studies were carried out to gain insight into the light-activated effects of EO-OPE-DABCO and PPE-DABCO on Candida species pathogens. Phenylene ethynylenes are unique in that their mechanism of action differs depending on the presence of light, in particular with respect to light intensity, emission wavelength, and duration (31). In the studies described here, duration of light exposure was the primary variable being studied. Light intensity was kept constant using a photoreactor with 14 interchangeable lamps. Lamps were chosen to have an emission wavelength overlapping the excitation spectrum of the phenylene ethynylene being used. UVA lamps that were centered on 350 nm were implemented for OPE testing, while 420-nm-centered lamps were used in PPE tests. With the light intensity and the spectrum being held constant for a given phenylene ethynylene, we focused on light exposure duration as the major focal point in an effort to discern the susceptibility of C. albicans to phenylene ethynylenes in the light versus that in the dark. Even though all samples were exposed to one of the two compounds for a total of 60 min, the duration of light exposure was varied by 4-min intervals and the balance of the 60 min of exposure was in the dark.
Figure 2A illustrates the biocidal activity of the two concentrations of EO-OPE-DABCO, 1 and 10 μg/ml. In the absence of light, a 1-μg/ml concentration of OPE killed 34% of C. albicans yeast cells; however, killing drastically increased with just minimal light exposure, as 2-log cell death was observed after just 8 min. Increasing the concentration to 10 μg/ml greatly improved the killing capacity of the OPE in the dark, resulting in 97% cell death. With minimal light exposure, 10 μg/ml EO-OPE-DABCO exhibited a profound biocidal effect, exceeding a 3-log reduction after just 4 min in UVA light. Both OPE concentrations exceeded 3-log kill (over 99.9% cell death) after 20 min of light exposure, and a 4-log reduction (99.99% cell death) was nearly achieved after 60 min of light exposure. Interestingly enough, lowering the concentration of OPE to just 1 μg/ml had little effect on light-activated biocidal activity but a far greater effect on killing in the dark. Some level of PE photodegradation was notable by 60 min (data not shown), which is why testing durations were limited to 1 h, as photodegradation limits 1O2 generation.
FIG 2.
C. albicans yeast viability as a function of light exposure duration and antimicrobial concentration. Viability is shown on a logarithmic scale for EO-OPE-DABCO (A) and PPE-DABCO (B) at 1 μg/ml (dashed lines) and 10 μg/ml (solid lines). Significant differences in viability are indicated by filled data markers (P value ≤ 0.01).
Figure 2B illustrates the viability of C. albicans following exposure to PPE-DABCO. It is quite evident that unlike EO-OPE-DABCO, its PPE counterpart is nontoxic in the absence of light; even at a relatively high concentration of 10 μg/ml, little to no cell death was observed even after 8 min of exposure to 420-nm light. After 52 min of continuous light exposure, 10 μg/ml PPE was able to kill 99% of all C. albicans yeast cells. In summary, we found the killing by the OPE in the dark to be concentration dependent, while the activity in the light was not. Conversely, the PPE's killing in the dark was not dependent on concentration, since it failed to elicit membrane damage in that case. The biocidal activity of PPE-DABCO is predicated on light exposure.
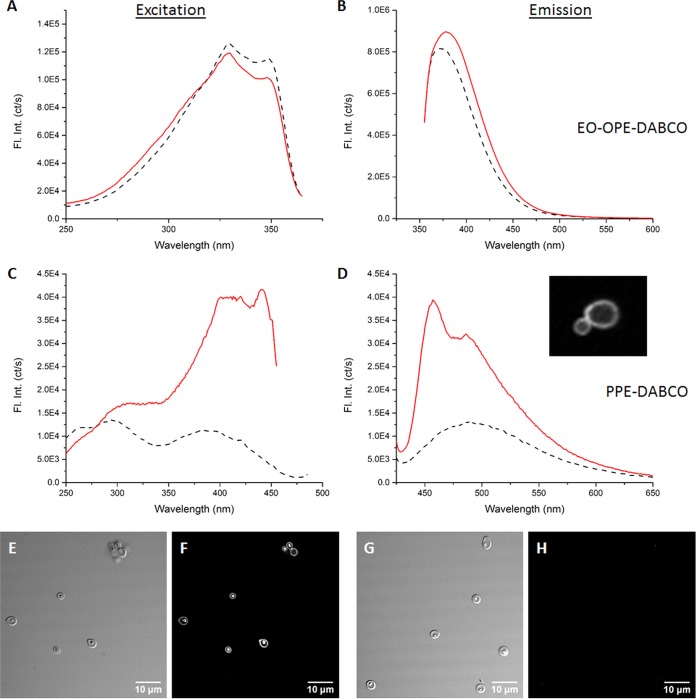
The inability of PPE-DABCO to kill C. albicans yeast cells in the dark led us to question whether the polymer may bind extensively to cell wall components, which might limit its ability to access the cell membrane. We evaluated the interactions between the two PEs with soluble β-(1,3)-glucan extracted from Saccharomyces cerevisiae yeast cell walls (32). The structures of S. cerevisiae β-(1,3)-glucan and C. albicans β-(1,3)-glucan are quite similar, and the polysaccharide is an important part of Candida drug resistance and pathogenicity, amounting to 40% of the cell wall (33). Size-fractionated β-glucan samples (low MW, 11 kDa; medium MW, 150 kDa; high MW, 450 kDa) were tested (Fig. 3A to D; see Fig. S1 in the supplemental material). Excitation and emission spectra of EO-OPE-DABCO and PPE-DABCO were evaluated in the absence and presence of the soluble β-glucan.
FIG 3.
Excitation (A, C) and emission (B, D) spectra illustrating the interactions between high-molecular-weight soluble β-glucan and EO-OPE-DABCO (A, B) or PPE-DABCO (bottom, D). Dashed lines represent the spectra of PE compounds alone, and solid lines represent the spectra observed in PE–β-glucan mixtures. The inset in panel D illustrates fluorescent PPE-DABCO concentrated in the cell wall of a C. albicans yeast cell, as shown in Fig. S2 in the supplemental material. Fl. Int. (ct/s), fluorescence intensity (counts/second). Additionally, confocal microscopy images illustrate PPE-DABCO bound to glucan microparticles. A transmitted light image (E) and reflected light image (F) are shown, with 405-nm excitation being used to generate fluorescence of bound PPE-DABCO. No fluorescent signal is observed in the absence of PPE-DABCO (G, H).
We observed enhanced emission of both PEs upon the introduction of the high-molecular-weight β-glucan, which is indicative of complexation (34). Emission enhancement is more profound in the case of PPE-DABCO (Fig. 3A to D), suggesting that complexation with soluble β-glucan promotes disaggregation of the PE polymer. In addition, a small degree of red-shifting was observed, implying that rotation of the conjugated regions of the PEs is restricted due to complexation with soluble β-glucan (35). The complexation between PPE-DABCO and β-glucan suggests that it may be largely sequestered in the cell wall; such positioning promotes interaction with components of the outer cell wall but may limit its ability to directly disrupt the plasma membrane.
These results shed light on the mechanisms by which EO-OPE-DABCO kills C. albicans yeast cells (Fig. 2A; see Discussion). With this in mind, the OPE became a focal point of this study from a biocidal perspective. With the determination that EO-OPE-DABCO was highly effective at killing standard laboratory strain C. albicans (SC5314), the question remained whether or not its biocidal efficacy would carry over to C. albicans clinical isolates.
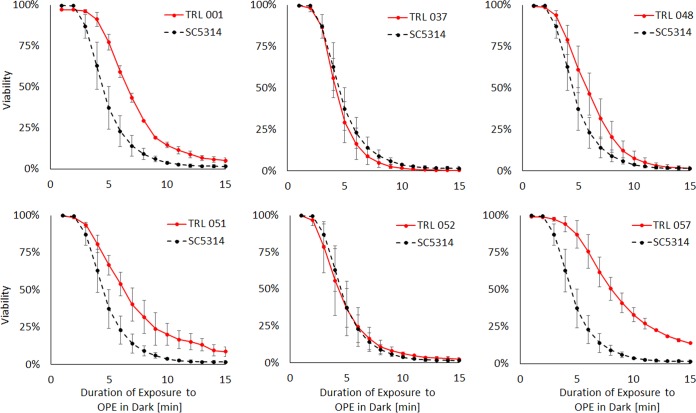
Using a modified biocidal assay, six C. albicans clinical isolates were surveyed for their susceptibility to 10 μg/ml EO-OPE-DABCO in the dark. In this instance, the cells were stained with SYTO 9 and TO-PRO-3 before the introduction of EO-OPE-DABCO. Taking a flow cytometry dual-fluorescence measurement of 10,000 events every minute allowed for real-time reporting of OPE-induced membrane perturbation. The susceptibility of clinical isolates was gauged relative to that of C. albicans SC5314, as shown in Fig. 4. Three of the six isolates, TRL 001 (P value = 0.006), TRL 051 (P value = 0.0013), and TRL 057 (P value = 0.0003), showed significantly increased levels of OPE resistance within 15 min in the form of slower kinetics of killing and higher residual viability after 15 min of treatment. Conversely, no OPE resistance was observed in TRL 037, TRL 040, and TRL 052.
FIG 4.
Susceptibility of various C. albicans clinical isolates to 10 μg/ml EO-OPE-DABCO in the dark. Laboratory strain SC5314 is shown for reference in all cases. Strains with the prefix “TRL” are recent (circa 2015) clinical isolates obtained as described in Materials and Methods.
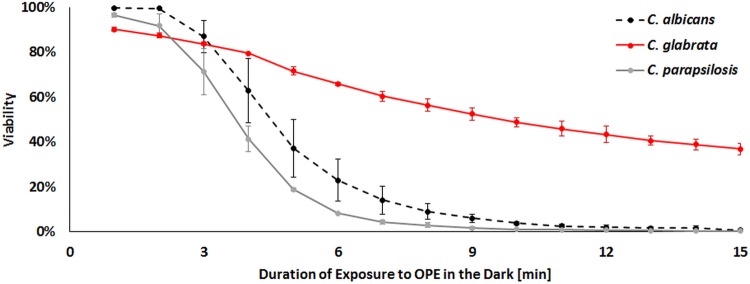
The variability in susceptibility to EO-OPE-DABCO among clinical isolates of one species (C. albicans) suggested that pathogenic non-albicans Candida species might also exhibit variable sensitivity to this biocide. To test this, we revised the above-mentioned 15-min flow cytometry assay to determine if EO-OPE-DABCO was more or less effective against C. parapsilosis and C. glabrata than it was to C. albicans SC5314. Figure 5 shows similar levels of biocidal activity against C. albicans and C. parapsilosis but less activity against C. glabrata, with about 50% surviving after 15 min of exposure. This result is consistent with the fact that C. albicans and C. parapsilosis share a closer phylogenetic relationship than is found between C. albicans and C. glabrata (36).
FIG 5.
Viability of C. albicans, C. glabrata, and C. parapsilosis in the presence of 10 μg/ml EO-OPE-DABCO in the dark.
β-Glucan is highly immunogenic upon recognition by the innate immunoreceptors Dectin-1 and Mac-1 (37). Several prominent genera of fungal pathogens, including Candida, are known to employ an innate immune evasion strategy of masking β-glucan to restrict its exposure on the cell wall surface (38–41). We hypothesize that PE antimicrobials bound to cell wall constituents and exposed to light will generate singlet oxygen, leading to local cell wall damage, unmasking β-glucan and increasing immunogenicity. Using an anti-β-(1,3)-glucan primary antibody in tandem with a secondary fluorescently labeled antibody allowed for the comparison of β-glucan exposures following treatment conditions: PE in the dark, PE in the light, and a 60-min light negative control. C. albicans yeast treated with EO-OPE-DABCO in the dark, using conditions associated with high biocidal activity (Fig. 2), exhibited no increase in β-glucan exposure. Likewise, we observed no β-glucan unmasking with light-activated EO-OPE-DABCO. We then treated C. albicans with PPE-DABCO and observed β-glucan exposure. PPE-DABCO clearly binds to the fungal cell wall, as evidenced by strong PPE-DABCO emission upon 405-nm excitation using confocal imaging (see Fig. S2 in the supplemental material). In the absence of stimulation by light, PPE-DABCO treatment results in no significant increase in β-glucan exposure. After illumination, PPE-DABCO-treated cell walls do show evidence of significant β-glucan unmasking (Fig. 6). These results suggest that β-glucan masking in Candida cell walls is light dependent and, presumably, mediated by 1O2 and other ROS.
FIG 6.
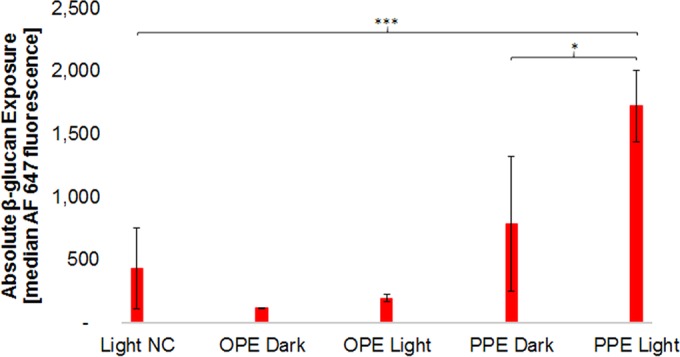
Absolute β-glucan exposure of C. albicans following various treatments. β-Glucan exposure was estimated from the median fluorescence signal of Alexa Fluor 647 (AF 647). The exposure duration for all samples was 60 min, with the exception of EO-OPE-DABCO exposure in the light, for which the exposure duration was limited to 10 min. NC, negative control; *, 0.01 ≤ P value < 0.05; ***, P value < 0.001.
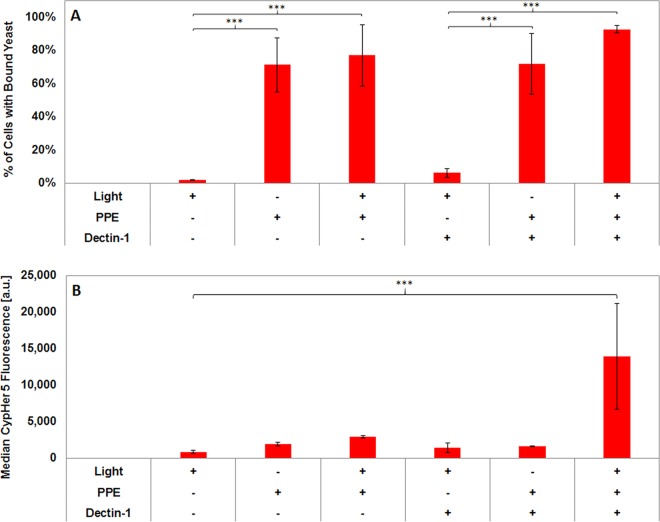
Given the evidence that PPE-DABCO can increase β-glucan exposure on C. albicans yeast cells, we tested whether the unmasking achieved by this treatment resulted in greater recognition of yeasts through the β-glucan receptor Dectin-1. HEK-293 cells were transfected with mApple-tagged human Dectin-1a, whereby expression is sufficient to drive phagocytosis of C. albicans yeast cells (42). Our transfection conditions resulted in Dectin-1-positive HEK-293 cells, discriminated by a positive mApple signal, and nontransfected cells, which were negative for mApple and served as an internal control to assess Dectin-1 dependence of binding and phagocytosis. We employed a flow cytometric assay of yeast cell binding to and internalization by HEK-293 transfectants. Yeast cells were labeled with the pH-sensitive dye CypHer5E, which increases dramatically in emission intensity after internalization within acidic phagosomal compartments (43, 44). The CypHer5E signal was used to measure binding and internalization of yeast cells. Flow cytometry data were gated on HEK-293 cell-containing events for analysis, as defined by a high side scatter signal, which was significantly greater than that of free yeast cells. Yeast cells that were bound to HEK-293 cells registered a low CypHer5E signal. If yeast cells were internalized, the CypHer5E signal was much higher. The percentage of HEK-293 cells with yeast bound (for mApple-Dectin-1-positive and -negative cells) was determined by the percentage of SSC-gated events with a low or high CypHer5E signal. The extent of phagocytosis was assessed by the median CypHer5E fluorescence intensity within these populations (see Fig. S3 in the supplemental material).
As can be seen in Fig. 7, we observed minimal binding between mApple-Dectin-1-negative HEK-293 cells and untreated C. albicans yeast cells. Even if the HEK-293 cell has been transfected and is expressing Dectin-1 (mApple positive), β-glucan masking permits very little β-glucan to be accessible at the cell wall surface for Dectin-1 binding (as seen in Fig. 6). Conversely, PPE-treated C. albicans yeast cells bind avidly to HEK-293 cells, and this binding is independent of excitation of PPE-DABCO or Dectin-1 expression by the HEK-293 cells (Fig. 7A). These data suggest that the binding of PPE-DABCO to Candida cell walls alters their surface properties in ways that promote Dectin-1-independent adhesion to human cells, perhaps through electrostatic and/or hydrophobic mechanisms. The extent of the interaction between the yeast cell and the HEK-293 cell is not dependent on the degree of incurred cell membrane damage, as C. albicans killed with light-activated PPE were no more likely to bind HEK-293 cells. Despite their ability to bind HEK-293, internalization of PPE-DABCO-treated C. albicans yeast cells required Dectin-1 expression and excitation of PPE-DABCO prior to binding (Fig. 7B). These data indicate that the β-glucan unmasking caused by light activation of PPE-DABCO on C. albicans cell walls results in the biological outcome of increased Dectin-1-dependent phagocytosis.
FIG 7.
PPE-DABCO induces C. albicans yeast phagocytosis by HEK-293 cells in a manner that requires illumination of PPE-DABCO and expression of the β-glucan receptor Dectin-1. Prior to the addition of HEK cells, samples were first treated with 10 μg/ml PPE-DABCO for 1 h and then subsequently stained with CypHer5E and SYTO 9. (A) PPE-DABCO treatment, with or without illumination, induces increased binding of C. albicans to HEK-293 cells in a Dectin-1-independent fashion. (B) Phagocytosis of C. albicans yeast bound to HEK-293 cells requires Dectin-1 expression and is induced by light treatment of PPE-DABCO. a.u., arbitrary units; ***, P value < 0.001.
DISCUSSION
Cationic phenylene ethynylenes exhibit biocidal effects against Candida yeast.
Despite its intrinsic resistance to cationic and oxidative stresses (45, 46), C. albicans was highly susceptible to EO-OPE-DABCO and, to a lesser extent, to PPE-DABCO. The biocidal activity of these compounds against C. albicans utilizes a dual mechanism combining light-independent cationic stress and light-dependent oxidative stress. These results resemble those of previous studies, which demonstrate the effectiveness of a dual-mechanism mode of biocidal action (47). Unlike other broad-spectrum antimicrobials, PEs exhibit low levels of in vitro toxicity against mammalian cells (48), making them attractive candidates in numerous clinical applications.
Therefore, it is relevant to note that all clinical isolate strains exhibited significant levels of killing during a 15-min exposure to EO-OPE-DABCO. The partial resistance of some clinical isolate strains may derive from adaptations of the pathogen to growth in the host, which may cause changes in cell wall structure and upregulation of mechanisms that permit growth under adverse conditions, such as leukocyte-derived ROS in the phagosomal environment (49, 50). Previous research has also suggested a correlation between Candida resistance to the antifungal drug amphotericin B and cell wall structure and composition (51, 52).
While C. parapsilosis was found to be as susceptible to EO-OPE-DABCO in the dark as C. albicans, C. glabrata displayed an inherent resistance. Candida spp. experience cationic stress as they interact with innate immune defenses. For example, cationic antimicrobial peptides, such as Histatin-5, are deployed in host defense against Candida spp. and are thought to work by disrupting fungal plasma membrane integrity (53). C. glabrata is noted for its resistance to killing by cationic antimicrobial peptides in comparison to C. albicans and other pathogenic Candida species (54–56). Furthermore, the decreased ability of EO-OPE-DABCO to kill C. glabrata resembles the results of a previous study, in which a 10-μg/ml concentration of the compound failed to kill 99% of S. cerevisiae yeast cells, even after an hour in the light (23). Although S. cerevisiae is benign, it is closely related to C. glabrata (32, 36). Finally, C. glabrata is also known to have robust antioxidative defenses that allow it to survive in the phagosome (57, 58) and that may impact its ability to resist oxidative killing by cationic phenylene ethynylenes.
Interactions with β-glucan.
An interesting result of this study is that PPE-DABCO strongly associates with soluble β-(1,3)-glucan (Fig. 3C and D), which is important for structural support of the cell wall of C. albicans (32), as well as glucan microparticles (Fig. 3E and F). We speculate that the PPE-DABCO–β-glucan interaction may directly cause more global disruption to the cell wall, and it is likely that the targeting of polymeric phenylene ethynylenes to cell wall polysaccharides places them in an ideal position to induce reactive oxygen-mediated damage to cell wall components after photoactivation. Using transmission electron microscopy and other techniques, Wang et al. demonstrated this behavior with PPE-DABCO and Gram-negative Escherichia coli (31). However, we also note the alternative possibility that glucan exposure upon treatment with PPE-DABCO and light might require cell wall remodeling downstream of cell wall stress responses due to ROS generated by excited PPE-DABCO.
Conversely, the OPE appears far less prone to complexation with the soluble β-(1,3)-glucan. Although this may limit the ability of OPE to unmask mannoproteins and reveal more β-(1,3)-glucan (Fig. 6), the lack of interaction with the glucan likely allows the molecule to quickly penetrate the cell wall and to access and damage the cell membrane. Abundant lateral noncovalent interactions between β-glucan and PPE-DABCO may promote PE–β-glucan complexation, analogous to the role of interpolymeric hydrogen bonding in stabilizing lateral interactions of individual β-glucan polymers in aqueous solution (59, 60). PPE-DABCO is far larger than its oligomeric counterpart and has numerous sites where weak interactions with β-glucan polymers may form; furthermore, extensive valency of laterally aggregated β-glucan would make this interaction very strong. Emission enhancement of the PE polymer (Fig. 3) resembles that of a previous study, in which PPE-DABCO was shown to exhibit similar photophysical effects in methanol, as opposed to water (29). Therefore, we suggest that abundant complexation with soluble β-glucan disaggregates PPE-DABCO in aqueous buffer.
These results help to rationalize the inability of PPE to kill C. albicans in the dark. It is likely that PPE-DABCO, which exhibits a strong propensity to interact and associate with β-glucan, is limited in its ability to fully penetrate the cell wall and that much of the compound is sequestered on β-glucan in the cell wall. Given the limited radius of destruction of singlet oxygen and the density of organic material in the cell wall capable of quenching singlet oxygen, this association may limit the depth of cell wall permeation by PPE-DABCO and its capacity to directly perturb the yeast's plasma membrane, in comparison to EO-OPE-DABCO. However, an advantage of PPE-DABCO's ability to bind β-glucan may be an increased specificity for and targeting to the fungal pathogen's cell wall, as opposed to host tissues, which are devoid of β-glucan. The oligomer, on the other hand, appears far less likely to interact with β-glucan, which may allow it to permeate the fungal cell wall more readily and to better access the yeast's plasma membrane.
PPE-DABCO unmasks β-glucan.
Furthermore, PPE-DABCO displays immunostimulatory attributes, particularly in the light. This polymer was found to unmask the outer cell wall of C. albicans yeast cells in such a way that β-(1,3)-glucan could more easily be recognized and bound by pattern recognition receptor Dectin-1. PPE-DABCO binds to yeast cell walls (see Fig. S2 in the supplemental material). The chemical basis of this binding may relate to direct interactions between PPE-DABCO and β-(1,3)-glucan, as discussed above. Additionally, PPE-DABCO may interact electrostatically with anionic moieties in the outer cell wall. Ultrastructural studies have described the presence of evenly dispersed anionic sites on the C. albicans yeast surface (61). Also, C. albicans N-linked mannans contain abundant oligomannose side chains attached via anionic phosphodiester linkages that could provide sites of electrostatic binding for polycations like PPE-DABCO (62, 63). In either configuration, PPE-DABCO would be ideally positioned in the outer cell wall to damage mannoproteins that are thought to provide β-glucan masking. Our results suggest that merely the binding of PPE-DABCO to C. albicans increases the adherence of the yeast to HEK-293 cells in a receptor-independent fashion, suggesting that PPE-DABCO alters cell wall surface characteristics in ways that impact the interaction with host cells nonspecifically (Fig. 7A). However, increases in both β-glucan exposure and Dectin-1-dependent phagocytosis require excitation of PPE-DABCO, which probably results in direct oxidative damage to the cell wall, leading to β-glucan unmasking. This is the first instance in which PEs have been demonstrated to elicit immunostimulatory attributes. Our work demonstrates that the biocidal and immunostimulatory properties of phenylene ethynylene antimicrobials make them promising candidates for novel antimicrobial applications to improve the health outcomes of patients with life-threatening fungal infectious diseases.
Potential antimicrobial applications.
Some antifungal medical applications envisioned for phenylene ethynylenes concern the treatment of medical devices or topical applications in wound care. For instance, prevention of catheter infections by the use of improved antimicrobial materials or preventive device treatment regimens could save lives, improve quality of life, and reduce costs associated with treating catheter-associated infections. However, providing light to activate phenylene ethynylenes in the catheter context is an important challenge. First, we showed that EO-OPE-DABCO has strong biocidal activity against C. albicans, even without light activation, so it is possible that this compound could be used in antifungal lock solutions even without illumination. Second, central venous catheters are susceptible to contamination via their hubs and injection ports. These device parts are external and accessible for illumination by external sources or integrated light-emitting diode (LED) sources. The port and hub lumen could be regularly filled with a phenylene ethynylene antimicrobial and subjected to light-induced disinfection as part of a routine infection prevention program. Third, phenylene ethynylene compounds could be grafted to external medical device materials (e.g., central venous catheter hubs, ports, and tubes) to incorporate surfaces that could be regularly disinfected by the application of light. In addition, phenylene ethynylenes could find medical use in the area of wound care as topically applied antimicrobials and by incorporation into wound dressings. In this setting, light activation could be applied manually from external sources during dressing changes or in a more automated fashion from an integrated dressing LED light source, to prevent contamination of the wound. Additional costs associated with the use of antimicrobial catheter treatments and associated illumination systems would be offset by prevention of the substantial costs associated with the treatment of catheter-associated infections, as well as by improvements in patient morbidity and mortality.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yue Zhang for her contributions to the biocidal experiments conducted at the onset of this study.
Support from the following sources was instrumental in the completion of this research: American Heart Association research grant 15BGIA25690020 to A.K.N. and H.C.P., NIH training grant T32AI007538 to M.S.G., and Defense Threat Reduction Agency research grant HDTRA1-08-1-0053 to D.G.W.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00317-16.
REFERENCES
- 1.Maki DG, Kluger DM, Crnich CJ. 2006. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Snydman DR, Gorbea HF, Pober BR, Majka JA, Murray SA PL. 1982. Predictive value of surveillance skin cultures in total-parenteral-nutrition-related infection. Lancet 320:1385–1388. doi: 10.1016/S0140-6736(82)91281-8. [DOI] [PubMed] [Google Scholar]
- 4.Bjornson HS, Colley R, Bower RH, Duty VP, Schwartz-Fulton JT, Fischer JE. 1982. Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery 92:720–727. [PubMed] [Google Scholar]
- 5.Cooper GL, Hopkins C. 1985. Rapid diagnosis of intravascular catheter-associated infection by direct Gram staining of catheter segments. N Engl J Med 312:1142–1147. doi: 10.1056/NEJM198505023121802. [DOI] [PubMed] [Google Scholar]
- 6.Raad I, Costerton W, Sabharwal U, Sadlowski M, Anaissie E, Bodey GP. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 168:400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]
- 7.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis 29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 8.Wingard JR. 1995. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis 20:115–125. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 9.Giri S, Kindo AJ. 2012. Review of Candida species causing blood stream infection. Indian J Med Microbiol 30:270. doi: 10.4103/0255-0857.99484. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Herrera J, Elorza MV, Valentín E, Sentandreu R. 2006. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res 6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 11.Klis FM, Groot PDE, Hellingwerf K. 2001. Molecular organization of the cell wall of Candida albicans. Med Mycol 39(Suppl 1):1–8. doi: 10.1080/mmy.39.1.1.8-0. [DOI] [PubMed] [Google Scholar]
- 12.Netea MG, Gow NAR, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van Der Meer JWM, Brown AJP, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin Microbiol Rev 17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nett JE, Andes DR. 2015. Fungal biofilms: in vivo models for discovery of anti-biofilm drugs. Microbiol Spectr 3(3):E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell KF, Taff HT, Cuevas MA, Reinicke EL, Sanchez H, Andes DR. 2013. Role of matrix β-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob Agents Chemother 57:1918–1920. doi: 10.1128/AAC.02378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brun-Buisson C, Doyon F, Sollet J-P, Cochard J-F, Cohen Y, Nitenberg G. 2004. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med 30:837–843. doi: 10.1007/s00134-004-2221-9. [DOI] [PubMed] [Google Scholar]
- 17.Ramritu P, Halton K, Collignon P, Cook D, Fraenkel D, Battistutta D, Whitby M, Graves N. 2008. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control 36:104–117. doi: 10.1016/j.ajic.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Kalfon P, De Vaumas C, Samba D, Boulet E, Lefrant JY, Eyraud D, Lherm T, Santoli F, Naija W, Riou B. 2007. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med 35:1032–1039. doi: 10.1097/01.CCM.0000259378.53166.1B. [DOI] [PubMed] [Google Scholar]
- 19.Groeger JS, Lucas AB, Coit D, LaQuaglia M, Brown AE, Turnbull A, Exelby P. 1993. A prospective, randomized evaluation of the effect of silver impregnated subcutaneous cuffs for preventing tunneled chronic venous. Ann Surg 218:206–210. doi: 10.1097/00000658-199308000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect JR. 2004. Antifungal resistance: the clinical front. Oncology (Williston Park) 18:15–22. [PubMed] [Google Scholar]
- 21.Zhou Z, Corbitt TS, Parthasarathy A, Tang Y, Ista LK, Schanze KS, Whitten DG. 2010. “End-only” functionalized oligo(phenylene ethynylene)s: synthesis, photophysical and biocidal activity. J Phys Chem Lett 1:3207–3212. doi: 10.1021/jz101088k. [DOI] [Google Scholar]
- 22.Wang Y, Zhou Z, Zhu J, Tang Y, Canady TD, Chi EY, Schanze KS, Whitten DG. 2011. Dark antimicrobial mechanisms of cationic phenylene ethynylene polymers and oligomers against Escherichia coli. Polymers (Basel) 3:1199–1214. doi: 10.3390/polym3031199. [DOI] [Google Scholar]
- 23.Wang Y, Chi EY, Natvig DO, Schanze KS, Whitten DG. 2013. Antimicrobial activity of cationic conjugated polyelectrolytes and oligomers against Saccharomyces cerevisiae vegetative cells and ascospores. ACS Appl Mater Interfaces 5:4555–4561. doi: 10.1021/am400220s. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Jones EM, Tang Y, Ji E, Lopez GP, Chi EY, Schanze KS, Whitten DG. 2011. Effect of polymer chain length on membrane perturbation activity of cationic phenylene ethynylene oligomers and polymers. Langmuir 27:10770–10775. doi: 10.1021/la201820k. [DOI] [PubMed] [Google Scholar]
- 25.Hill EH, Pappas HC, Whitten DG. 2014. Activating the antimicrobial activity of an anionic singlet-oxygen sensitizer through surfactant complexation. Langmuir 30:5052–5056. doi: 10.1021/la501230m. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Tang Y, Zhou Z, Ji E, Lopez GP, Chi EY, Schanze KS, Whitten DG. 2010. Membrane perturbation activity of cationic phenylene ethynylene oligomers and polymers: selectivity against model bacterial and mammalian membranes. Langmuir 26:12509–12514. doi: 10.1021/la102269y. [DOI] [PubMed] [Google Scholar]
- 27.King RD, Lee JC, Morris AL. 1980. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun 27:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dascier D, Ji E, Parthasarathy A, Schanze KS, Whitten DG. 2012. Efficacy of end-only-functionalized oligo(arylene-ethynylene)s in killing bacterial biofilms. Langmuir 28:11286–11290. doi: 10.1021/la302476s. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Pinto MR, Hardison LM, Mwaura J, Müller J, Jiang H, Witker D, Kleiman VD, Reynolds JR, Schanze KS. 2006. Variable band gap poly(arylene ethynylene) conjugated polyelectrolytes. Macromolecules 39:6355–6366. doi: 10.1021/ma0611523. [DOI] [Google Scholar]
- 30.Lowman DW, Greene RR, Bearden DW, Kruppa MD, Pottier M, Monteiro MA, Soldatov DV, Ensley HE, Cheng S-C, Netea MG, Williams DL. 2014. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem 289:3432–3443. doi: 10.1074/jbc.M113.529131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Schanze K, Chi E, Whitten D. 2013. When worlds collide: interactions at the interface between biological systems and synthetic cationic conjugated polyelectrolytes and oligomers. Langmuir 29:10635–10647. doi: 10.1021/la4012263. [DOI] [PubMed] [Google Scholar]
- 32.Moran G, Coleman D, Sullivan D, Butler G, Hoyer LL, Romani L, Netea MG, Gow NAR, Spellberg B, Fu Y, Ibrahim AS, Jang WS, Edgerton M, Murno C, Richard ML, Brown AJP, Haynes K, Quinn J, Zordan R, Cormack B, Vylkova S, Lorenz MC, Deepu A, Calderone R, Li D, Pfaller MA, Diekema DJ. 2012. Candida and candidiasis, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 33.Brown JA, Catley BJ. 1992. Monitoring polysaccharide synthesis in Candida albicans. Carbohydr Res 227:195–202. doi: 10.1016/0008-6215(92)85071-7. [DOI] [Google Scholar]
- 34.Hong Y, Lam JWY, Tang BZ. 2011. Aggregation-induced emission. Chem Soc Rev 40:5361–5388. doi: 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- 35.Donabedian PL, Pham TK, Whitten DG, Chi EY. 2015. Oligo(p-phenylene ethynylene) electrolytes: a novel molecular scaffold for optical tracking of amyloids. ACS Chem Neurosci 6:1526–1535. doi: 10.1021/acschemneuro.5b00086. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick DA, Logue ME, Stajich JE, Butler G. 2006. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol 6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsoni SV, Brown GD. 2008. β-Glucans and Dectin-1. Ann N Y Acad Sci 1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 38.Carrion S d J, Leal SM, Ghannoum MA, Aimanianda V, Latge J-P, Pearlman E. 2013. The RodA hydrophobin on Aspergillus fumigatus spores masks Dectin-1- and Dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol 191:2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rappleye CA, Eissenberg LG, Goldman WE. 2007. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A 104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler RT, Kombe D, Agarwala SD, Fink GR. 2008. Dynamic, morphotype-specific Candida albicans β-glucan exposure during infection and drug treatment. PLoS Pathog 4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheeler RT, Fink GR. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adie EJ, Kalinka S, Smith L, Francis MJ, Marenghi A, Cooper ME, Briggs M, Michael NP, Milligan G, Game S. 2002. A pH-sensitive fluor, CypHer 5, used to monitor agonist-induced G protein-coupled receptor internalization in live cells. Biotechniques 33:1152–1154, 1156–1157. [DOI] [PubMed] [Google Scholar]
- 44.Adie EJ, Francis MJ, Davies J, Smith L, Marenghi A, Hather C, Hadingham K, Michael NP, Milligan G, Game S. 2003. CypHer 5: a generic approach for measuring the activation and trafficking of G protein-coupled receptors in live cells. Assay Drug Dev Technol 1:251–259. doi: 10.1089/15406580360545062. [DOI] [PubMed] [Google Scholar]
- 45.Jamieson DJ, Stephen DW, Terrière EC. 1996. Analysis of the adaptive oxidative stress response of Candida albicans. FEMS Microbiol Lett 138:83–88. doi: 10.1111/j.1574-6968.1996.tb08139.x. [DOI] [PubMed] [Google Scholar]
- 46.Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, Brown AJP. 2009. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol 9:44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaloriti D, Jacobsen M, Yin Z, Patterson M, Tillmann A, Smith D a, Cook E, You T, Grimm MJ, Bohovych I, Grebogi C, Segal BH, Gow N a, Haynes RK, Quinn J, Brown AJP. 2014. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. mBio 5:1–11. doi: 10.3391/mbi.2014.5.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilde KNK, Whitten DGD, Canavan HE. 2013. In vitro cytotoxicity of antimicrobial conjugated electrolytes: interactions with mammalian cells. ACS Appl Mater Interfaces 5:9305–9311. doi: 10.1021/am402476g. [DOI] [PubMed] [Google Scholar]
- 49.Sasada M, Johnston RB. 1980. Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med 152:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miramón P, Kasper L, Hube B. 2013. Thriving within the host: Candida spp. interactions with phagocytic cells. Med Microbiol Immunol 202:183–195. doi: 10.1007/s00430-013-0288-z. [DOI] [PubMed] [Google Scholar]
- 51.Bahmed K, Bonaly R, Coulon J. 2003. Relation between cell wall chitin content and susceptibility to amphotericin B in Kluyveromyces, Candida and Schizosaccharomyces species. Res Microbiol 154:215–222. doi: 10.1016/S0923-2508(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 52.Mesa-Arango AC, Rueda C, Román E, Quintin J, Terrón MC, Luque D, Netea MG, Pla J, Zaragoza O. 2016. Cell wall changes in amphotericin B-resistant strains from Candida tropicalis and relationship with the immune responses elicited by the host. Antimicrob Agents Chemother 60:2326–2335. doi: 10.1128/AAC.02681-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mochon AB, Liu H. 2008. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog 4:e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikawa H, Jin C, Fukushima H, Makihira S, Hamada T. 2001. Antifungal activity of histatin-5 against non-albicans Candida species. Oral Microbiol Immunol 16:250–252. doi: 10.1034/j.1399-302X.2001.160409.x. [DOI] [PubMed] [Google Scholar]
- 55.Helmerhorst EJ, Venuleo C, Beri A, Oppenheim FG. 2005. Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast 22:705–714. doi: 10.1002/yea.1241. [DOI] [PubMed] [Google Scholar]
- 56.Raman N, Lee M-R, Lynn DM, Palecek SP. 2015. Antifungal activity of 14-helical β-peptides against planktonic cells and biofilms of Candida species. Pharmaceuticals (Basel) 8:483–503. doi: 10.3390/ph8030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briones-Martin-del-Campo M, Orta-Zavalza E, Cañas-Villamar I, Gutiérrez-Escobedo G, Juárez-Cepeda J, Robledo-Márquez K, Arroyo-Helguera O, Castaño I, De Las Peñas A. 2015. The superoxide dismutases of Candida glabrata protect against oxidative damage and are required for lysine biosynthesis, DNA integrity and chronological life survival. Microbiology 161:300–310. doi: 10.1099/mic.0.000006. [DOI] [PubMed] [Google Scholar]
- 58.Kasper L, Seider K, Hube B. 2015. Intracellular survival of Candida glabrata in macrophages: immune evasion and persistence. FEMS Yeast Res 15:fov042. doi: 10.1093/femsyr/fov042. [DOI] [PubMed] [Google Scholar]
- 59.Lowman D, Ensley H, Williams D. 2005. Introduction to the chemistry and immunobiology of beta-glucans, p 1–34. In Young S-H, Castranova V (ed), Toxicology of 1-3-beta-glucans. Informa Healthcare, Boca Raton, Florida. [Google Scholar]
- 60.Young SH, Dong WJ, Jacobs RR. 2000. Observation of a partially opened triple-helix conformation in 1→3-beta-glucan by fluorescence resonance energy transfer spectroscopy. J Biol Chem 275:11874–11879. doi: 10.1074/jbc.275.16.11874. [DOI] [PubMed] [Google Scholar]
- 61.Horisberger M, Clerc MF. 1988. Ultrastructural localization of anionic sites on the surface of yeast, hyphal and germ-tube forming cells of Candida albicans. Eur J Cell Biol 46:444–452. [PubMed] [Google Scholar]
- 62.Cutler JE. 2001. N-glycosylation of yeast, with emphasis on Candida albicans. Med Mycol 39(Suppl 1):S75–S86. [PubMed] [Google Scholar]
- 63.Hall RA, Gow NAR. 2013. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol 90:1147–1161. doi: 10.1111/mmi.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.