Abstract
The usage of fluconazole and amphotericin B in clinical settings is often limited by, among other things, drug resistance development and undesired side effects. Thus, there is a constant need to find new drugs to better manage fungal infections. Toward this end, the study described in this paper considered the repurposing of aspirin (acetylsalicylic acid) and ibuprofen as alternative drugs to control the growth of cryptococcal cells. In vitro susceptibility tests, including a checkerboard assay, were performed to assess the response of Cryptococcus neoformans and Cryptococcus gattii to the above-mentioned anti-inflammatory drugs. Next, the capacity of these two drugs to induce stress as well as their mode of action in the killing of cryptococcal cells was determined. The studied fungal strains revealed a response to both aspirin and ibuprofen that was dose dependent, with ibuprofen exerting greater antimicrobial action. More importantly, the MICs of these drugs did not negatively (i) affect growth or (ii) impair the functioning of macrophages; rather, they enhanced the ability of these immune cells to phagocytose cryptococcal cells. Ibuprofen was also shown to act in synergy with fluconazole and amphotericin B. The treatment of cryptococcal cells with aspirin or ibuprofen led to stress induction via activation of the high-osmolarity glycerol (HOG) pathway, and cell death was eventually achieved through reactive oxygen species (ROS)-mediated membrane damage. The presented data highlight the potential clinical application of aspirin and ibuprofen as candidate anti-Cryptococcus drugs.
INTRODUCTION
The advent of HIV/AIDS has led to the emergence of species such as Cryptococcus neoformans and Cryptococcus gattii as important disease-causing microbes (1–3). To demonstrate this point, these species are reported to cause over 1 million infections worldwide, with the highest burden of infections being localized in resource-poor settings (4). In their paper, Perfect et al. argued that the management of fungal diseases is strongly dependent on capital resources available to a specific region (5). Based on the latter, Sebolai and Ogundeji further argued that it is not surprising for countries in sub-Saharan Africa, given the complex geopolitical and socioeconomic challenges that prevail in this region, to be unable to provide the necessary life-saving drugs, which are often expensive (6). Toward this end, a solution may be to repurpose already FDA-approved drugs that are cheap, such as aspirin (acetylsalicylic acid) and ibuprofen.
We have previously reported that aspirin affected Cryptococcus cells in a number of ways: it (i) decreased capsule shedding, (ii) inhibited the production and trafficking of capsule-associated 3-hydroxy fatty acids, and (iii) inhibited cellular growth (7). In the current study, we build further on our prior antimicrobial work by drawing a comparison between the effects of aspirin and ibuprofen. Importantly, we also attempt to elucidate the mode of action employed by the two drugs in the killing of cryptococcal cells. The answers to the above-described objectives could assist in making a case to repurpose aspirin and ibuprofen as antimicrobial drugs, which, in turn, could help in the management of cryptococcal infections, more so in resource-poor settings, like Africa.
MATERIALS AND METHODS
Strains, cultivation, and standardization of cells.
Ten clinical Cryptococcus strains, including five C. neoformans strains (LMPE 028, LMPE 030, LMPE 043, LMPE 046, and LMPE 047) and five C. gattii strains (LMPE 045, LMPE 048, LMPE 052, LMPE 054, and LMPE 070), were used in this study. These strains were obtained from Universitas Academic Hospital, Bloemfontein, South Africa. The strains were maintained on yeast-malt extract (YM) agar (Merck, South Africa), after which a loopful of cells (0.01 ml loop) was taken from a 48-h-old YM agar plate and cultivated in a 250-ml conical flask containing 100 ml of yeast nitrogen base (YNB; 6.7 g/liter; Difco Laboratories, USA) broth supplemented with 4% (wt/vol) glucose (Merck, South Africa) while shaking at 160 rpm at 30°C. The cells were allowed to grow overnight before 0.1 ml of the old culture medium was inoculated into another conical flask containing 100 ml of fresh YNB broth. The cells were grown until the mid-exponential phase and were immediately washed twice, using phosphate-buffered saline (PBS; Oxoid, South Africa). Using a hemocytometer, the cells were subsequently standardized to 1 × 106 cells/ml in 15-ml centrifuge tubes (Becton Dickinson Labware, USA) containing 10 ml of RPMI 1640 medium (Sigma-Aldrich, South Africa). The cells were kept on ice for further use.
For drug sensitivity testing, the strains were maintained on Sabouraud dextrose agar (Merck, South Africa) at 35°C for 24 h, after which a loopful of cells (five distinct colonies on a 0.01-ml loop) was taken from a 24-h-old agar plate and suspended in 5 ml of distilled water. The turbidity of each strain suspension was adjusted using a spectrophotometer (EZ Read 800 Research; Biochrom, United Kingdom) to a final concentration of between 0.5 × 105 and 2.5 × 105 CFU/ml (8).
A macrophage cell line, RAW 264.7, was also used. This murine cell line was cultured in RPMI 1640 medium (Sigma-Aldrich, South Africa) supplemented with 10% fetal bovine serum (Biochrom, Germany), 20 U/ml penicillin (Sigma-Aldrich, South Africa), 20 g/ml streptomycin (Sigma-Aldrich, USA), and 2 mM l-glutamine (Sigma-Aldrich, South Africa) in a CO2 incubator (5%) at 37°C. The cell line (a kind donation from P. Masoko and R. Makola, University of Limpopo, South Africa) was originally obtained from the American Type Culture Collection (USA). Before use, cells were standardized to 1 × 105 cells/ml and seeded into the wells of a sterile, disposable 96-well flat-bottom microtiter plate(s) (Greiner Bio-One, Germany).
Drugs.
Standard powders of aspirin (acetylsalicylic acid; Sigma-Aldrich, South Africa), ibuprofen (Sigma-Aldrich, South Africa), fluconazole (Sigma-Aldrich, South Africa), and amphotericin B (Sigma-Aldrich, South Africa) were used in this study. Aspirin was prepared in absolute ethanol (Merck, South Africa) to yield a stock solution of 1,000 μg/ml, and ibuprofen was dissolved in dimethyl sulfoxide (DMSO; final stock solution of 1,000 μg/ml; Merck, South Africa). Fluconazole was reconstituted in distilled water (final stock solution of 1,000 μg/ml), while amphotericin B was dissolved in dimethyl sulfoxide (Merck, South Africa) to yield a stock concentration of 1,000 μg/ml.
Drug susceptibility testing.
Drug susceptibility tests were performed using broth microdilution protocols according to the method outlined by the European Committee on Antimicrobial Susceptibility Testing (8). For drug susceptibility testing, the effects of aspirin and ibuprofen were independently assessed in triplicate for each strain in a direct comparative study using a final drug concentration gradient in RPMI 1640 medium of 0.01 mM (1.80 μg/ml for aspirin and 2.06 μg/ml for ibuprofen), 0.1 mM (18.0 μg/ml for aspirin and 20.6 μg/ml for ibuprofen), and 1 mM (180 μg/ml for aspirin and 206 μg/ml for ibuprofen). These 10-fold microdilution plates (Greiner Bio-One, Germany) were incubated at 35°C for 48 h. The MICs were determined spectrophotometrically after 48 h. In this study, the MIC endpoints were defined as the lowest drug concentration that resulted in a reduction in growth of 50% or more compared with the growth (i.e., the optical density [OD]) in a drug-free control well (8). In anticipation of the checkerboard assay, OD readings were also measured for fluconazole (2 μg/ml, 4 μg/ml, 8 μg/ml, 16 μg/ml, and 32 μg/ml) and amphotericin B (0.25 μg/ml, 0.5 μg/ml, 1 μg/ml, 2 μg/ml, and 4 μg/ml), after incubating the microdilution plates at 35°C for 48 h.
Effect of aspirin and ibuprofen at their MICs on macrophage growth and function.
To determine if aspirin (180 μg/ml) and ibuprofen (206 μg/ml) at their MICs, i.e., the concentrations that led to 50% or more reduction in growth compared with the growth (i.e., OD) of a drug-free control, were toxic to macrophages, standardized macrophages in RPMI 1640 medium (1 × 105 cells/ml in 100 μl) were seeded into wells and treated with aspirin (360 μg/ml in 100 μl of RPMI 1640 medium) or ibuprofen (412 μg/ml in 100 μl of RPMI 1640 medium). The microtiter plates were incubated in a CO2 incubator (5%) for 24 h at 37°C. The OD was measured using a spectrophotometer. Appropriate drug vehicle controls (ethanol and DMSO for aspirin and ibuprofen, respectively) were also included for comparison.
We also determined if macrophage exposure to aspirin or ibuprofen would alter their function, i.e., ability to phagocytose cryptococcal cells (9). Here, we used the phagocytosis stain pHrodo green zymosan A BioParticles (Life Technologies, USA). The stain fluoresces only when it is excited at acidic pH, such as inside the phagosome. Drug-free, standardized cryptococcal cells (1 × 105 CFU/ml) in PBS (which has a neutral pH) were stained (1 μl of stain, 999 μl of cells) for 1 h at room temperature while they were slowly agitated. Next, cryptococcal cells were washed with PBS, spun down, and suspended in 1,000 μl of sterile PBS. A 100-μl suspension of cells was then transferred to microtiter plate wells (Greiner Bio-One, Germany) that had already been seeded with macrophages (100 μl; 1 × 105 cells/ml in RPMI 1640 medium). Aspirin (720 μg/ml in 100 μl of RPMI 1640 medium) or ibuprofen (824 μg/ml in 100 μl of RPMI 1640 medium) was added to the Cryptococcus-macrophage coculture wells at 0 h, and the contents of the wells were allowed to interact in a CO2 incubator (5%) for 2 h or 6 h at 37°C. At the end of the incubation period, the induced fluorescence was measured (excitation wavelength, 492 nm; emission wavelength, 538 nm) using a Fluoroskan Ascent FL (Thermo Scientific, USA) microplate reader, which converts logarithmic signals to relative fluorescence units. Wells with only Cryptococcus-macrophage (i.e., wells without drugs) were included for comparison. In addition, the fluorescence of macrophages alone was also measured in order to normalize the readings (9).
Checkerboard assay.
A checkerboard assay (with pairings of ibuprofen with fluconazole and ibuprofen with amphotericin B), which was performed in a sterile, disposable 96-well flat-bottom microtiter plate (Greiner Bio-One, Germany), was also designed. These microdilution plates were likewise incubated, and at the end of the incubation period (48 h), OD readings were taken, and subsequently, the fractional inhibitory concentration (FIC) index (FICI) was calculated. Fractional inhibitory concentration index (that is, the sum of the FICs [ΣFIC]) was defined as FICA + FICB, where FICA is the MIC of drug A in combination/MIC of drug A alone and FICB is the MIC of drug B in combination/MIC of drug B alone (10). Fractional inhibitory concentration index values were determined to establish if there was synergism (≤0.5), no interaction (>0.5 to 4), or antagonism (>4).
Effect of aspirin and ibuprofen on cellular ultrastructure.
Scanning electron microscopy (SEM) was performed to determine the effect of aspirin and ibuprofen on the outer ultrastructure of the cell. Material for SEM was obtained from cells, i.e., nontreated cells (0 μg/ml), aspirin (180 μg/ml)-treated cells, and ibuprofen (206 μg/ml)-treated cells, that were grown for 48 h at 30°C. After 48 h, cells from the different experimental conditions were transferred to 1.5-ml plastic tubes (Merck). The material was prepared for SEM according to the method of van Wyk and Wingfield (11). In brief, the material was chemically fixed using sodium phosphate-buffered 3% glutardialdehyde (Merck, South Africa) and sodium phosphate-buffered 3% osmium tetroxide (Merck, South Africa), followed by dehydration in a graded ethanol (Merck) series. Next, the material was dried (Bio-Rad Microscience Division, England), mounted on stubs, and coated with gold using an SEM coating system (Bio-Rad Microscience Division, England) (11). Preparations were examined using a Shimadzu Superscan SSX 550 scanning electron microscope (Japan).
Nano-scanning auger microscopy (nano-SAM) was performed to determine the effect of aspirin and ibuprofen on the inner cellular ultrastructure. Here, the same SEM stubs described above were recoated with gold and reexamined using a nano-scanning auger microscope in SEM mode linked to argon (Ar+ ion) etching according to a method previously described by Swart et al. (12). Cells from each experimental condition were then examined with a PHI 700 nanoprobe (Japan) equipped with SEM and scanning auger microscopy (SAM) facilities. For SEM and SAM analyses in the field emission mode, an electron gun was used and was set with a 2.788-A filament current, 3.56-kV extractor voltage, and 175-μA extractor current. A 25-kV, 1-nA electron beam was obtained with these settings for the auger analyses and SEM imaging. The electron beam had a diameter of 12 nm. The electron gun unit had an upper pressure of 8.7E−10 torr, and the pressure of the main chamber was 4.4E−10 torr. Aperture A was used for all the measurements. For SEM the field of view (FOV) was 2 μm. Four cycles per survey, 1 eV per step, and 50 ms per step were used to obtain auger point analyses. The Ar+ ion sputtering gun, which the nanoprobe was also equipped with, was set at a 2-kV beam voltage, 5-μA ion beam current, and a 1- by 1-mm raster area, giving a sputter rate of 15 nm/min (12).
Effect of aspirin and ibuprofen on stress induction.
In order to determine if our two test drugs could lead to stress induction, we performed a Hog1 mitogen-activated protein kinase (MAPK) Western blot assay (13, 14) that was complemented with a phospho-p38 MAPK enzyme-linked immunosorbent assay (ELISA). For Western blot analysis, cultures were treated with 180 μg/ml aspirin, 206 μg/ml ibuprofen, 58,000 μg/ml (1 M) sodium chloride (NaCl; Sigma-Aldrich, South Africa), or 34 μg/ml (1 mM) hydrogen peroxide (H2O2; Sigma-Aldrich, South Africa) for 20 min (grown at 30°C in YNB broth). Immediately after this, the cells were washed twice in PBS, harvested, and suspended in a Tris-EDTA buffer solution, pH 7.4 (Sigma-Aldrich, South Africa). Next, the cells were ruptured using a French press cell disrupter (Constant Cell Disruption Systems, United Kingdom) at a pressure of 36 kips per in.2. The protein content was measured using the Bradford method, equal amounts of protein (50 μg) were loaded onto a 12.5% SDS-polyacrylamide gel, and the gel was stained with Coomassie brilliant blue (Sigma-Aldrich, South Africa). The separated proteins were then transferred to an Immun-Blot polyvinylidene difluoride membrane (Bio-Rad, USA), incubated overnight at 4°C with a rabbit primary antibody specific for Hog1 (Santa Cruz Biotechnology, USA), and then tagged with a secondary anti-rabbit IgG horseradish peroxide-conjugated antibody (Sigma-Aldrich, South Africa) for 2 h at room temperature. The blot was developed using a Gel Doc EZ system (Bio-Rad, USA). This test was done in duplicate. To determine the phosphorylation state of our MAPK protein, we performed a phospho-p38 MAPK ELISA (Sigma-Aldrich, South Africa). The assay was performed on lysates (obtained from nontreated cells and cells treated with 180 μg/ml aspirin or 206 μg/ml ibuprofen) according to the manufacturer's instruction. A positive control, supplied by the manufacturer, was also included for referencing. The OD readings were measured using a spectrophotometer. The obtained OD readings were normalized using the corresponding pan-p38 MAPK readings.
Effect of aspirin and ibuprofen on accumulation of ROS.
We first examined whether aspirin and ibuprofen caused the loss of the mitochondrial membrane potential, which could in part account for the accumulation of reactive oxygen species (ROS). Toward this end, nontreated cells and treated cells (treated with 180 μg/ml aspirin and 206 μg/ml ibuprofen) were seeded at 1 × 106 cells/ml in a sterile black 96-well flat-bottom microtiter plate (Greiner Bio-One, Germany) and incubated for 48 h at 30°C (15). At the end of the incubation period, the mitochondrial membrane potential was measured using the dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) according to the JC-1 mitochondrial membrane potential assay kit instructions (Life Technologies, USA). The mitochondrial membrane potential was calculated as the ratio of the J aggregates (healthy cells; excitation wavelength, 540 nm; emission wavelength, 570 nm) and monomeric forms (unhealthy cells; excitation wavelength, 485 nm; emission wavelength, 535 nm). The induced fluorescence was measured using a Fluoroskan Ascent FL (Thermo Scientific, USA) microplate reader. For measurement of reactive oxygen species accumulation, cells were prepared as described above. Following incubation, 10 μl of a fluorescent dye, i.e., 2′,7-dichlorofluorescin diacetate (DCFHDA; 1 μg/ml; Sigma-Aldrich, South Africa), was reacted with 90 μl of cells for 30 min in the dark at room temperature. The Fluoroskan Ascent FL (Thermo Scientific, USA) microplate reader was used to measure the fluorescence induced at 485 nm/535 nm.
Effect of aspirin and ibuprofen on membrane function.
In order to determine if the membrane function was impaired or maintained after the cells were challenged with aspirin and ibuprofen, we assessed membrane integrity using (i) propidium iodide (PI) stain, (ii) a Toxilight bioassay, and (iii) 4′,6-diamidino-2-phenylindole (DAPI) stain. For all these experiments, cells were once again treated with 180 μg/ml aspirin and 206 μg/ml ibuprofen in a sterile 96-well flat-bottom microtiter plate (Greiner Bio-One, Germany) and incubated for 48 h at 30°C (15). For PI staining, cells were washed twice with PBS, and 99 μl of cells (from each experimental condition) was reacted with 1 μl of PI (Life Technologies) in the dark for 1 h at room temperature. Thereafter, microscope slides were prepared in the presence of an antifade compound, 1,4-diazabicyclo[2.2.2]-octane (DABCO; Sigma-Aldrich, South Africa), before they were viewed using a confocal laser scanning microscope (CLSM; Nikon TE 2000; Nikon, Tokyo, Japan). The Toxilight bioassay (Lonza Rockland, Inc., USA) was performed according to the manufacturer's instructions. This assay quantitatively measures the release of adenylate kinase from cells with damaged membranes into the extracellular environment. The supernatant was collected and reacted with the Toxilight reagent in a new sterile white 96-well flat-bottom microtiter plate (Greiner Bio-One, Germany) for 15 min. The emitted light intensity was measured using a Fluoroskan Ascent FL (Thermo Scientific, USA) microplate reader. For DAPI staining, cells were likewise washed with PBS, and 99 μl of cells (from each experimental condition) was reacted with 1 μl of DAPI (Life Technologies, USA) in the dark for 1 h at room temperature. DABCO was added to prepared slides before the cells were viewed using a CLSM (Nikon TE 2000; Nikon, Tokyo, Japan).
Statistical analysis.
All data, unless stated otherwise, represent mean values from three biological replicates for each experimental condition studied. Where appropriate, standard deviations were calculated and Student t tests were performed to determine the statistical significance of the difference in the data between the different experimental conditions. A P value equal to or below 0.05 was regarded as statistically significant.
RESULTS AND DISCUSSION
Ibuprofen has a greater inhibitory effect than aspirin.
The need for effective antifungal drugs for the better management of cryptococcal infections necessitated this study into the activity of anti-inflammatory drugs, namely, aspirin and ibuprofen. Table 1 details the effect of aspirin and ibuprofen on the metabolic activity of five C. neoformans strains and five C. gattii strains. All 10 tested strains showed a dose-dependent response to both test drugs compared to the response of the drug-free control. However, a closer examination of the comparative data in Table 1 revealed that (i) C. neoformans strains were more sensitive than C. gattii strains and (ii) ibuprofen had a greater inhibitory effect than aspirin on all strains at each drug concentration, i.e., 0.01 mM (1.80 μg/ml for aspirin and 2.06 μg/ml for ibuprofen), 0.1 mM (18.0 μg/ml for aspirin and 20.6 μg/ml for ibuprofen), and 1 mM (180 μg/ml for aspirin and 206 μg/ml for ibuprofen). The greatest growth reduction was achieved at the highest concentration of both drugs. For aspirin, 180 μg/ml is within the recommended concentration in blood (16), while 206 μg/ml ibuprofen is four times the recommended concentration (17). However, the literature documents that ibuprofen is more rapidly metabolized in plasma than aspirin (18). Importantly, patients who are known to have overdosed on ibuprofen (in one case, the patient consumed more than 20 times the peak plasma concentration seen after a single dose of 400 mg ibuprofen [17, 19]) are reported to have experienced no to mild symptoms without experiencing sequelae (17, 20).
TABLE 1.
Effects of aspirin and ibuprofen on metabolic activity of C. neoformans and C. gattii
| Species and strain | OD562a of nontreated cells | % growth reductionb |
|||||
|---|---|---|---|---|---|---|---|
| Aspirin-treated cells |
Ibuprofen-treated cells |
||||||
| 1.80 μg/ml | 18.0 μg/ml | 180 μg/ml | 2.06 μg/ml | 20.6 μg/ml | 206 μg/ml | ||
| C. neoformans | |||||||
| LMPE 028 | 0.403 (0.009) | 11 (0.008) | 32 (0.044) | 71 (0.011) | 13 (0.014) | 36 (0.007) | 75 (0.021) |
| LMPE 030 | 0.411 (0.014) | 10 (0.022) | 34 (0.007) | 72 (0.007) | 12 (0.009) | 37 (0.021) | 74 (0.022) |
| LMPE 043 | 0.398 (0.005) | 12 (0.041) | 31 (0.005) | 70 (0.018) | 13 (0.009) | 34 (0.034) | 75 (0.015) |
| LMPE 046 | 0.402 (0.032) | 14 (0.036) | 38 (0.052) | 74 (0.009) | 16 (0.017) | 40 (0.015) | 76 (0.009) |
| LMPE 047 | 0.401 (0.012) | 13 (0.049) | 35 (0.051) | 73 (0.016) | 14 (0.034) | 37 (0.022) | 74 (0.013) |
| C. gattii | |||||||
| LMPE 045 | 0.401 (0.026) | 10 (0.007) | 30 (0.029) | 70 (0.012) | 11 (0.023) | 32 (0.015) | 73 (0.011) |
| LMPE 048 | 0.400 (0.036) | 12 (0.018) | 32 (0.022) | 72 (0.009) | 13 (0.007) | 33 (0.043) | 74 (0.032) |
| LMPE 052 | 0.390 (0.009) | 10 (0.021) | 29 (0.014) | 69 (0.021) | 12 (0.016) | 31 (0.012) | 71 (0.013) |
| LMPE 054 | 0.401 (0.015) | 11 (0.009) | 31 (0.008) | 70 (0.007) | 12 (0.030) | 32 (0.006) | 72 (0.016) |
| LMPE 070 | 0.394 (0.007) | 10 (0.008) | 30 (0.022) | 70 (0.011) | 11 (0.031) | 31 (0.041) | 71 (0.008) |
OD562, OD at 562 nm.
Percent growth reduction was calculated as 100% − [(OD of treated cells/OD of nontreated cells) · 100%]. Values represent the mean values from three biological replicates, and values in parentheses represent standard deviations.
All subsequent tests were performed on C. neoformans strain LMPE 046, as this strain showed the greatest sensitivity toward all test drugs, including fluconazole and amphotericin B (see Tables S1 and S2 in the supplemental material).
In the present study, it was determined that aspirin at 180 μg/ml and ibuprofen at 206 μg/ml were nontoxic to macrophages, as neither drug yielded a 50% reduction in the growth of the macrophages (Fig. 1). To the point, aspirin effected only an 11% reduction in macrophage growth, while ibuprofen effected a 13% reduction. Importantly, the observed drug effect was not due to the drug vehicles. For this study, macrophages were chosen as a model cell line, as cryptococcal cells have been reported to take up residency within these immune cells without inducing an immunological response (21). Further to the point, these drugs enhanced the ability of macrophages to phagocytose cryptococcal cells (including beads) in the presence of aspirin or ibuprofen (which yielded significantly higher relative fluorescence unit [RFU] values) compared to their ability in the absence of these drugs (which yielded significantly lower RFU values) after 2 h and 6 h (Fig. 2). The phagocytic capability of macrophages was also shown to increase over time when studying the RFU values at both 2 h and 6 h. Once more, the observed effect was not due to the drug vehicles. The findings presented in Figure 2 further support the argument that aspirin and ibuprofen, at their respective MICs, do not negatively affect macrophages, as shown in Fig. 1.
FIG 1.
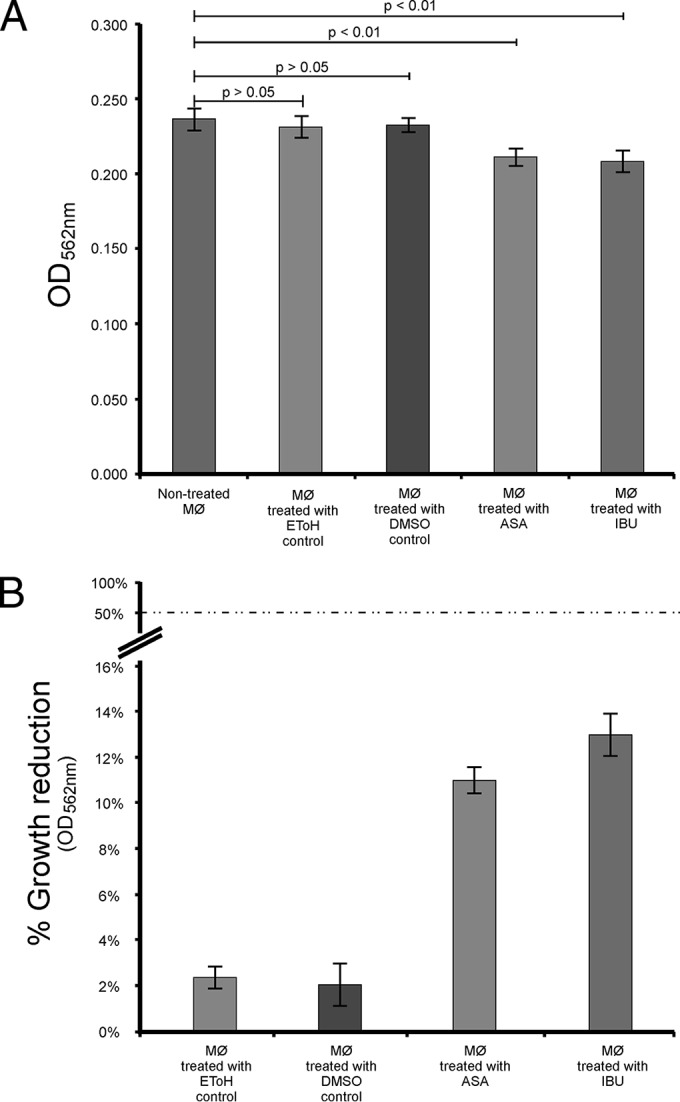
Direct effect of aspirin and ibuprofen on macrophage (MØ) growth (A) and the corresponding expression of this effect as the percent reduction in growth (B). Analysis of the data revealed that aspirin at 180 μg/ml (1 mM) effected only an 11% reduction in growth, while ibuprofen at 206 μg/ml (1 mM) effected a 13% reduction. The findings suggest that aspirin and ibuprofen are nontoxic to macrophages at the tested concentrations, as they did not yield a 50% reduction in growth. Ethanol (EToH) and DMSO were included as controls. ASA, acetylsalicylic acid (aspirin); IBU, ibuprofen.
FIG 2.
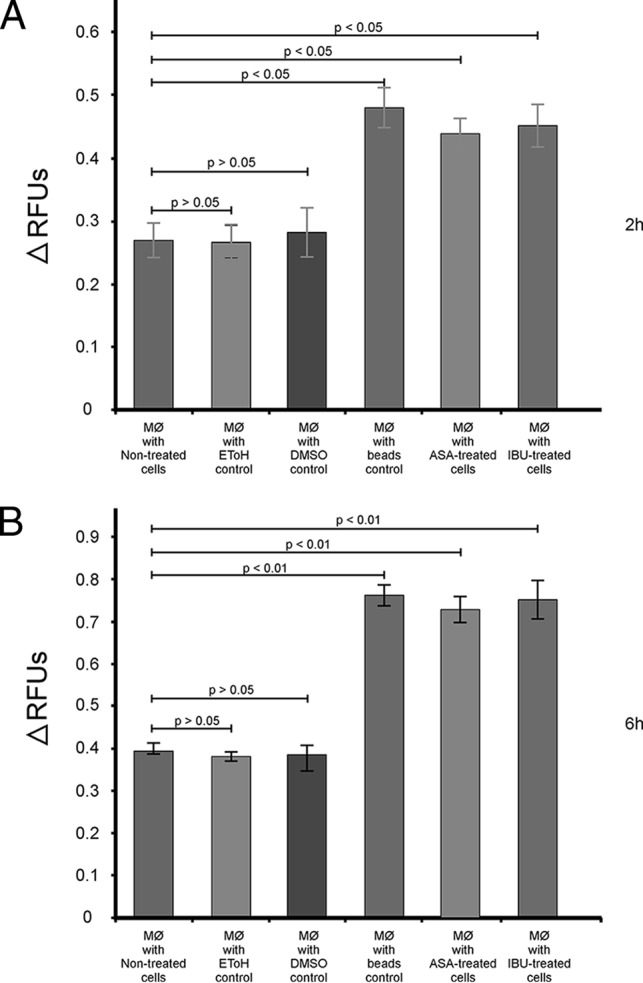
The chemosensitization of macrophages (MØ) by aspirin and ibuprofen. The ability of macrophages to internalize cryptococcal cells in the absence (nontreated cells) and presence of aspirin or ibuprofen was measured using the phagocytosis stain pHrodo green zymosan A BioParticles after 2 h (A) and 6 h (B). Addition of aspirin and ibuprofen significantly enhanced (P > 0.05) the ability of macrophages to internalize cryptococci compared to that of no treatment at both times. This further indicates that the two drugs do not negatively affect macrophages, as suggested in Fig. 1. Ethanol (EToH), DMSO, and beads were included as controls.
Ibuprofen acts in synergy with fluconazole and amphotericin B.
In our study, the MICs for fluconazole and amphotericin B were defined to be 8 μg/ml and 1 μg/ml, respectively (see Tables S1 and S2 in the supplemental material). Since ibuprofen was more effective than aspirin, it was subsequently paired with fluconazole or with amphotericin B (Table 2). The combined effect of all drug pairings did not yield total growth inhibition. Nonetheless, synergistic outcomes were observed. More encouragingly, all drugs (ibuprofen, fluconazole, and amphotericin B) were able to effect synergism at concentrations that were lower than their individually defined MICs. The latter findings thus speak to the possible clinical application of ibuprofen with amphotericin B or ibuprofen with fluconazole in combined therapy. At the same time, however, it is important to point out that careful consideration should be taken when designing such studies in order to realize the desired therapeutic outcome to the exclusion of adverse effects.
TABLE 2.
Combined effects of ibuprofen and amphotericin B and ibuprofen and fluconazole on C. neoformans strain LMPE 046a
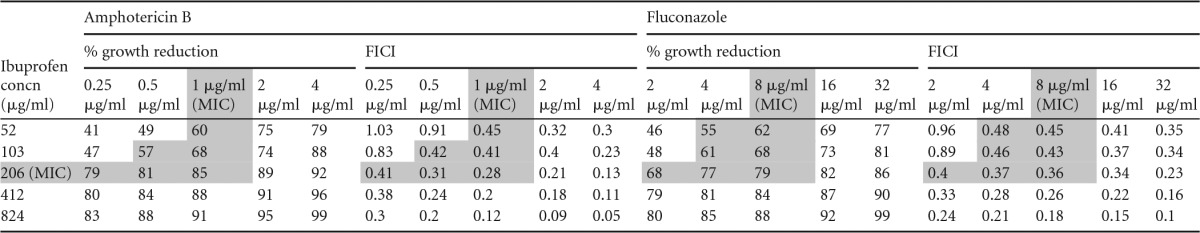
Shading indicates synergism. The other shaded cells represent the corresponding % growth reduction values in relation to the FICI.
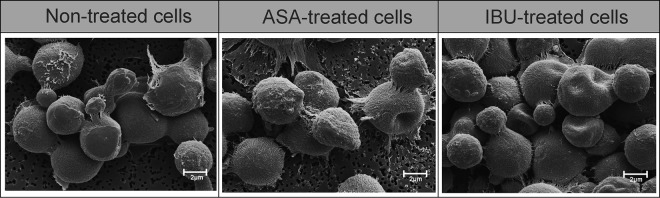
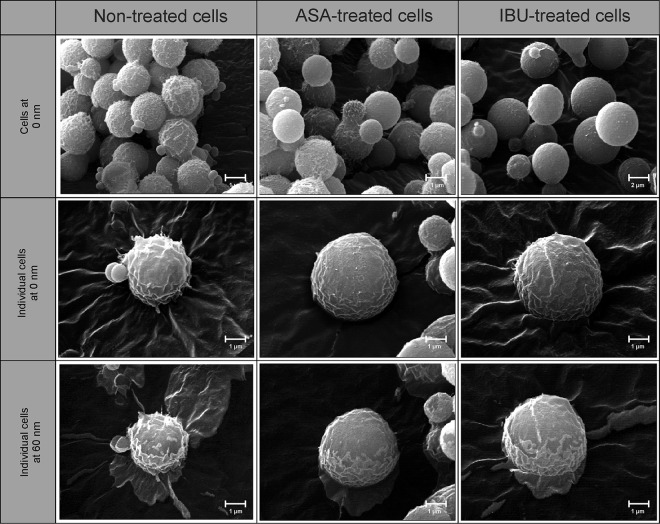
When considering the effects of aspirin and ibuprofen on the outer ultrastructure of strain LMPE 046, we noted no differences with respect to how cells looked, i.e., the degree of smoothness or roughness, across the different experimental conditions (Fig. 3). However, both drugs were able to drastically reduce the size (P < 0.05) of treated cells relative to that of nontreated cells (see Table S3 in the supplemental material). More to the point, nontreated cells were, on average, 3.96 ± 0.08 μm in diameter, whereas aspirin-treated cells were 2.90 ± 0.07 μm in diameter and ibuprofen-treated cells were 3.12 ± 0.08 μm in diameter. However, when examining the inner ultrastructure of cells using nano-SAM (Fig. 4), we could differentiate treated cells from nontreated cells based on their appearances. To be specific, the topography of nontreated cells at depths of 0 nm (outer ultrastructure) and 60 nm (inner ultrastructure) appeared to be rougher than that of aspirin-treated cells and ibuprofen-treated cells, which were less rough. The observed altered organization of the cell membrane/wall may result in the expression of a different physiological outcome by cells in response to aspirin and ibuprofen treatment.
FIG 3.
Scanning electron micrographs depicting the effect of aspirin and ibuprofen on the outer ultrastructure of cells. Cells from the different experimental conditions, i.e., nontreated, aspirin-treated, and ibuprofen-treated cells, could not be differentiated on the basis of their degree of smoothness or roughness. However, they could be differentiated on the basis of their cell diameter (see Table S3 in the supplemental material).
FIG 4.
Nano-scanning auger micrographs depicting the effect of aspirin and ibuprofen on the outer and inner ultrastructure of cells. Cells from the different experimental conditions, i.e., nontreated, aspirin-treated, and ibuprofen-treated cells, could be differentiated on the basis of their appearances. For example, nontreated cells appeared to be rough with spiky protuberances, whereas aspirin- and ibuprofen-treated cells were less rough. The images were taken at depths of 0 nm and 60 nm following etching of thin slices off the cell using an argon laser at a sputter rate of 15 nm/min.
Aspirin and ibuprofen induce stress in treated cells.
The high-osmolarity glycerol (HOG) pathway is well studied in Saccharomyces cerevisiae (22), and the pathway is said to be activated in response to, among other things, osmotic stress, high temperature, and UV irradiation (23). Bahn et al. reported that the phosphorylation of Hog1 MAPK activates downstream target genes to effect adaptation to environmental stressors (24). The Hog1 signaling pathway is, interestingly, also reported to be responsible for the maintenance of cell membrane/wall integrity (13, 14). Thus, in our study, we sought to establish if the treatment of cells with aspirin and ibuprofen can cause membrane damage and, in turn, lead to activation of Hog1 MAPK. When exposed to NaCl, H2O2, aspirin, and ibuprofen, the cells yielded an expected protein band of 50 kDa that was detected using an antibody specific for Hog1 MAPK (Fig. 5A). However, in order to determine the phosphorylation state and activation levels of this MAPK protein, we performed a phospho-p38 MAPK ELISA (Fig. 5B). On the basis of our data, the MAPK levels that were induced by aspirin and ibuprofen were highly comparable (P > 0.05) to the levels induced by the positive control. Additionally, the levels induced by the two test anti-inflammatory drugs, including the positive control, were significantly higher (P < 0.05) than the levels in nontreated cells, which provided a baseline for referencing. The latter points to activation of Hog1 MAPK possibly as a result of an assault on the membrane caused by aspirin and ibuprofen treatment.
FIG 5.
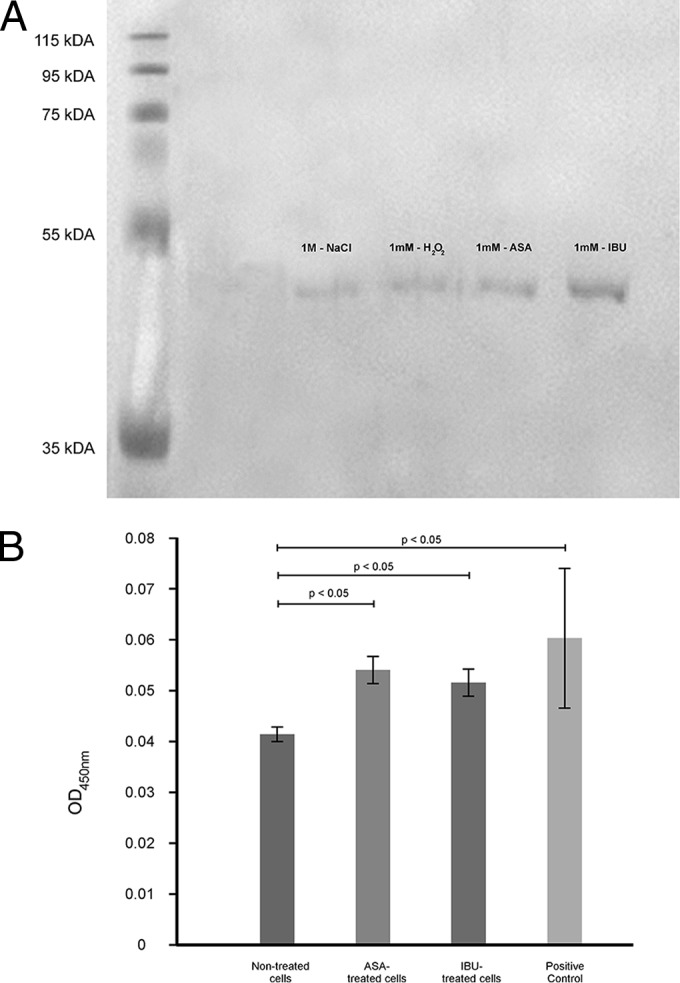
Aspirin and ibuprofen treatment activates a stress response pathway. (A) Western blot analysis using antibodies specific for Hog1 MAPK. The cells were treated for 20 min, and a representative blot depicting the expected protein band of 50 kDa is shown. NaCl and H2O2 were included as positive controls. (B) Quantitative analysis examining the phosphorylation status of MAPK. The levels of phosphorylated p38 of aspirin- and ibuprofen-treated cells were highly comparable (P > 0.05) to those for the positive control (supplied by the manufacturer) and were at the same time significantly higher (P < 0.05) than those for the nontreated cells.
Aspirin and ibuprofen kill cells via ROS-mediated damage.
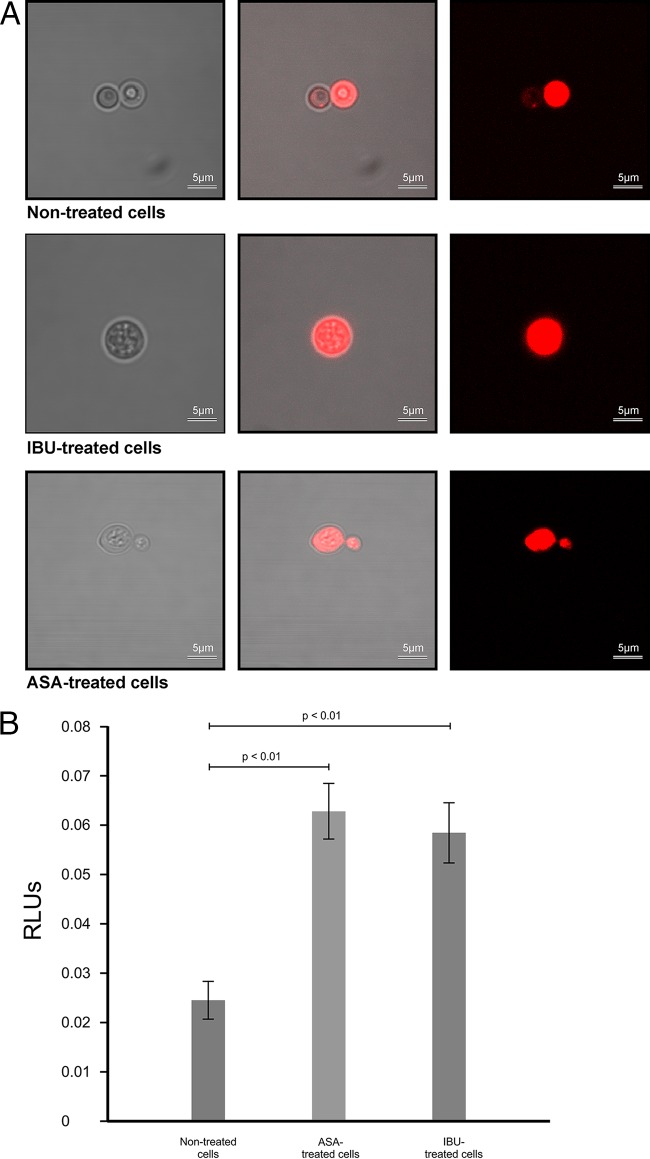
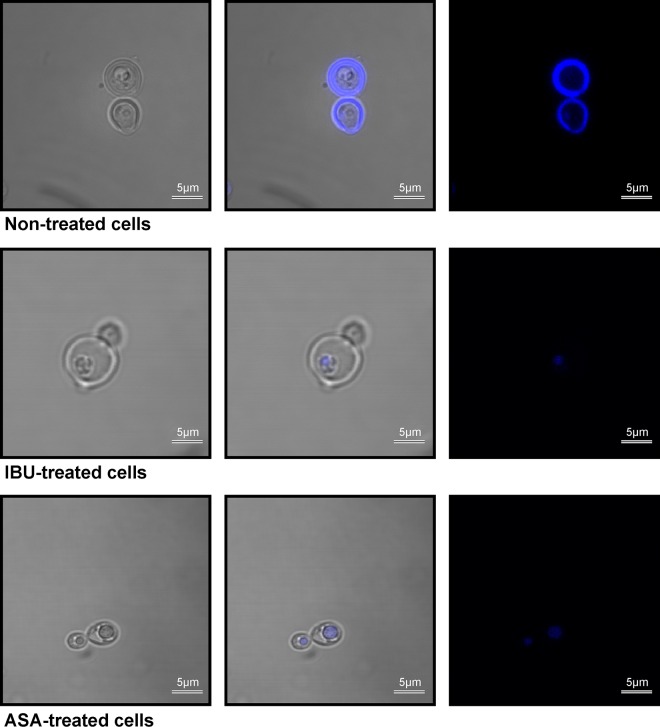
In addition to regulating the transportation of materials needed for survival, the membrane is documented to serve as a site for energy generation (25), which is critical in respiring cells like those of Cryptococcus species. In particular, Joshi and Bakowska proposed that the membrane potential is important for maintaining the function of the respiratory chain in order to generate energy (26), and thus, the loss of membrane potential may prove to have dire consequences. In this part of the study, we showed that aspirin- and ibuprofen-treated cells were characterized by a significant loss of membrane potential (P < 0.01) compared to that for nontreated cells (Fig. 6A). Under normal physiological conditions, respiring cells are reported to produce ROS as part of their oxygen metabolism (27). Further to the point, it is reasonable to conclude that an increase in ROS levels may also manifest when a stressor targeting the site of respiration, i.e., the membrane, alters the membrane potential as well as its capacity to recycle cofactors. To demonstrate this point, we found that the loss of membrane potential was associated with a concomitant increase in ROS levels (Fig. 6B). Here, aspirin- and ibuprofen-treated cells significantly (P < 0.01) accumulated ROS compared to the level of accumulation by nontreated cells. This accumulation of ROS underlies the inescapable fate of cells, wherein they die as a result of oxidative damage to the membrane (Fig. 7 and 8). For both staining experiments, i.e., experiments with PI and DAPI staining, the microscope settings were adjusted by normalizing the gain of blue, green, and red lights to a unit of 1.0, in order to compare the light intensity of images across the different experimental conditions. Figure 7A represents cells (nontreated cells, aspirin- and ibuprofen-treated cells) that were stained with PI. PI was used as an exclusion stain, wherein cells with impaired membrane function were expected to accumulate the stain. The merged white and fluorescent images (Fig. 7A, middle) and fluorescent-only images (Fig. 7A, right) show the clear accumulation of PI inside all examined cells. Similar imaging results were obtained for other PI-stained biological replicates. Interestingly, for nontreated cells, we chose a budding cell that clearly showed a mother cell with the intracellular accumulation of PI and a daughter cell that accumulated minimal amounts of PI and fluoresced less intensely. Conversely, the micrograph showing aspirin-treated cells shows both the mother cell and daughter cell with more intracellular accumulation and that they fluoresced more intensely than the nontreated budding cell. Figure 7B shows the measurement of intracellular metabolites that leaked into the culture supernatants across the different experimental conditions. Aspirin and ibuprofen caused a significant (P < 0.01) accumulation of adenylate kinase in supernatants compared to that achieved by no treatment. We also assessed membrane permeability using DAPI to determine if the stain could cross the cell membrane and reach the nuclei in order to react with the DNA (Fig. 8). Interestingly, in nontreated cells, the stain localized at the membrane and could not be transported across healthy intact membranes. However, in cells with damaged membranes (aspirin- and ibuprofen-treated cells), the stain was able to cross the membrane and eventually reacted with the DNA, as depicted in the combined white and fluorescent images (Fig. 8, middle) and fluorescent-only images (Fig. 8, right). Similar imaging results were obtained for other DAPI-stained biological replicates.
FIG 6.
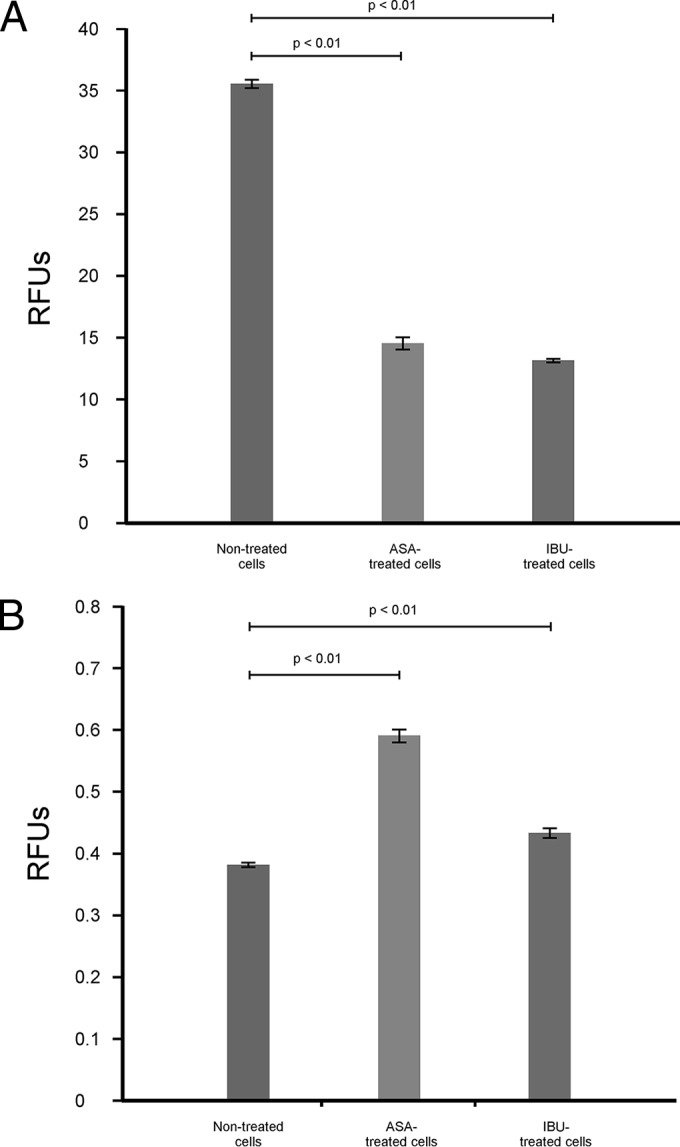
Aspirin- and ibuprofen-treated cells subjected to oxidative stress. (A) Evidence for the loss of membrane potential in aspirin- and ibuprofen-treated cells on account of a larger amount of the monomeric form compared to the amount of the J aggregate. The loss of membrane potential is concomitantly associated with ROS accumulation, as the membrane potential is a driving force that leads to energy production in respiring cells. (B) Evidence for ROS accumulation in treated cells. Aspirin- and ibuprofen-treated cells accumulated more ROS than nontreated cells.
FIG 7.
Effect of aspirin and ibuprofen on membrane function. (A) Confocal micrographs depicting cells from different experimental conditions stained with PI. When cells were treated with aspirin or ibuprofen, more of the PI stain accumulated inside the cells, based on the fluorescence intensity after the gain of blue, green, and red light, which was normalized to 1.0. (Left) White light micrographs; (middle) white light superimposed with fluorescent light micrographs; (right) fluorescent light micrographs. (B) Toxilight bioassay results. When exposed to aspirin and ibuprofen, cells secreted significantly (P < 0.01) more intracellular metabolites than nontreated cells.
FIG 8.
Confocal micrographs depicting cells from different experimental conditions stained with DAPI. When cells were treated with aspirin or ibuprofen, the DAPI stain crossed the damaged cell membrane to reach the cell nuclei and react with DNA. However, in nontreated cells with intact cell membranes, the DAPI stain localized at the membrane and could not be transported inside the cell. (Left) White light micrographs; (middle) white light superimposed with fluorescent light micrographs; (right) fluorescent light micrographs.
Conclusions.
Previously registered nonantimicrobial drugs have been given a new lease on life following the discovery that they also possess antimicrobial qualities (28). Aspirin and ibuprofen are such drugs. This study successfully demonstrated in vitro the anti-Cryptococcus activity of aspirin and ibuprofen. Also encouraging was the finding that ibuprofen acted in synergy with fluconazole and amphotericin B. When considered in totality, this study's findings highlight that the membrane is a target site for aspirin and ibuprofen action. Through alteration of membrane integrity, from disruption of the membrane potential to activity that compromises transportation across the bilayer, these two anti-inflammatory drugs may have revealed a mechanism that is efficient in killing respiring cells, although in eukaryotic organisms of a lower order. It therefore becomes important to demonstrate that the repurposing of these drugs will work in higher eukaryotic host cells. Toward this end, animal studies should be performed to model the treatment of cryptococcal infections using aspirin and ibuprofen in order to establish their therapeutic benefits. The duality in function of these drugs, i.e., as anti-inflammatory drugs and now as antifungal agents, may provide an additional beneficial therapeutic outcome. Therefore, it will also be interesting to see the clinical effect of these drugs on inflammatory conditions induced by pathogens, such as in the treatment of pathogen-induced immune reconstitution inflammatory syndrome. It will also be important to determine if the demonstrated antimicrobial activity of aspirin and ibuprofen could be expanded to other medically important pathogens, in particular, microbes such as Pseudomonas species and Mycobacterium species, which, like Cryptococcus species, are highly aerobic.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the services and assistance offered by the following colleagues at the University of the Free State: (i) H. G. O'Neill for Western blot analysis, (ii) E. Coetsee-Hugo for nano-SAM work, and (iii) C. W. Swart and P. W. van Wyk for SEM and fluorescence microscopy work.
This work was supported by the National Research Foundation of South Africa (grant number UID 87903).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02810-15.
REFERENCES
- 1.Levitz SM, Boekhout T. 2006. Cryptococcus: the once-sleeping giant is fully awake. FEMS Yeast Res 6:461–462. doi: 10.1111/j.1567-1364.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper CR., Jr 2011. Yeasts pathogenic to humans, p 9–20. In Kurtzman CP, Fell JW, Boekhout T (ed), The yeasts, a taxonomic study, 5th ed Elsevier Sciences B.V, Amsterdam, Netherlands. [Google Scholar]
- 3.Kwon-Chung KJ. 2011. Filobasidiella Kwon-Chung. 1975, p 1443–1456. In Kurtzman CP, Fell JW, Boekhout T (ed), The yeast, a taxonomic study, 5th ed Elsevier Sciences B.V, Amsterdam, Netherlands. [Google Scholar]
- 4.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 5.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen M, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebolai OM, Ogundeji AO. 2015. New antifungal discovery from existing chemical compound collections, p 143–158. In Coste AT, Vandeputte P (ed), Antifungals: from genomics to resistance and the development of novel agents. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 7.Sebolai OM, Pohl CH, Botes PJ, van Wyk PW, Kock JL. 2008. The influence of acetylsalicylic acid on oxylipin migration in Cryptococcus neoformans var. neoformans UOFS Y-1378. Can J Microbiol 54:91–96. doi: 10.1139/W07-114. [DOI] [PubMed] [Google Scholar]
- 8.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ, and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2015. EUCAST definitive document E. Def 7.3: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. EUCAST, Copenhagen, Denmark: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_Yeast_testing_definitive.pdf. [Google Scholar]
- 9.Madu UL, Ogundeji AO, Mochochoko BM, Pohl CH, Albertyn J, Swart CW, Allwood JW, Southam AD, Dunn WB, May RC, Sebolai OM. 2015. Cryptococcal 3-hydroxy fatty acids protect cells against amoebal phagocytosis. Front Microbiol 6:1351. doi: 10.3389/fmicb.2015.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meletiadis J, Mouton JW, TeDorsthorst DT, Meis JF, Mouton JW. 2005. Assessing combination of antifungal drugs against yeasts and filamentous fungi: comparison of different drugs interaction models. Med Mycol 43:133–152. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 11.Van Wyk PWJ, Wingfield MJ. 1991. Ascospores ultrastructure and development in Ophiostoma cucullatum. Mycologia 83:698–707. doi: 10.2307/3760427. [DOI] [Google Scholar]
- 12.Swart CW, Dithebe K, Pohl CH, Swart HC, Coetsee E, van Wyk PW, Swarts JC, Lodolo EJ, Kock JL. 2012. Gas bubble formation in the cytoplasm of a fermenting yeast. FEMS Yeast Res 12:867–869. doi: 10.1111/j.1567-1364.12004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman MJ, Spatt D, Winston F. 2011. The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188:325–338. doi: 10.1534/genetics.111.128322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto M, Furuichi Y, Komiyama T. 2012. The high-osmolarity glycerol- and cell wall integrity-MAP kinase pathways of Saccharomyces cerevisiae are involved in adaptation to the action of killer toxin HM-I. Yeast 29:475–485. doi: 10.1002/yea.2927. [DOI] [PubMed] [Google Scholar]
- 15.Thibane VS, Ells R, Hugo A, Albertyn J, van Rensburg WJ, Van Wyk PW, Kock JL, Pohl CH. 2012. Polyunsaturated fatty acids cause apoptosis in C. albicans and C. dubliniensis biofilms. Biochim Biophys Acta 1820:1463–1468. doi: 10.1016/j.bbagen.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Levy G. 1976. Pharmacokinetics of aspirin in man. J Investig Dermatol 67:667–668. doi: 10.1111/1523-1747.ep12544495. [DOI] [Google Scholar]
- 17.Seifert SA, Bronstein AC, McGuire T. 2000. Massive ibuprofen ingestion with survival. J Toxicol Clin Toxicol 38:55–57. doi: 10.1081/CLT-100100917. [DOI] [PubMed] [Google Scholar]
- 18.Paulus HE. 1990. FDA Arthritis Advisory Committee meeting: guidelines for approving nonsteroidal antiinflammatory drugs for over-the-counter use. Arthritis Rheum 33:1056–1058. doi: 10.1002/art.1780330722. [DOI] [PubMed] [Google Scholar]
- 19.Volans G. 2015. Human toxicity of ibuprofen, p 500–519. In Rainsford KD. (ed), Ibuprofen: discovery, development and therapeutics. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 20.Volans G, Monaghan J, Colbridge M. 2003. Ibuprofen overdose. Int J Clin Pract Suppl 135:54–60. [PubMed] [Google Scholar]
- 21.Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot Cell 9:835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rourke SM, Herskowitz I, O'Shea EK. 2002. Yeast go the whole HOG for the hyperosmotic response. Trends Genet 18:405–412. doi: 10.1016/S0168-9525(02)02723-3. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez C, Berl T, Rivard CJ, Edelstein CL, Capasso JM. 2004. Phosphorylation of MAP kinase-like proteins mediates the response of the halotolerant alga Dunaliella viridis to hypertonic shock. Biochim Biophys Acta 1644:61–69. [DOI] [PubMed] [Google Scholar]
- 24.Bahn Y-S, Kojima K, Cox GM, Heitman J. 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell 16:2285–2300. doi: 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberts B, Bray D, Hopkin K. 2009. Essential cell biology, 3rd ed Garland Sciences, New York, NY. [Google Scholar]
- 26.Joshi DC, Bakowska JC. 2011. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp 51:2704. doi: 10.3791/2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkinezos IG, Moraes CT. 2001. Reactive oxygen species and mitochondrial diseases. Cell Dev Biol 12:449–457. doi: 10.1006/scdb.2001.0282. [DOI] [PubMed] [Google Scholar]
- 28.Cederlund H, Mardh PA. 1993. Antimicrobial activities of non-antibiotic drugs. J Antimicrob Chemother 32:355–365. doi: 10.1093/jac/32.3.355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.